Preparation of Anti-Aristolochic Acid I Monoclonal Antibody and Development of Chemiluminescent Immunoassay and Carbon Dot-Based Fluoroimmunoassay for Sensitive Detection of Aristolochic Acid I
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Animals
2.2. Instruments
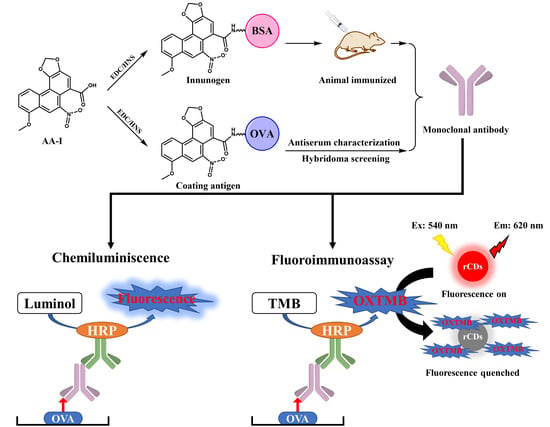
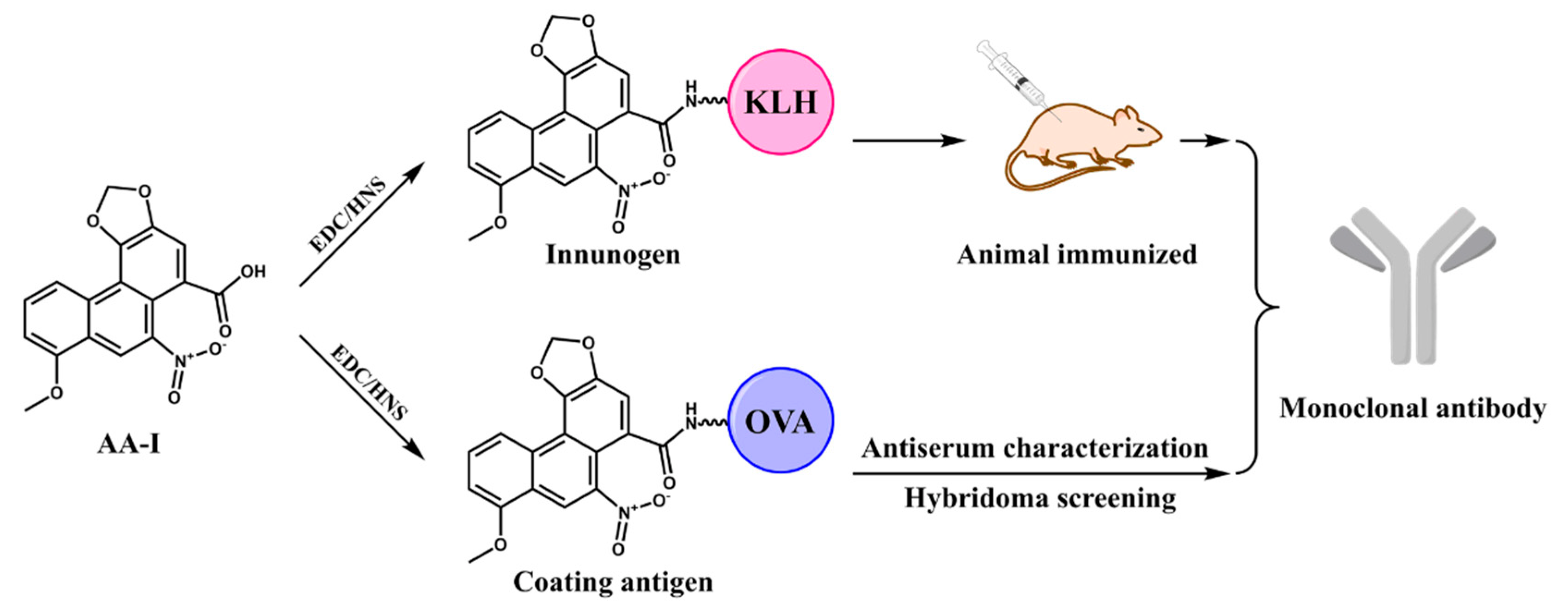
2.3. Production of Monoclonal Antibody
2.4. Development of Chemiluminescent Immunoassay
2.5. Development of Fluoroimmunoassay
2.6. Recovery Test
3. Results
3.1. Characterization of Antisera
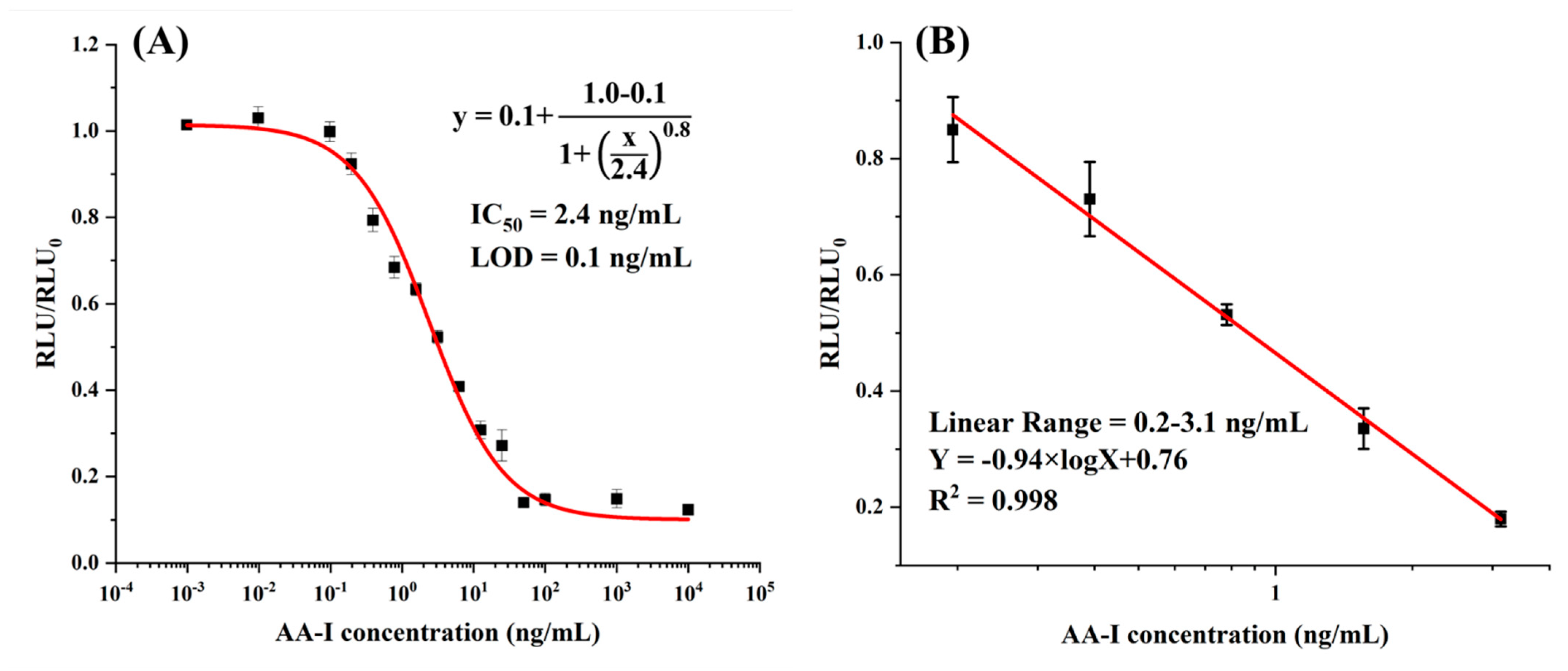
3.2. Development of CLEIA
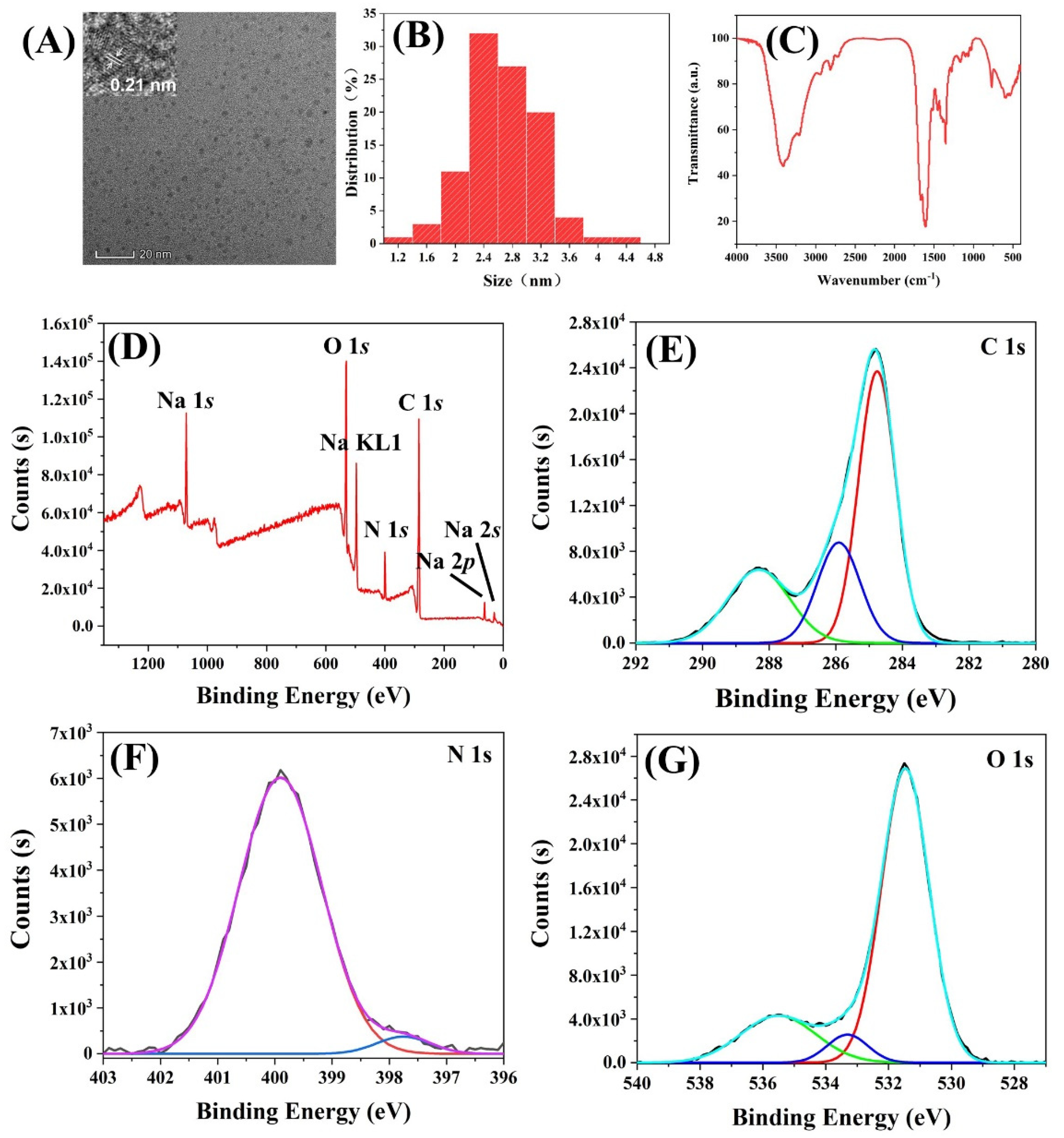
3.3. Characterization of rCDs
3.4. Development of FIA
3.5. Recovery Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, F.Y.; Lin, Y.H.; Su, C.C. A sensitive enzyme-linked immunosorbent assay for detecting carcinogenic aristolochic acid in herbal remedies. J. Agric. Food Chem. 2006, 54, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.H.; Lee, Y.T.; Hsieh, H.S.; Hwang, D.F. Short-term toxicity of aristolochic acid, aristolochic acid-I and aristolochic acid-II in rats. Food Chem. Toxicol. 2008, 46, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Wu, M.L.; Deng, J.F.; Hwang, D.F. High-performance liquid chromatographic determination for aristolochic acid in medicinal plants and slimming products. J. Chromatogr. B 2002, 766, 169–174. [Google Scholar] [CrossRef]
- Oraby, H.F.; Alarfaj, N.A.; El-Tohamy, M.F. Gold nanoparticle-enhanced luminol/ferricyanide chemiluminescence system for aristolochic acid-I detection in medicinal plants and slimming products. Green Chem. Lett. Rev. 2017, 10, 138–147. [Google Scholar] [CrossRef]
- Ioset, J.R.; Raoelison, G.E.; Hostettmann, K. Detection of aristolochic acid in Chinese phytomedicines and dietary supplements used as slimming regimens. Food Chem. Toxicol. 2003, 41, 29–36. [Google Scholar] [CrossRef]
- Nault, J.; Letouzé, E. Mutational processes in hepatocellular carcinoma: The story of aristolochic acid. Semin. Liver Dis. 2019, 39, 334–340. [Google Scholar] [CrossRef]
- Nortier, J.L.; Martinez, M.M.; Schmeiser, H.H.; Arlt, V.M.; Bieler, C.A.; Petein, M.; Depierreux, M.F.; De Pauw, L.; Abramowicz, D.; Vereerstraeten, P.; et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N. Engl. J. Med. 2000, 342, 1686–1692. [Google Scholar] [CrossRef] [Green Version]
- Cosyns, J.; Jadoul, M.; Squifflet, J.; van Cangh, P.; van Ypersele de Strihou, C. Urothelial malignancy in nephropathy due to Chinese herbs. Lancet 1994, 344, 188. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhao, X.H.; Sun, Z.H.; Li, G.C.; Liu, G.C.; Sun, L.R.; Hou, J.Q.; Zhou, W. Recognition of the toxicity of aristolochic acid. J. Clin. Pharm. Ther. 2019, 44, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Jadot, I.; Declèves, A.; Nortier, J.; Caron, N. An integrated view of aristolochic acid nephropathy: Update of the literature. Int. J. Mol. Sci. 2017, 18, 297. [Google Scholar] [CrossRef] [Green Version]
- Kocic, G.; Gajic, M.; Tomovic, K.; Hadzi Djokic, J.; Anderluh, M.; Smelcerovic, A. Purine adducts as a presumable missing link for aristolochic acid nephropathy-related cellular energy crisis, potential anti-fibrotic prevention and treatment. Brit. J. Pharmacol. 2021, 178, 4411–4427. [Google Scholar]
- Koyama, N.; Yonezawa, Y.; Nakamura, M.; Sanada, H. Evaluation for a mutagenicity of aristolochic acid by Pig-a and PIGRET assays in rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. Environ. Mutagenesis 2016, 811, 80–85. [Google Scholar] [CrossRef]
- Vanherweghem, J.; Tielemans, C.; Abramowicz, D.; Depierreux, M.; Vanhaelen-Fastre, R.; Vanhaelen, M.; Dratwa, M.; Richard, C.; Vandervelde, D.; Verbeelen, D.; et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 1993, 341, 387–391. [Google Scholar] [CrossRef]
- Depierreux, M.; Van Damme, B.; Vanden Houte, K.; Vanherweghem, J.L. Pathologic aspects of a newly described nephropathy related to the prolonged use of Chinese herbs. Am. J. Kidney Dis. 1994, 24, 172–180. [Google Scholar] [CrossRef]
- Koh, H.L.; Wang, H.; Zhou, S.; Chan, E.; Woo, S.O. Detection of aristolochic acid I, tetrandrine and fangchinoline in medicinal plants by high performance liquid chromatography and liquid chromatography/mass spectrometry. J. Pharmaceut. Biomed. 2006, 40, 653–661. [Google Scholar] [CrossRef]
- Guo, L.; Yue, H.; Cai, Z.W. A novel pre-column fluorescent derivatization method for the sensitive determination of aristolochic acids in medicinal herbs by high-performance liquid chromatography with fluorescence detection. J. Pharmaceut. Biomed. 2010, 53, 37–42. [Google Scholar] [CrossRef]
- Wang, Y.A.; Chan, W. Determination of aristolochic acids by high-performance liquid chromatography with fluorescence detection. J. Agric. Food Chem. 2014, 62, 5859–5864. [Google Scholar] [CrossRef]
- Wang, X.R.; Wang, Y.Y.; Wang, Y.D.; Chen, Q.; Liu, X. Nanobody-alkaline phosphatase fusion-mediated phosphate-triggered fluorescence immunoassay for ochratoxin a detection. Spectrochim. Acta A 2020, 226, 117617. [Google Scholar] [CrossRef]
- Inui, H.; Takeuchi, T.; Uesugi, A.; Doi, F.; Takai, M.; Nishi, K.; Miyake, S.; Ohkawa, H. Enzyme-linked immunosorbent assay with monoclonal and single-chain variable fragment antibodies Selective to Coplanar Polychlorinated Biphenyls. J. Agric. Food Chem. 2012, 60, 1605–1612. [Google Scholar] [CrossRef]
- Zhou, J.J.; Ren, M.S.; Wang, W.J.; Huang, L.; Lu, Z.C.; Song, Z.Y.; Foda, M.F.; Zhao, L.; Han, H.Y. Pomegranate-inspired silica nanotags enable sensitive dual-modal detection of rabies virus nucleoprotein. Anal. Chem. 2020, 92, 8802–8809. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zhao, G.X.; Wang, P.; Liu, J.; Zhang, H.C.; Wang, J.P. Production of the broad specific monoclonal antibody against sarafloxacin for rapid immunoscreening of 12 fluoroquinolones in meat. J. Environ. Sci. Heal. B 2013, 48, 139–146. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.Q.; Kuang, H.; Xu, C.L. Preparing monoclonal antibodies and developing immunochromatographic strips for paraquat determination in water. Food Chem. 2020, 311, 125897.1–125897.9. [Google Scholar] [CrossRef]
- Wu, Y.P.; Wang, J.; Zhou, Y.; Qi, Y.; Ma, L.C.; Wang, X.N.; Tao, X.Q. Quantitative determination of nitrofurazone metabolites in animal-derived foods based on a background fluorescence quenching immunochromatographic Assay. Foods 2021, 10, 1668. [Google Scholar] [CrossRef]
- Chen, X.R.; Miao, X.T.; Ma, T.T.; Leng, Y.K.; Hao, L.W.; Duan, H.; Yuan, J.; Li, Y.; Huang, X.L.; Xiong, Y.H. Gold nanobeads with enhanced absorbance for improved sensitivity in competitive lateral flow immunoassays. Foods 2021, 10, 1488. [Google Scholar] [CrossRef]
- Tao, X.Q.; Zhou, S.; Yuan, X.M.; Li, H.J. Determination of chloramphenicol in milk by ten chemiluminescent immunoassays: Influence of assay format applied. Anal. Methods 2016, 8, 4445–4451. [Google Scholar] [CrossRef]
- Xu, L.; Suo, X.Y.; Zhang, Q.; Li, X.P.; Chen, C.; Zhang, X.Y. ELISA and chemiluminescent enzyme immunoassay for sensitive and specific determination of lead (II) in Water, Food and Feed Samples. Foods 2020, 9, 305. [Google Scholar] [CrossRef] [Green Version]
- Yi, K.Y.; Zhang, X.T.; Zhang, L. Eu3+@metal–organic frameworks encapsulating carbon dots as ratiometric fluorescent probes for rapid recognition of anthrax spore biomarker. Sci. Total Environ. 2020, 743, 140692. [Google Scholar] [CrossRef]
- Li, T.X.; Li, Z.; Huang, T.Z.; Tian, L. Carbon quantum dot-based sensors for food safety. Sens. Actuators A Phys. 2021, 331, 113003. [Google Scholar] [CrossRef]
- Song, P.; Liu, Q.; Zhang, Y.; Liu, W.; Meng, M.; Yin, Y.M.; Xi, R.M. The chemical redox modulated switch-on fluorescence of carbon dots for probing alkaline phosphatase and its application in an immunoassay. RSC Adv. 2018, 8, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Ni, P.J.; Xie, J.F.; Chen, C.X.; Jiang, Y.Y.; Lu, Y.Z.; Hu, X. Fluorometric determination of the activity of alkaline phosphatase and its inhibitors based on ascorbic acid-induced aggregation of carbon dots. Microchim. Acta 2019, 202, 186. [Google Scholar] [CrossRef]
- Li, G.L.; Fu, H.L.; Chen, X.J.; Gong, P.W.; Chen, G.; Xia, L.; Wang, H.; You, J.M.; Wu, Y.N. Facile and sensitive fluorescence sensing of alkaline phosphatase activity with photoluminescent carbon dots based on inner filter effect. Anal. Chem. 2016, 88, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Liu, X.X.; Xiao, Z.L.; Fu, H.J.; Huang, Y.P.; Huang, S.Y.; Shen, Y.D.; He, F.; Yang, X.X.; Hammock, B.; et al. Production of a specific monoclonal antibody for 1-naphthol based on novel hapten strategy and development of an easy-to-use ELISA in urine samples. Ecotox. Environ. Saf. 2020, 196, 110533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Zhou, K.; Ha, W.Z.; Chen, P.H.; Fu, H.J.; Shen, Y.D.; Sun, Y.M.; Xu, Z.L. Development of a low-cost, simple, fast and quantitative lateral-flow immunochromatographic assay (ICA) strip for melatonin in health foods. Food Agric. Immunol. 2019, 30, 497–509. [Google Scholar] [CrossRef]

| Mouse | Immunogen | Coating Antigen 1 | Titer | IC50 (ng/mL) |
|---|---|---|---|---|
| 1 | AA-I-BSA | AA-I-OVA | 16K | 120.3 ± 13.0 |
| 2 | AA-I-BSA | AA-I-OVA | 32K | 148.8 ± 11.8 |
| 3 | AA-I-BSA | AA-I-OVA | 16K | 156.1 ± 16.2 |
| 4 | AA-I-KLH | AA-I-OVA | 64K | 27.3 ± 4.3 |
| 5 | AA-I-KLH | AA-I-OVA | 64K | 56.6 ± 7.7 |
| 6 | AA-I-KLH | AA-I-OVA | 32K | 35.8 ± 3.6 |
| Compounds | Structure | IC50 (ng/mL) | CR 1 (%) |
|---|---|---|---|
| AA-I | 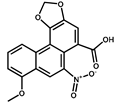 | 1.8 | 100 |
| AA-II |  | 2.1 | 86 |
| AA-III |  | 120.0 | 1.5 |
| AA-IV |  | 450.0 | 0.4 |
| Abietic acid | 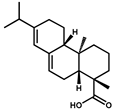 | >1000 | <0.1 |
| Asarinin |  | >1000 | <0.1 |
| Colchicine | 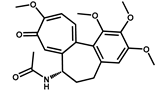 | >1000 | <0.1 |
| Methyleugenol | 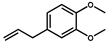 | >1000 | <0.1 |
| Ephedrine hydrochloride |  | >1000 | <0.1 |
| Method | IC50 (ng/mL) | Linear Range (ng/mL) | LOD (ng/mL) | Reference |
|---|---|---|---|---|
| icELISA | 1.2 | ND 1 | 0.1 | 1 |
| Injection analysis chemiluminescence | ND 1 | 10–20000 | 3 | 4 |
| CLEIA | 2.4 | 0.2–3.1 | 0.1 | This work |
| FIA | 0.41 | 0.08–2.50 | 0.06 | This work |
| Sample No. | Spiked (ng/g) | CLEIA | FIA | UPLC-QQQ-MS/MS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measured (ng/mL) (Mean ± SD 1) | Recovery (%) | CV 2 (%) | Measured (ng/mL) (Mean ± SD) | Recovery (%) | CV (%) | Measured (ng/mL) (Mean ± SD) | Recovery (%) | CV (%) | ||
| Pleurotus ostreatus | 600 | 511.4 ± 41.4 | 86.7 | 7.7 | 655.2 ± 92.4 | 109.2 | 14.1 | 648.3 ± 11.9 | 108.1 | 1.8 |
| 300 | 165.3 ± 21.9 | 83.0 | 13.3 | 355.2 ± 55.2 | 118.4 | 15.5 | 303.7 ± 12.9 | 101.2 | 4.2 | |
| 150 | 135.1 ± 14 | 90.0 | 12.8 | 129 ± 18 | 86 | 14.0 | 127.7 ± 9.6 | 85.1 | 7.5 | |
| Slimming capsule | 600 | 520.1 ± 11.3 | 86.7 | 10.4 | 642 ± 92.4 | 107 | 14.4 | 571.3 ± 12.9 | 95.2 | 2.3 |
| 300 | 237.9 ± 30.4 | 119.0 | 12.6 | 306 ± 28.8 | 102 | 9.4 | 313.7 ± 16.5 | 104.6 | 5.3 | |
| 150 | 116 ± 41.1 | 116.0 | 36.2 | 147 ± 21 | 98 | 14.3 | 136 ± 5.6 | 90.7 | 4.1 | |
| Slimming tea | 600 | 517.4 ± 11.8 | 86.7 | 3.8 | 678 ± 62.4 | 113 | 9.2 | 644.3 ± 7.5 | 107.4 | 1.2 |
| 300 | 204.9 ± 45.7 | 102.0 | 22.5 | 312 ± 15.6 | 104 | 5.0 | 305.3 ± 11.9 | 101.8 | 3.9 | |
| 150 | 114.3 ± 13.3 | 114.0 | 12.3 | 138 ± 9 | 92 | 6.5 | 155.3 ± 6.5 | 103.5 | 4.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, A.-F.; Chen, Z.-J.; Zhang, Y.-F.; He, Q.-Y.; Xu, Z.-L.; Zhao, S.-Q. Preparation of Anti-Aristolochic Acid I Monoclonal Antibody and Development of Chemiluminescent Immunoassay and Carbon Dot-Based Fluoroimmunoassay for Sensitive Detection of Aristolochic Acid I. Foods 2021, 10, 2647. https://doi.org/10.3390/foods10112647
Ou A-F, Chen Z-J, Zhang Y-F, He Q-Y, Xu Z-L, Zhao S-Q. Preparation of Anti-Aristolochic Acid I Monoclonal Antibody and Development of Chemiluminescent Immunoassay and Carbon Dot-Based Fluoroimmunoassay for Sensitive Detection of Aristolochic Acid I. Foods. 2021; 10(11):2647. https://doi.org/10.3390/foods10112647
Chicago/Turabian StyleOu, Ai-Fen, Zi-Jian Chen, Yi-Feng Zhang, Qi-Yi He, Zhen-Lin Xu, and Su-Qing Zhao. 2021. "Preparation of Anti-Aristolochic Acid I Monoclonal Antibody and Development of Chemiluminescent Immunoassay and Carbon Dot-Based Fluoroimmunoassay for Sensitive Detection of Aristolochic Acid I" Foods 10, no. 11: 2647. https://doi.org/10.3390/foods10112647
APA StyleOu, A.-F., Chen, Z.-J., Zhang, Y.-F., He, Q.-Y., Xu, Z.-L., & Zhao, S.-Q. (2021). Preparation of Anti-Aristolochic Acid I Monoclonal Antibody and Development of Chemiluminescent Immunoassay and Carbon Dot-Based Fluoroimmunoassay for Sensitive Detection of Aristolochic Acid I. Foods, 10(11), 2647. https://doi.org/10.3390/foods10112647