Fabrication, Structure and Functional Characterizations of pH-Responsive Hydrogels Derived from Phytoglycogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
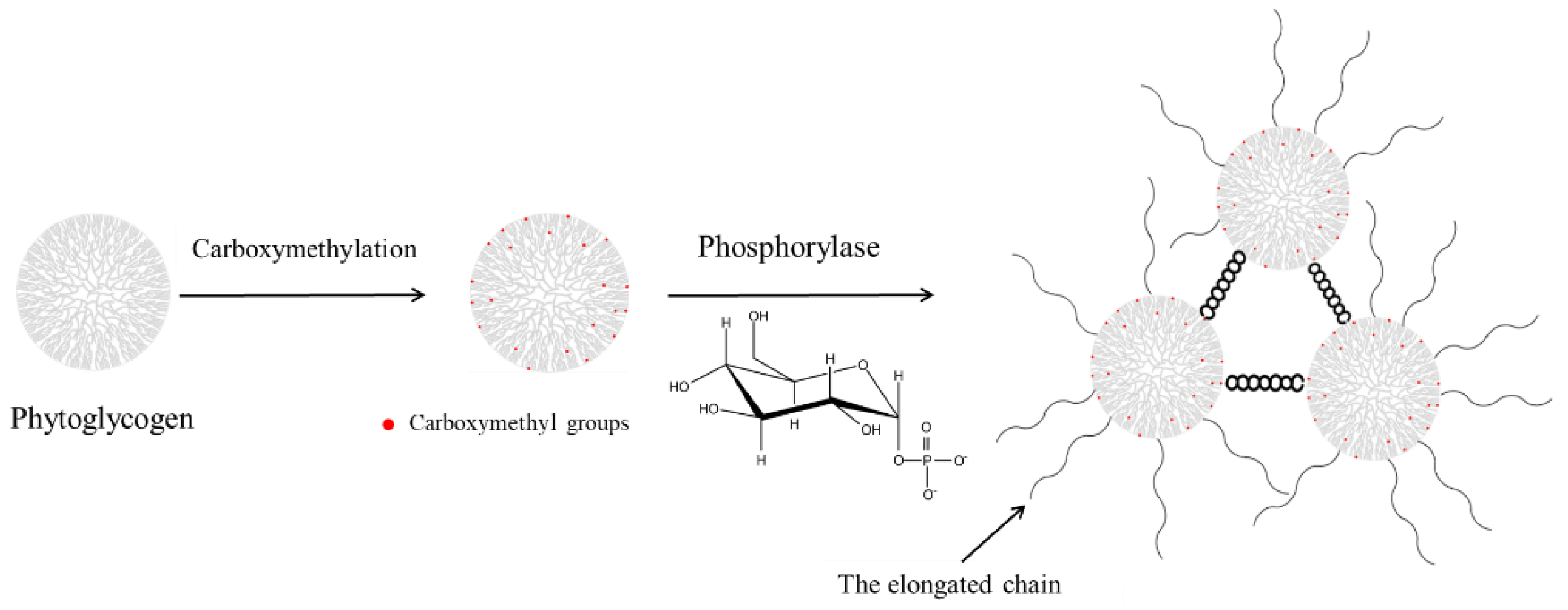
2.2. Preparation of pH-Responsive Hydrogels
2.3. Iodine Staining Analysis
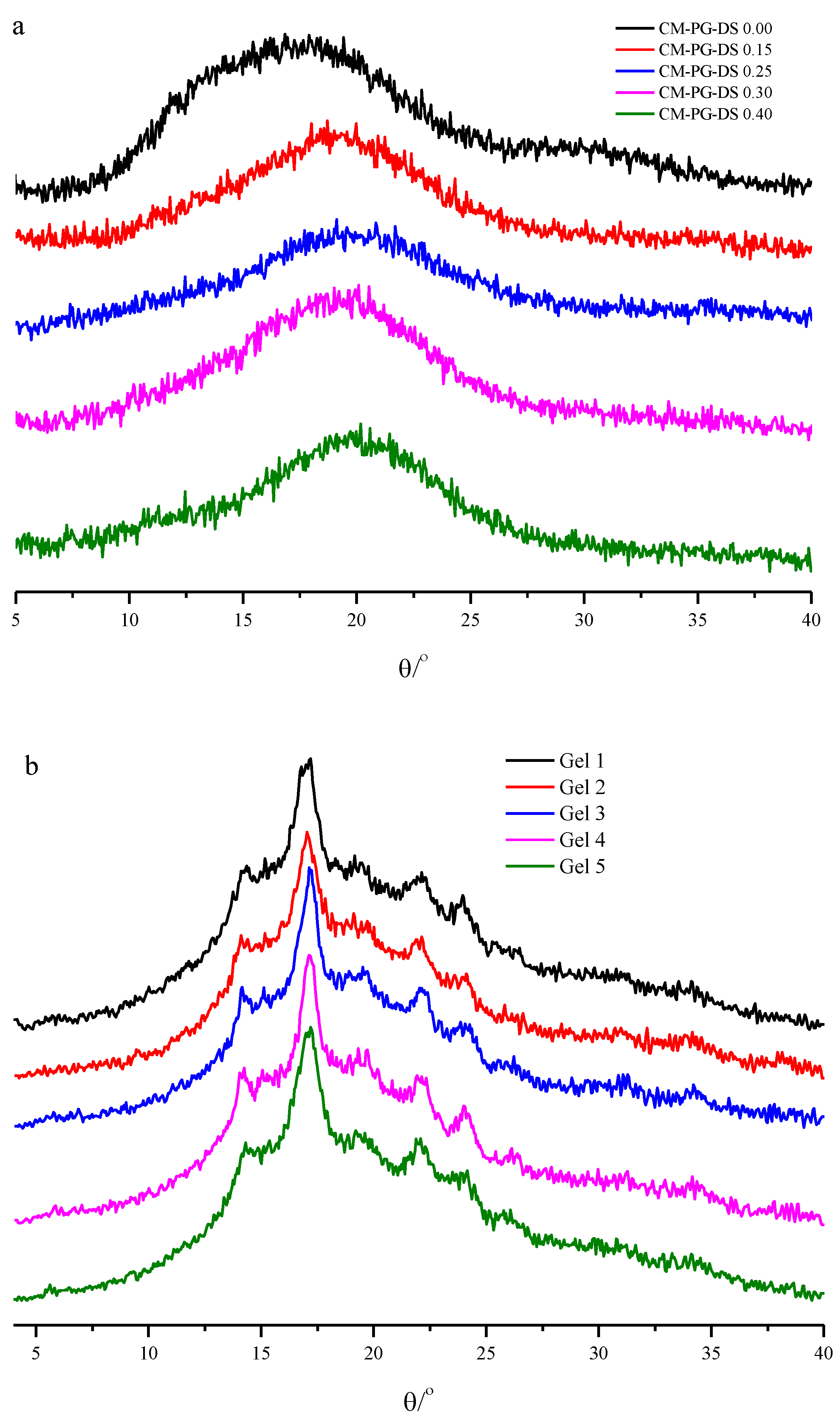
2.4. X-ray Diffraction (XRD) Analysis
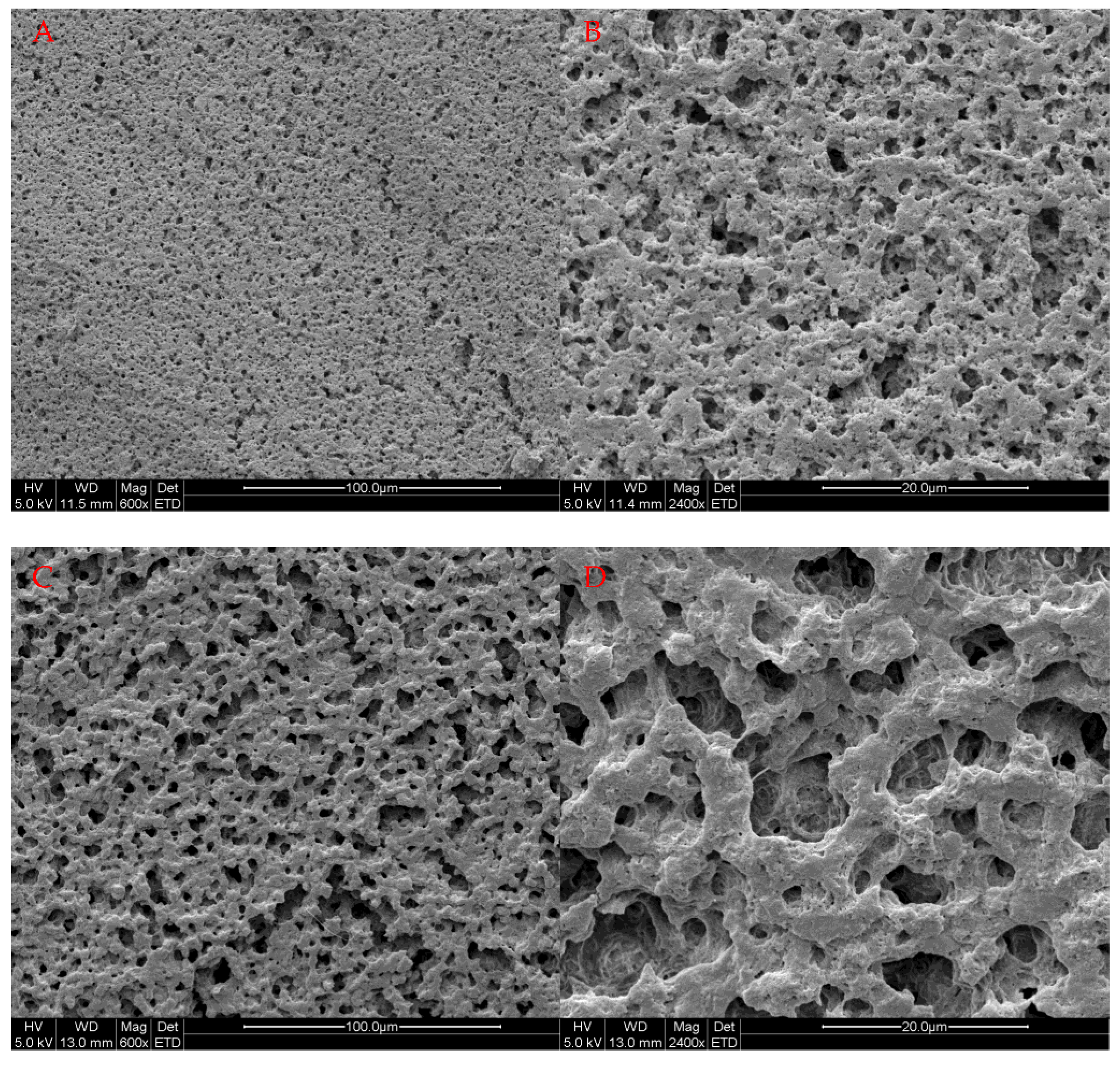
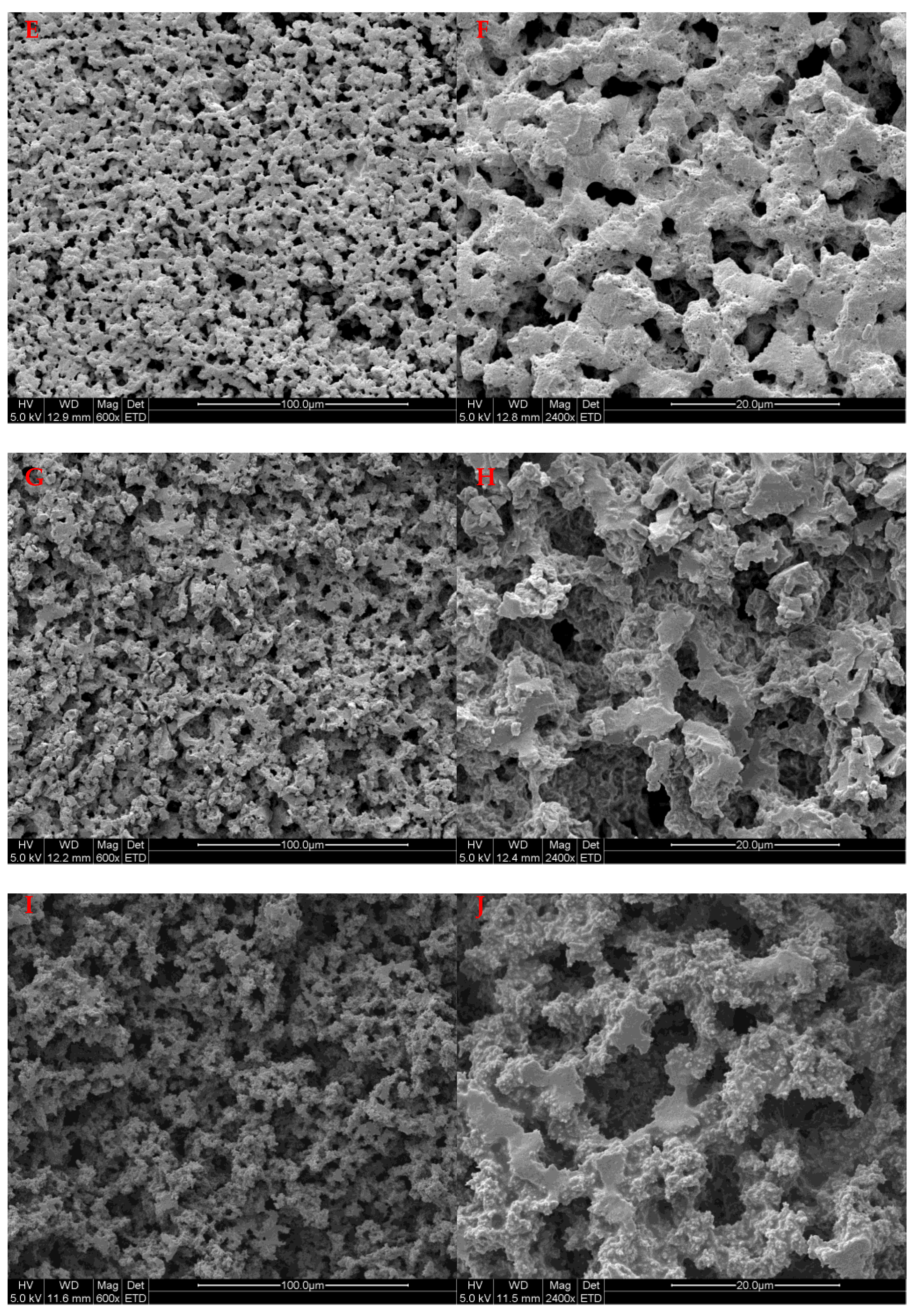
2.5. Scanning Electron Microscope (SEM) Analysis
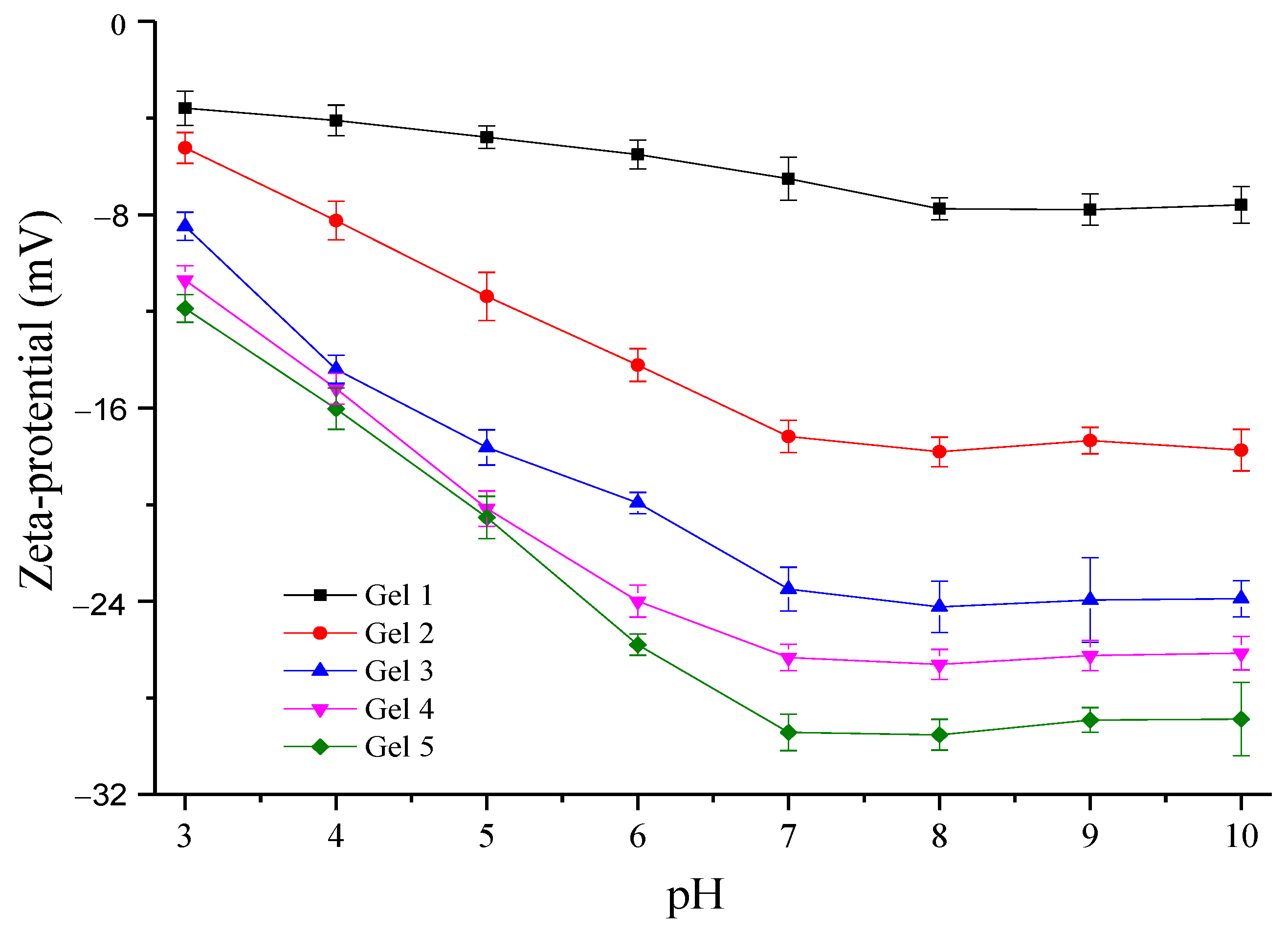
2.6. Assay of Zeta-Potential
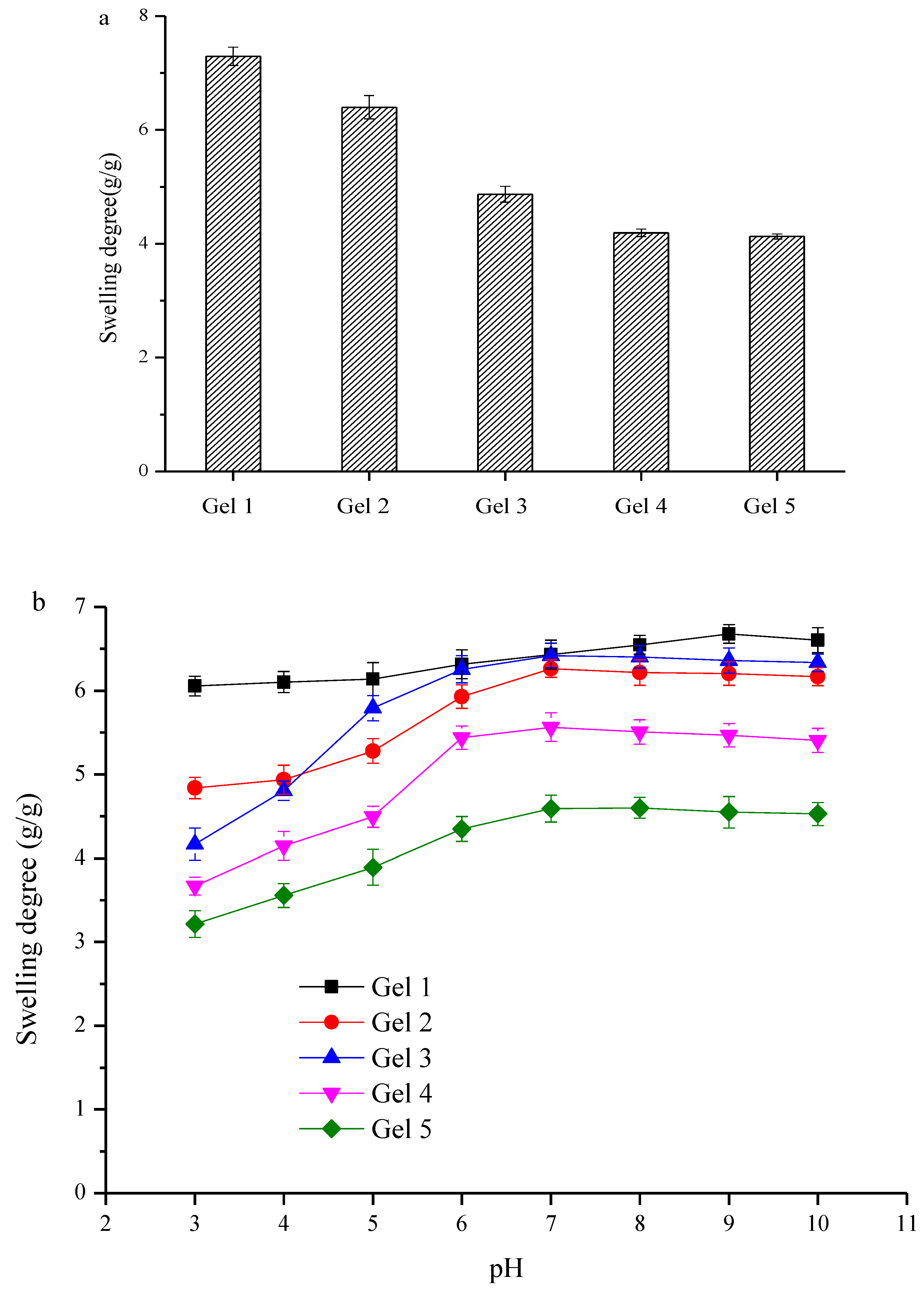
2.7. Swelling Degree and pH-Responsive Behavior
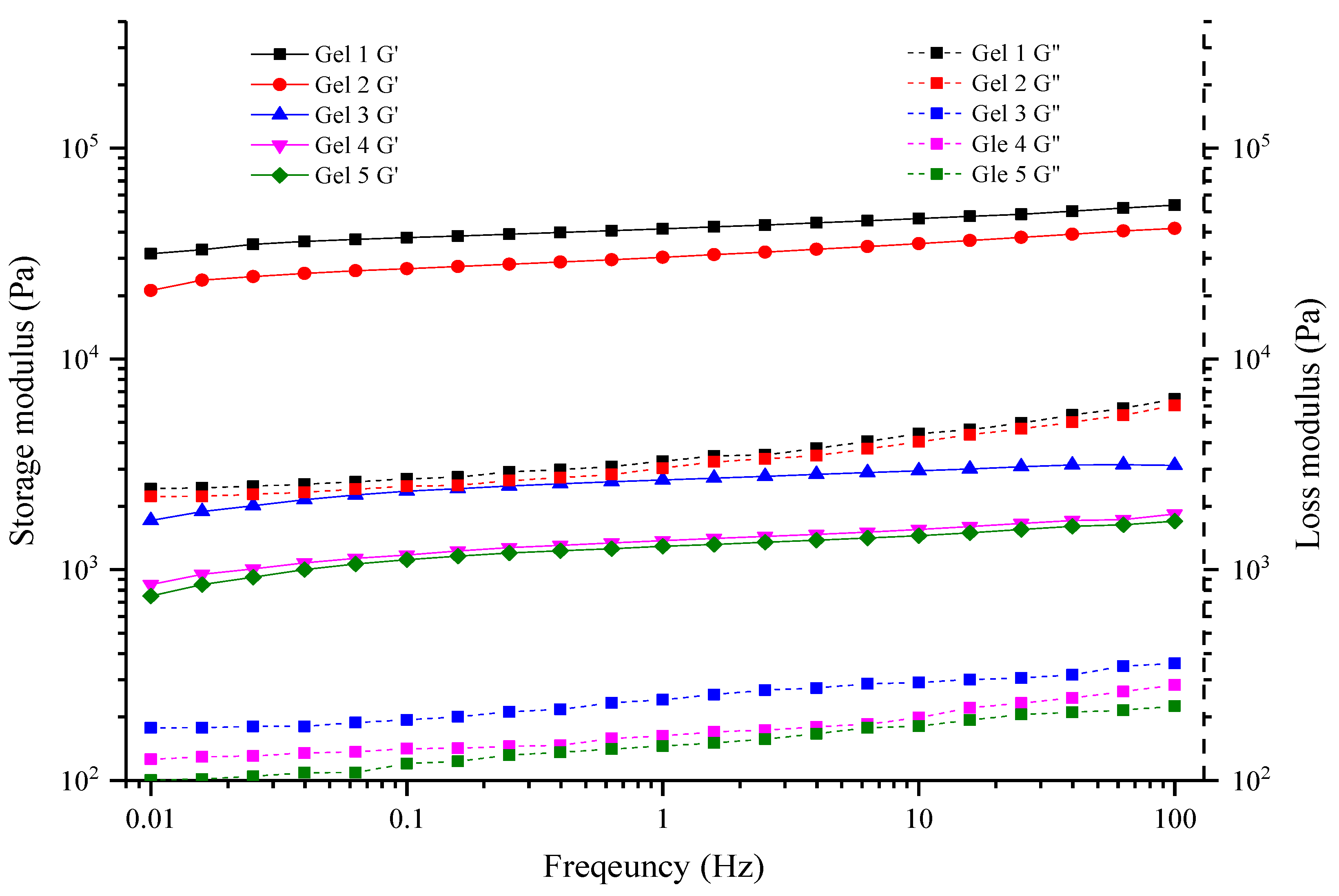
2.8. Rheological Properties
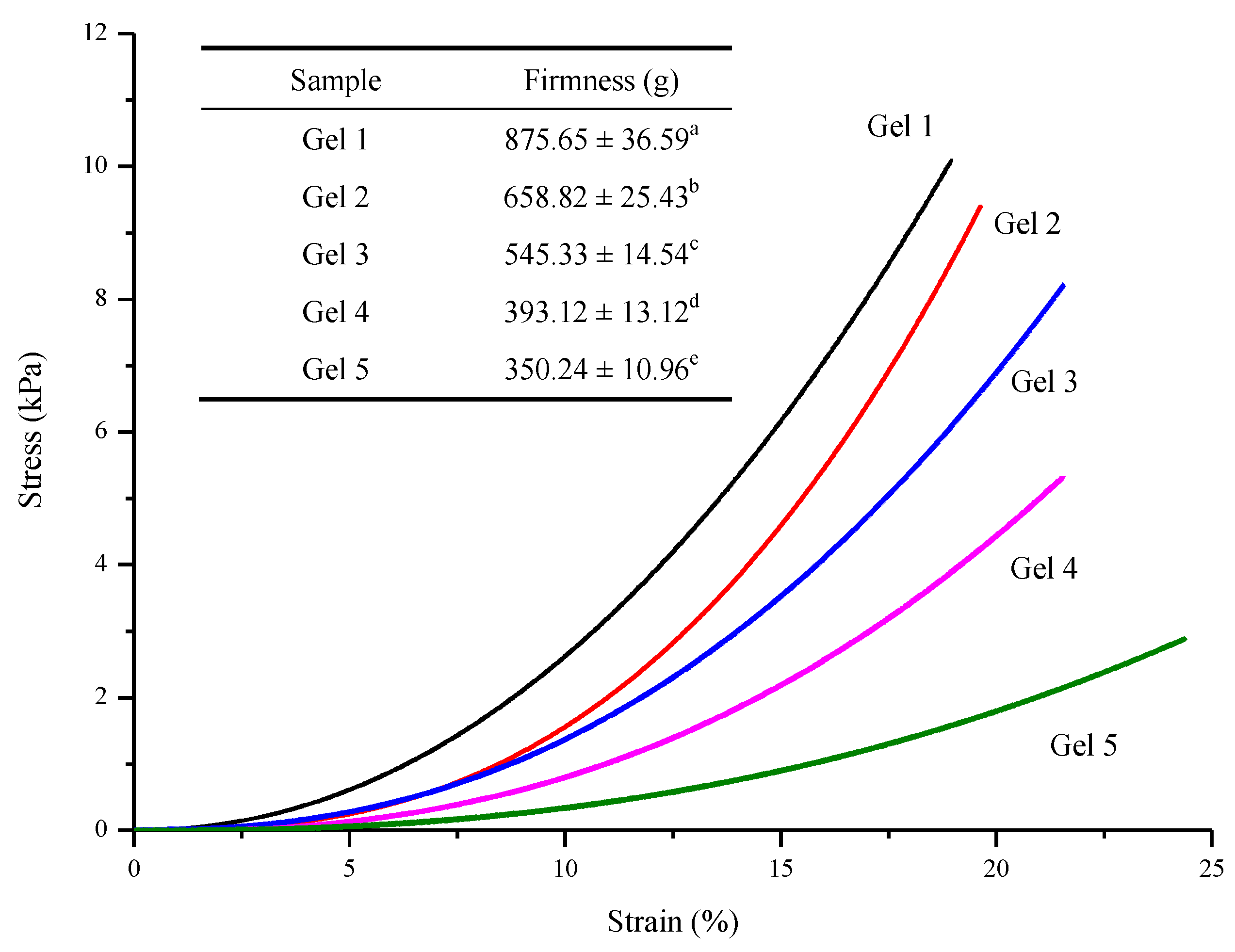
2.9. Mechanical Properties
2.10. Statistical Analysis
3. Results and Discussion
3.1. Iodine Staining Analysis
3.2. XRD Analysis
3.3. Formation of the pH-Responsive Hydrogel
3.4. Zeta-Potential of Hydrogels at Different pHs
3.5. Swelling Degree and pH-Responsive Behavior
3.6. Rheological Properties
3.7. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. Recent Advances in Edible Polymer Based Hydrogels as a Sustainable Alternative to Conventional Polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, D.; Ma, W.; Wu, C.; Tian, Y.; Wang, S.; Du, M. Strong fish gelatin hydrogels enhanced by carrageenan and potassium sulfate. Food Hydrocoll. 2021, 119, 106841. [Google Scholar] [CrossRef]
- González, K.; Guaresti, O.; Palomares, T.; Alonso-Varona, A.; Eceiza, A.; Gabilondo, N. The role of cellulose nanocrystals in biocompatible starch-based clicked nanocomposite hydrogels. Int. J. Biol. Macromol. 2020, 143, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.K.; Stevens, M.M. Tailoring gelation mechanisms for advanced hydrogel applications. Adv. Funct. Mater. 2020, 30, 2002759. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, B.; Hu, X.; Jin, Z.; Xu, X.; Tian, Y. Preparation and characterization of carboxymethyl starch microgel with different crosslinking densities. Carbohydr. Polym. 2015, 124, 245–253. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, Y.; Barboiu, M.; Maumus, M.; Noël, D.; Jorgensen, C.; Li, S. Biobased pH-responsive and self-healing hydrogels prepared from O-carboxymethyl chitosan and a 3-dimensional dynamer as cartilage engineering scaffold. Carbohydr. Polym. 2020, 244, 116471. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Bilal, M.; Cao, C.; Zhao, P.; Zhang, H.; Huang, Q. Multifunctional manganese-based carboxymethyl chitosan hydrogels for pH-triggered pesticide release and enhanced fungicidal activity. Carbohydr. Polym. 2021, 262, 117933. [Google Scholar] [CrossRef]
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Lu, K.; Zhang, T. Structure and digestibility of endosperm water-soluble α-glucans from different sugary maize mutants. Food Chem. 2014, 143, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Miao, M.; Cui, S.; Jiang, B.; Jin, Z.; Li, X. Characterisations of oil-in-water Pickering emulsion stabilized hydrophobic phytoglycogen nanoparticles. Food Hydrocoll. 2018, 76, 78–87. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, Z.; Gao, Y.; Liu, C.; Wu, J.; Hu, X. Crystallization of short-chain amylose: Effect of the precipitant. Starch-Starke 2019, 71, 1900007. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Z.; Zeng, Z.; Deng, C.; Luo, S.; Liu, C. Improving resistance of crystallized starch by narrowing molecular weight distribution. Food Hydrocoll. 2020, 103, 105641. [Google Scholar] [CrossRef]
- Liu, G.; Ji, N.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Molecular interactions in debranched waxy starch and their effects on digestibility and hydrogel properties. Food Hydrocoll. 2018, 84, 166–172. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Wei, H.; Zhang, C.; Huang, S.; Chen, Y.; Lu, Y.; Li, Y. Effects of molecular interactions in debranched high amylose starch on digestibility and hydrogel properties. Food Hydrocoll. 2020, 101, 105498. [Google Scholar] [CrossRef]
- Kitaoka, M.; Hayashi, K. Carbohydrate-prcoessing phosphorolytic enzymes. Trends Glycosci. Glycotechnol. 2002, 14, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Yanase, M.; Takaha, T.; Kuriki, T. α-Glucan phosphorylase and its use in carbohydrate engineering. J. Sci. Food Agric. 2006, 86, 1631–1635. [Google Scholar] [CrossRef]
- Kazłowski, B.; Ko, Y.-T. Reaction of phosphorylase-a with α-d-glucose 1-phosphate and maltodextrin acceptors to give products with degree of polymerization 6–89. Carbohydr. Polym. 2014, 106, 209–216. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.C.; Field, R.A. Enzymatic synthesis using glycoside phosphorylases. Carbohydr. Res. 2015, 403, 23–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Lu, K.; Hu, X.; Jin, Z.; Miao, M. Structure, properties and potential applications of phytoglycogen and waxy starch subjected to carboxymethylation. Carbohydr. Polym. 2020, 234, 115908. [Google Scholar] [CrossRef]
- Banks, W.; Greenwood, C.; Khan, K. The interaction of linear, amylose oligomers with iodine. Carbohydr. Res. 1971, 17, 25–33. [Google Scholar] [CrossRef]
- Izawa, H.; Nawaji, M.; Kaneko, Y.; Kadokawa, J.-I. Preparation of glycogen-based polysaccharide materials by phosphorylase-catalyzed chain elongation of glycogen. Macromol. Biosci. 2009, 9, 1098–1104. [Google Scholar] [CrossRef]
- Kaneko, Y.; Matsuda, S.-I.; Kadokawa, J.-I. chemoenzymatic syntheses of amylose-grafted chitin and chitosan. Biomacromolecules 2007, 8, 3959–3964. [Google Scholar] [CrossRef]
- Matsuda, S.-I.; Kaneko, Y.; Kadokawa, J.-I. Chemoenzymatic synthesis of amylose-grafted chitosan. Macromol. Rapid Commun. 2007, 28, 863–867. [Google Scholar] [CrossRef]
- Omagari, Y.; Matsuda, S.-I.; Kaneko, Y.; Kadokawa, J.-I. Chemoenzymatic synthesis of amylose-grafted cellulose. Macromol. Biosci. 2009, 9, 450–455. [Google Scholar] [CrossRef]
- Kadokawa, J.-I.; Shoji, T.; Yamamoto, K. Preparation of amylose-carboxymethyl cellulose conjugated supramolecular networks by phosphorylase-catalyzed enzymatic polymerization. Catalysts 2019, 9, 211. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Bratlie, K.M. pH sensitive methacrylated chitosan hydrogels with tunable physical and chemical properties. Biochem. Eng. J. 2018, 132, 38–46. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Zheng, X.; Zhang, A.; Zhang, X.; Tang, K. Pullulan dialdehyde crosslinked gelatin hydrogels with high strength for biomedical applications. Carbohydr. Polym. 2019, 216, 45–53. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and characterization of pH- and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C 2013, 33, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, R.; Yu, S.; Li, J.; Wang, Y.; Song, Y.; Yang, X.; Pan, W.; Li, S. Nanostructured lipid carrier-based pH and temperature dual-responsive hydrogel composed of carboxymethyl chitosan and poloxamer for drug delivery. Int. J. Biol. Macromol. 2018, 114, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, H.; Park, W.H. Effect of pH and precursor salts on in situ formation of calcium phosphate nanoparticles in methylcellulose hydrogel. Carbohydr. Polym. 2018, 191, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, J.; Shoemaker, C. Measurement of thixotropy of model food colloidal suspensions with step change shear rate. J. Food Eng. 1992, 16, 17–24. [Google Scholar] [CrossRef]
- Ranjan, R.; Rawat, K.; Bohidar, H. Interface versus bulk gelation and UCST in hydrophobically assembled TX-100 molecular gels. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 499, 113–122. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Liu, Y.; Chen, Y.; Zhang, T.; Miao, M. Fabrication, Structure and Functional Characterizations of pH-Responsive Hydrogels Derived from Phytoglycogen. Foods 2021, 10, 2653. https://doi.org/10.3390/foods10112653
Hu X, Liu Y, Chen Y, Zhang T, Miao M. Fabrication, Structure and Functional Characterizations of pH-Responsive Hydrogels Derived from Phytoglycogen. Foods. 2021; 10(11):2653. https://doi.org/10.3390/foods10112653
Chicago/Turabian StyleHu, Xiuting, Yao Liu, Yimei Chen, Tao Zhang, and Ming Miao. 2021. "Fabrication, Structure and Functional Characterizations of pH-Responsive Hydrogels Derived from Phytoglycogen" Foods 10, no. 11: 2653. https://doi.org/10.3390/foods10112653
APA StyleHu, X., Liu, Y., Chen, Y., Zhang, T., & Miao, M. (2021). Fabrication, Structure and Functional Characterizations of pH-Responsive Hydrogels Derived from Phytoglycogen. Foods, 10(11), 2653. https://doi.org/10.3390/foods10112653






