Encapsulation of Lutein via Microfluidic Technology: Evaluation of Stability and In Vitro Bioaccessibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
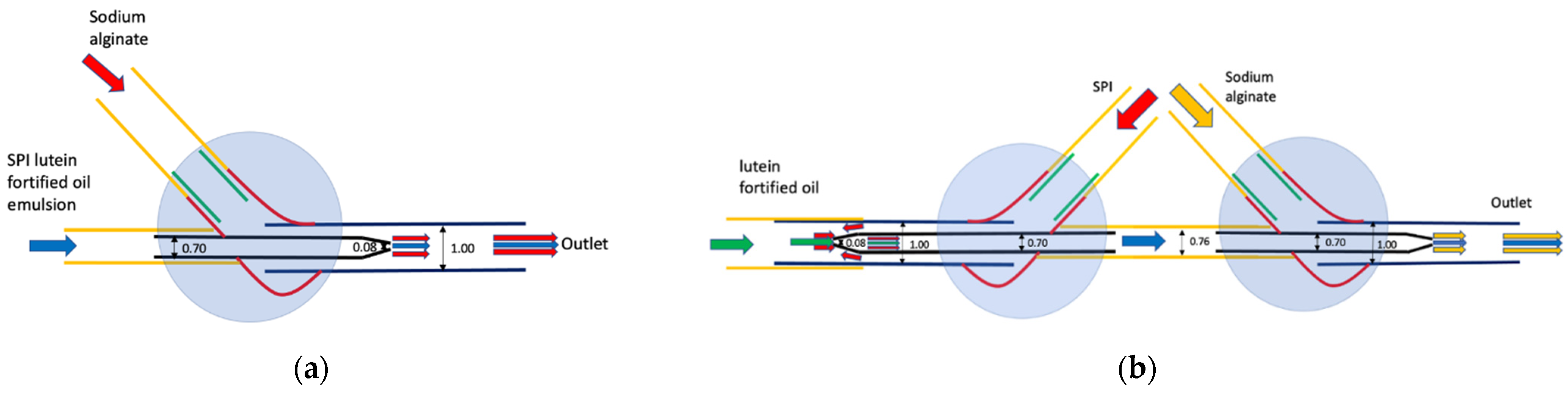
2.3. Assembly of Microfluidic Devices
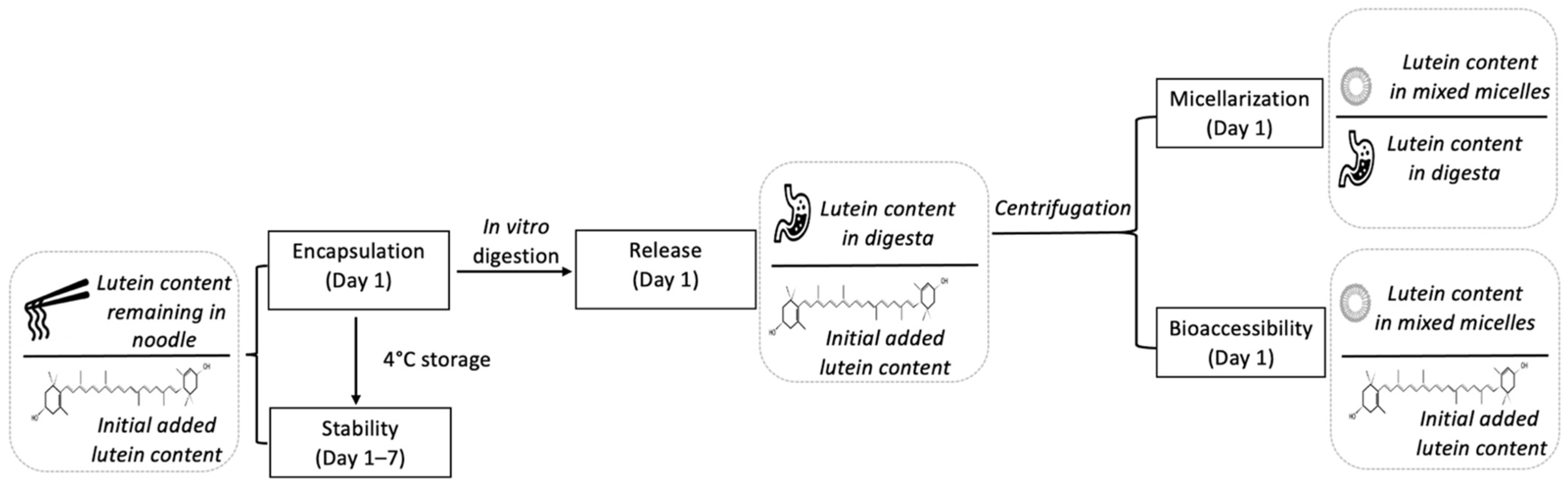
2.4. In Vitro Digestion
2.5. Extraction and Quantification of Lutein
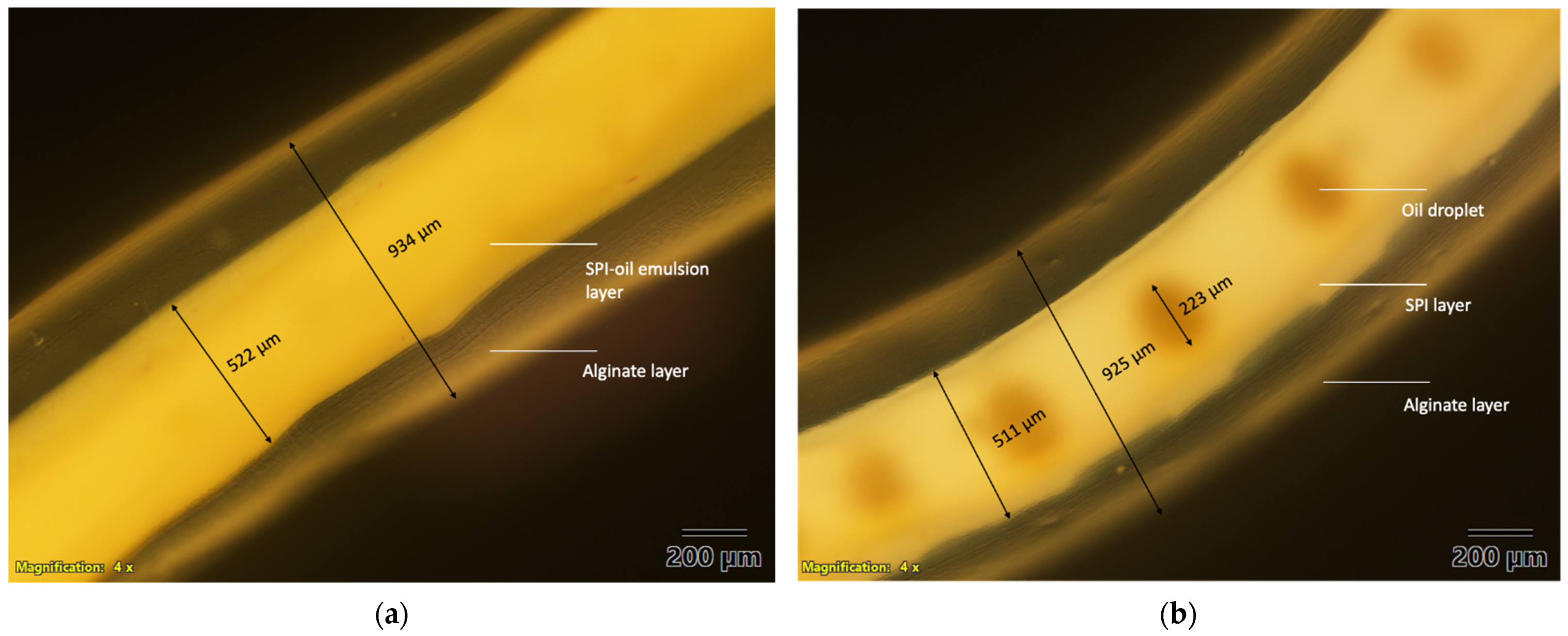
2.6. Optical Microscopy
2.7. Storage Stability
2.8. Bioaccessibility, Release and Micellarization of Lutein
2.9. Statistical Analysis
3. Results and Discussion
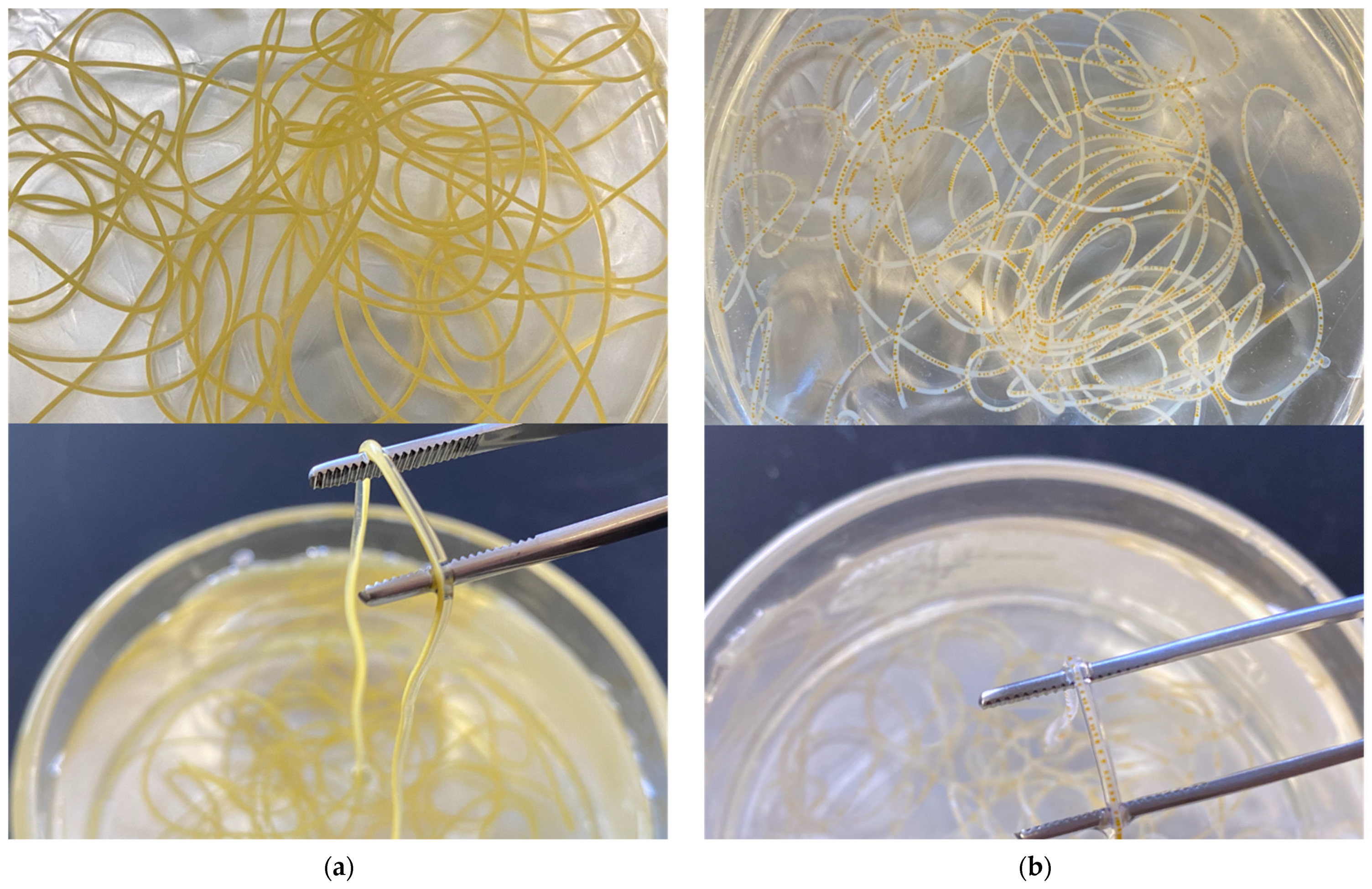
3.1. Structure Characteristics of the Microfluidic Noodle
3.2. Stability of Lutein
3.3. Bioaccessibility, Release and Micellarization of Lutein
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Young, A.J.; Lowe, G.L. Carotenoids—Antioxidant properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Perera, C.O.; Yen, G.M. Functional properties of carotenoids in human health. Int. J. Food Prop. 2007, 10, 201–230. [Google Scholar] [CrossRef]
- Di Pietro, N.; Di Tomo, P.; Pandolfi, A. Carotenoids in cardiovascular disease prevention. JSM Atheroscler. 2016, 1, 1002. [Google Scholar]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.E.; Dennison, J. The photobiology of lutein and zeaxanthin in the eye. J. Ophthalmol. 2015, 2015, 687173. [Google Scholar] [CrossRef] [Green Version]
- Koushan, K.; Rusovici, R.; Li, W.; Ferguson, L.R.; Chalam, K.V. The role of lutein in eye-related disease. Nutrients 2013, 5, 1823–1839. [Google Scholar] [CrossRef]
- Junghans, A.; Sies, H.; Stahl, W. Biophysics. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 2001, 391, 160–164. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef]
- Brown, L.; Rimm, E.B.; Seddon, J.M.; Giovannucci, E.L.; Chasan-Taber, L.; Spiegelman, D.; Willett, W.C.; Hankinson, S.E. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am. J. Clin. Nutr. 1999, 70, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Moeller, S.M.; Voland, R.; Tinker, L.; Blodi, B.A.; Klein, M.L.; Gehrs, K.M.; Johnson, E.J.; Snodderly, D.M.; Wallace, R.B.; Chappell, R. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study (CAREDS), an ancillary study of the women’s health initiative. Arch. Ophthalmol. 2008, 126, 354–364. [Google Scholar] [CrossRef]
- Sy, C.; Gleize, B.; Dangles, O.; Landrier, J.F.; Veyrat, C.C.; Borel, P. Effects of physicochemical properties of carotenoids on their bioaccessibility, intestinal cell uptake, and blood and tissue concentrations. Mol. Nutr. Food Res. 2012, 56, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Díaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Yao, K.; McClements, D.J.; Xiang, J.; Zhang, Z.; Cao, Y.; Xiao, H.; Liu, X. Improvement of carotenoid bioaccessibility from spinach by co-ingesting with excipient nanoemulsions: Impact of the oil phase composition. Food Funct. 2019, 10, 5302–5311. [Google Scholar] [CrossRef]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro-and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Zhao, C.; Wei, L.; Yin, B.; Liu, F.; Li, J.; Liu, X.; Wang, J.; Wang, Y. Encapsulation of lycopene within oil-in-water nanoemulsions using lactoferrin: Impact of carrier oils on physicochemical stability and bioaccessibility. Int. J. Biol. Macromol. 2020, 153, 912–920. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Dias, M.I.; Ferreira, I.C.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Joseph, N.; Feng, S.; Jellicoe, M.; Raston, C.L. Application of microfluidic technology in food processing. Food Funct. 2020, 11, 5726–5737. [Google Scholar] [CrossRef]
- Cheng, S.; Deng, J.; Zheng, W.; Jiang, X. Microfluidics for biomedical applications. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 368–383. [Google Scholar]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary microfluidics in microchannels: From microfluidic networks to capillaric circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J.; Zou, L.; Zhang, R.; Salvia-Trujillo, L.; Kumosani, T.; Xiao, H. Enhancing nutraceutical performance using excipient foods: Designing food structures and compositions to increase bioavailability. Compr. Rev. Food Sci. Food Saf. 2015, 14, 824–847. [Google Scholar] [CrossRef]
- Mashurabad, P.C.; Palika, R.; Jyrwa, Y.W.; Bhaskarachary, K.; Pullakhandam, R. Dietary fat composition, food matrix and relative polarity modulate the micellarization and intestinal uptake of carotenoids from vegetables and fruits. J. Food Sci. Technol. 2017, 54, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Pullakhandam, R.; Failla, M.L. Micellarization and intestinal cell uptake of β-carotene and lutein from drumstick (Moringa oleifera) leaves. J. Med. Food. 2007, 10, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Nagao, A. Effects of mixed micellar lipids on carotenoid uptake by human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2012, 76, 875–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Casado, S.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. In vitro bioaccessibility of colored carotenoids in tomato derivatives as affected by ripeness stage and the addition of different types of oil. J. Food Sci. 2018, 83, 1404–1411. [Google Scholar] [CrossRef] [Green Version]
- Teixé-Roig, J.; Oms-Oliu, G.; Ballesté-Muñoz, S.; Odriozola-Serrano, I.; Martín-Belloso, O. Improving the in vitro bioaccessibility of β-carotene using pectin added nanoemulsions. Foods. 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Enhanced delivery of lipophilic bioactives using emulsions: A review of major factors affecting vitamin, nutraceutical, and lipid bioaccessibility. Food Funct. 2018, 9, 22–41. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- Trif, M.; Vodnar, D.C.; Mitrea, L.; Rusu, A.V.; Socol, C.T. Design and development of oleoresins rich in carotenoids coated microbeads. Coatings 2019, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Liu, X.; McClements, D.J.; Cao, Y.; Xiao, H. Enhancement of phytochemical bioaccessibility from plant-based foods using excipient emulsions: Impact of lipid type on carotenoid solubilization from spinach. Food Funct. 2018, 9, 4352–4365. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; López-López, I.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Cofrades, S.; Pérez-Sacristán, B.; Delgado-Pando, G.; Jiménez-Colmenero, F. Lutein-enriched frankfurter-type products: Physicochemical characteristics and lutein in vitro bioaccessibility. Food Chem. 2010, 120, 741–748. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Loh, W.W.; Sutanto, C.N.; Yao, Y.; Kim, J.E. Skin carotenoids status and plasma carotenoids: Biomarkers of dietary carotenoids, fruits and vegetables for middle-aged and older Singaporean adults. Br. J. Nutr. 2021, 126, 1398–1407. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, W.; Jin, W.; Shah, B.R.; Li, Y.; Li, B. Influence of anionic alginate and cationic chitosan on physicochemical stability and carotenoids bioaccessibility of soy protein isolate-stabilized emulsions. Food Res. Int. 2015, 77, 419–425. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; McClements, D.J.; Zou, L. Encapsulation of β-carotene-loaded oil droplets in caseinate/alginate microparticles: Enhancement of carotenoid stability and bioaccessibility. J. Funct. Foods 2018, 40, 527–535. [Google Scholar] [CrossRef]
- Matalanis, A.; McClements, D.J. Impact of encapsulation within hydrogel microspheres on lipid digestion: An in vitro study. Food Biophys. 2012, 7, 145–154. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Savic Gajic, I.M.; Savic, I.M.; Gajic, D.G.; Dosic, A. Ultrasound-assisted extraction of carotenoids from orange peel using olive oil and its encapsulation in ca-alginate beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef]
- Steiner, B.M.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci. Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Xu, D.; Aihemaiti, Z.; Cao, Y.; Teng, C.; Li, X. Physicochemical stability, microrheological properties and microstructure of lutein emulsions stabilized by multilayer membranes consisting of whey protein isolate, flaxseed gum and chitosan. Food Chem. 2016, 202, 156–164. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin). Food Hydrocoll. 2015, 45, 175–185. [Google Scholar] [CrossRef]
- Kenmogne-Domguia, H.B.; Meynier, A.; Viau, M.; Llamas, G.; Genot, C. Gastric conditions control both the evolution of the organization of protein-stabilized emulsions and the kinetic of lipolysis during in vitro digestion. Food Funct. 2012, 3, 1302–1309. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.; Al Juhaimi, F. Fatty acid composition and tocopherol profiles of safflower (Carthamus tinctorius L.) seed oils. Nat. Prod. Res. 2015, 29, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Kotake-Nara, E.; Hase, M. Effects of fats and oils on the bioaccessibility of carotenoids and vitamin E in vegetables. Biosci. Biotechnol. Biochem. 2013, 77, 1055–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, R.M.; Yao, L.; She, L.; Furr, H.C. A comparison of lycopene and astaxanthin absorption from corn oil and olive oil emulsions. Lipids 2000, 35, 803–806. [Google Scholar] [CrossRef]
- Verkempinck, S.; Salvia-Trujillo, L.; Moens, L.; Carrillo, C.; Van Loey, A.; Hendrickx, M.; Grauwet, T. Kinetic approach to study the relation between in vitro lipid digestion and carotenoid bioaccessibility in emulsions with different oil unsaturation degree. J. Funct. Foods 2018, 41, 135–147. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of particle size on lipid digestion and β-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Enhancement of carotenoid bioaccessibility from carrots using excipient emulsions: Influence of particle size of digestible lipid droplets. Food Funct. 2016, 7, 93–103. [Google Scholar] [CrossRef]
- Jeffery, J.L.; Turner, N.D.; King, S.R. Carotenoid bioaccessibility from nine raw carotenoid-storing fruits and vegetables using an in vitro model. J. Sci. Food Agric. 2012, 92, 2603–2610. [Google Scholar] [CrossRef]
- Achir, N.; Randrianatoandro, V.A.; Bohuon, P.; Laffargue, A.; Avallone, S. Kinetic study of β-carotene and lutein degradation in oils during heat treatment. Eur. J. Lipid Sci. Technol. 2010, 112, 349–361. [Google Scholar] [CrossRef]
- Ahmad, F.T.; Asenstorfer, R.E.; Soriano, I.R.; Mares, D.J. Effect of temperature on lutein esterification and lutein stability in wheat grain. J. Cereal Sci. 2013, 58, 408–413. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Liu, C.; Dai, T.; Zhang, R.; Zhang, Z.; McClemnets, D.J. Enhancement of carotenoid bioaccessibility from tomatoes using excipient emulsions: Influence of particle size. Food Biophys. 2017, 12, 172–185. [Google Scholar] [CrossRef]
- Yonekura, L.; Nagao, A. Soluble fibers inhibit carotenoid micellization in vitro and uptake by Caco-2 cells. Biosci. Biotechnol. Biochem. 2009, 73, 196–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhöfer, G.; Schmidt-Erfurth, U. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar] [CrossRef]
- Murray, I.J.; Makridaki, M.; van der Veen, R.L.; Carden, D.; Parry, N.R.; Berendschot, T.T. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: The CLEAR study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1781–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.C.; Wu, C.R.; Wang, Z.L.; Wang, L.Y.; Han, Y.; Sun, S.L.; Li, Q.S.; Ma, L. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac. J. Clin. Nutr. 2017, 26, 406–411. [Google Scholar]
- Ma, L.; Hao, Z.-X.; Liu, R.-R.; Yu, R.-B.; Shi, Q.; Pan, J.-P. A dose–response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract. Graefe Arch. Clin. Exp. Ophthalmol. 2014, 252, 63–70. [Google Scholar] [CrossRef]
- Shao, A.; Hathcock, J.N. Risk assessment for the carotenoids lutein and lycopene. Regul. Toxicol. Pharmacol. 2006, 45, 289–298. [Google Scholar] [CrossRef]

| Co-Flow + OL (%) | Co-Flow + SO (%) | Combination-Flow + OL (%) | Combination-Flow + SO (%) |
|---|---|---|---|
| 83.3 ± 1.5 ab | 88.7 ± 3.6 ab | 80.0 ± 0.6 b | 92.1 ± 1.6 a |
| Lutein | Lutein in Micelles (μg) | Lutein in Digesta (μg) | Bioaccessibility (%) | Release (%) | Micellarization (%) |
|---|---|---|---|---|---|
| Co-flow + OL | 29.8 ± 2.2 | 640.8 ± 21.3 | 3.4 ± 0.3 ab | 73.7 ± 2.5 a | 4.6 ± 0.3 c |
| Combination-flow + OL | 27.1 ± 1.6 | 401.8 ± 12.4 | 3.1 ± 0.2 ab | 46.2 ± 1.4 bc | 6.8 ± 0.4 b |
| Co-flow + SO | 23.7 ± 1.8 | 477.6 ± 24.1 | 2.7 ± 0.2 b | 54.9 ± 2.8 b | 5.0 ± 0.4 bc |
| Combination-flow + SO | 34.8 ± 1.7 | 369.1 ± 22.2 | 4.0 ± 0.2 a | 42.4 ± 2.6 c | 9.4 ± 0.5 a |
| Device Type | - | - | p = 0.051 | p < 0.05 | p < 0.05 |
| Oil Type | - | - | p > 0.05 | p < 0.05 | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Lin, J.J.; Chee, X.Y.J.; Liu, M.H.; Khan, S.A.; Kim, J.E. Encapsulation of Lutein via Microfluidic Technology: Evaluation of Stability and In Vitro Bioaccessibility. Foods 2021, 10, 2646. https://doi.org/10.3390/foods10112646
Yao Y, Lin JJ, Chee XYJ, Liu MH, Khan SA, Kim JE. Encapsulation of Lutein via Microfluidic Technology: Evaluation of Stability and In Vitro Bioaccessibility. Foods. 2021; 10(11):2646. https://doi.org/10.3390/foods10112646
Chicago/Turabian StyleYao, Yuanhang, Jiaxing Jansen Lin, Xin Yi Jolene Chee, Mei Hui Liu, Saif A. Khan, and Jung Eun Kim. 2021. "Encapsulation of Lutein via Microfluidic Technology: Evaluation of Stability and In Vitro Bioaccessibility" Foods 10, no. 11: 2646. https://doi.org/10.3390/foods10112646






