UPLC-MS/MS Profiling, Antioxidant, α-Glucosidase Inhibitory, Cholinesterase Inhibitory, and Cardiovascular Protection Potentials of Jialing 20 (Morus multicaulis Perr.) Mulberry Branch Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Extraction
2.2. Chemicals
2.3. UPLC-MS/MS Analysis
2.3.1. Chromatographic Conditions
2.3.2. Mass Spectrometry Conditions
2.3.3. Mass Spectrometry Data Processing
2.4. Antioxidant Activity
2.4.1. Scavenging of DPPH Radicals
2.4.2. Scavenging of ABTS Radicals
2.5. Inhibition of α-Glucosidase Activity
2.6. Inhibition of Cholinesterase Activity
2.7. Protection of MBE against ox-LDL-Induced HUVECs Damage
2.7.1. Cell Culture
2.7.2. Cytotoxicity
2.7.3. Cell Vitality
2.7.4. Determination of Intracellular Reactive Oxygen Species (ROS)
3. Results and Discussion
3.1. UPLC-MS/MS Fingerprints
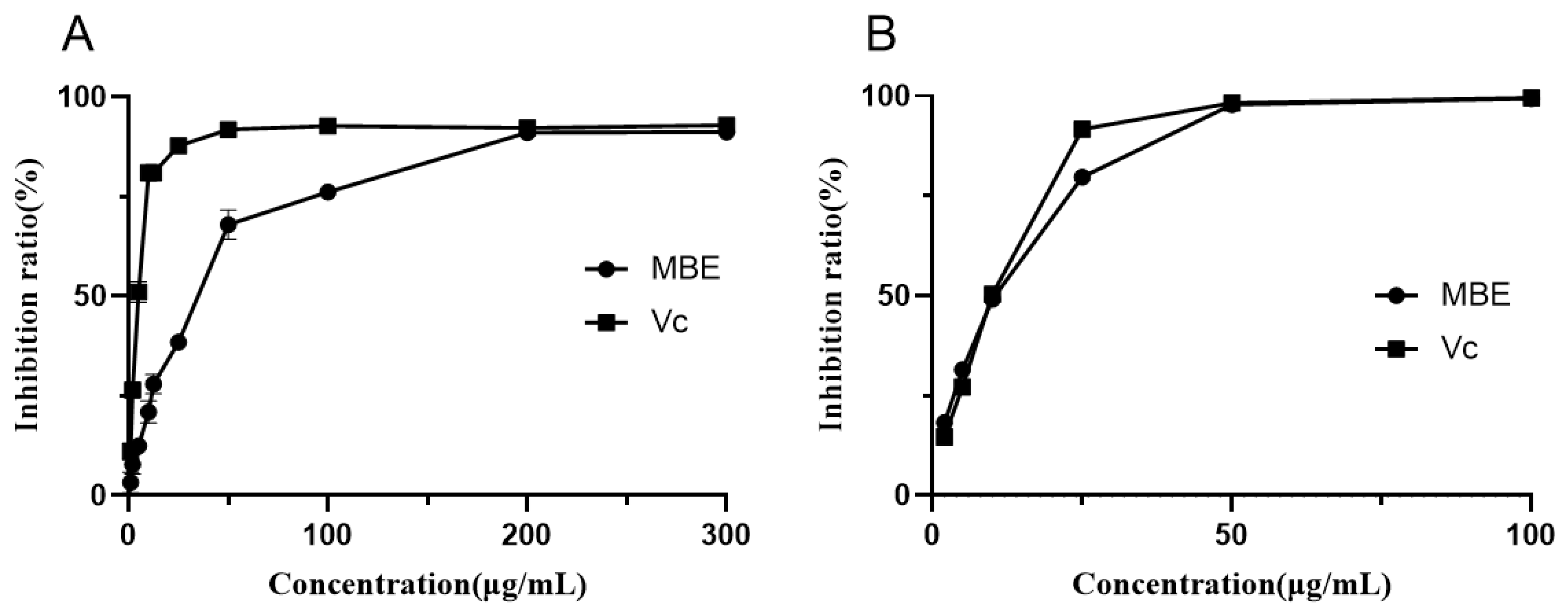
3.2. Antioxidant Activity
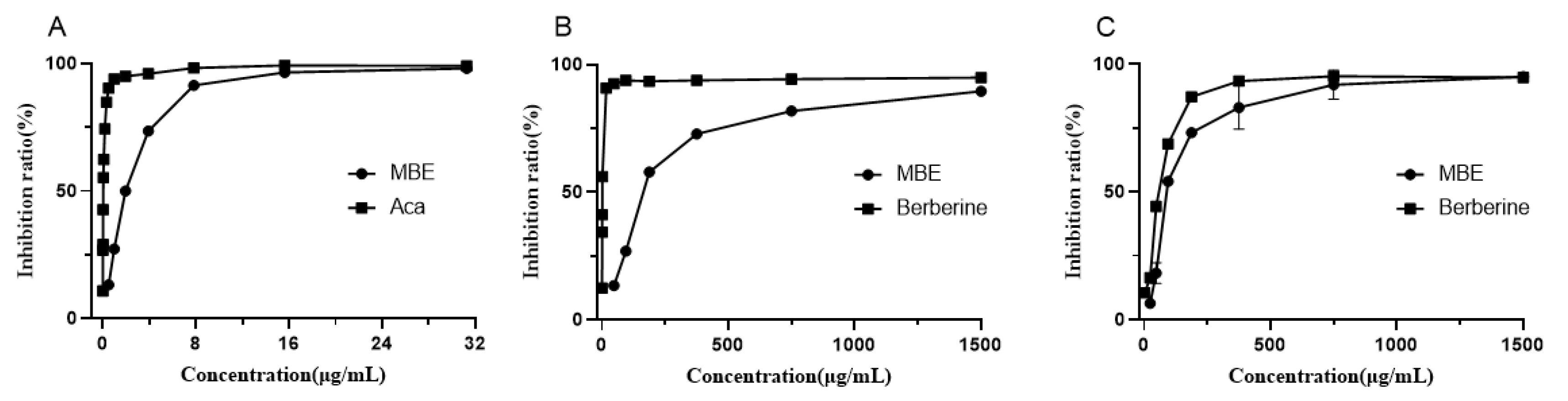
3.3. Inhibition of α-Glucosidase Activity
3.4. Inhibition of Cholinesterase Activities
3.5. Protection of MBE against ox-LDL-Induced HUVECs Damage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.; Wang, B.L.; Xia, X.J.; Li, X.; Wang, R.Y.; Sheng, L.; Li, D.; Liu, Y.L.; Li, Y. Simultaneous quantification of three active alkaloids from a traditional Chinese medicine Ramulus Mori (Sangzhi) in rat plasma using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. 2015, 109, 177–183. [Google Scholar] [CrossRef]
- Yu, W.S.; Chen, H.; Xiang, Z.H.; He, N.J. Preparation of Polysaccharides from Ramulus mori, and Their Antioxidant, Anti-Inflammatory and Antibacterial Activities. Molecules 2019, 24, 856. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Kim, J.K.; Kim, I.H.; Lim, Y.H. Inhibitory effect of ethanolic extract of Ramulus mori on adipogenic differentiation of 3T3-L1 cells and their antioxidant activity. J. Food Biochem. 2018, 42, e12469. [Google Scholar] [CrossRef]
- Choi, S.W.; Jang, Y.J.; Lee, Y.J.; Leem, H.H.; Kim, E.O. Analysis of Functional Constituents in Mulberry (Morus alba L.) Twigs by Different Cultivars, Producing Areas, and Heat Processings. Prev. Nutr. Food Sci. 2013, 18, 256–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.P.; Jia, Y.N.; Yu, Y.; Xu, L. DNA protection activity of a hydroethanol extract and six polyphenol monomers from Morus alba L. (mulberry) twig. Int. J. Food Prop. 2017, 20, 2207–2219. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Zhao, Z.; Chang, Y.; Ning, N.; Guo, H.; et al. Structures, bioactivities and future prospective of polysaccharides from Morus alba (white mulberry): A review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.G.; Linhardt, R.J.; Liao, S.T.; Wu, H.; Zou, Y.X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Tech. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Xu, L.; Yang, F.; Wang, J.; Huang, H.; Huang, Y. Anti-diabetic effect mediated by Ramulus mori polysaccharides. Carbohydr. Polym. 2015, 117, 63–69. [Google Scholar] [CrossRef]
- Radojkovic, M.M.; Zekovic, Z.P.; Vidovic, S.S.; Kocar, D.D.; Maskovic, P.Z. Free radical scavenging activity and total phenolic and flavonoid contents of mulberry (Morus spp. L., Moraceae) extracts. Hem. Ind 2012, 66, 545–550. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, L. Detection of antioxidant active compounds in mori ramulus by HPLC-MS-DPPH. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2012, 37, 800–802. [Google Scholar]
- Gu, Y. Comparison test of new Artificial Triploid Mulberry Variety Jialing 20 in Yubei District. Newsl. Sericultural Sci. 2011, 31, 9–10. [Google Scholar]
- Li, Y.H.; Huang, L.; Sun, J.H.; Wei, X.H.; Wen, J.H.; Zhong, G.P.; Huang, M.; Bi, H.C. Mulberroside A suppresses PXR-mediated transactivation and gene expression of P-gp in LS174T cells. J. Biochem. Mol. Toxicol. 2017, 31, e21884. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Guo, H.L.; Zhao, T.T.; Fu, J.; Xu, Y.H. Mulberroside A from Cortex Mori Enhanced Gut Integrity in Diabetes. Evid.-Based Complementary Altern. Med. 2021, 2021, 6655555. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Jin, J.; Shi, L.G. Protective function of cis-mulberroside A and oxyresveratrol from Ramulus mori against ethanol-induced hepatic damage. Environ. Toxicol. Pharmacol. 2008, 26, 325–330. [Google Scholar] [CrossRef]
- Jia, Y.N.; Peng, Y.L.; Zhao, Y.P.; Cheng, X.F.; Zhou, Y.; Chai, C.L.; Zeng, L.S.; Pan, M.H.; Xu, L. Comparison of the Hepatoprotective Effects of the Three Main Stilbenes from Mulberry Twigs. J. Agr. Food Chem. 2019, 67, 5521–5529. [Google Scholar] [CrossRef] [PubMed]
- Belikov, A.V. Age-related diseases as vicious cycles. Ageing Res. Rev. 2019, 49, 11–26. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wu, D.; Yu, Y.; Hu, N.; Wang, H.; Li, X.; Wu, Y. Emerging strategies for the activity assay and inhibitor screening of alpha-glucosidase. Food Funct. 2020, 11, 66–82. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Chen, J.J.; Li, T.Z.; Zhang, X.K.; Guo, Y.Q.; Zhang, X.M.; Hu, J.; Geng, C.A. Nineteen New Flavanol-Fatty Alcohol Hybrids with alpha-Glucosidase and PTP1B Dual Inhibition: One Unusual Type of Antidiabetic Constituent from Amomum tsao-ko. J. Agric. Food Chem. 2020, 68, 11434–11448. [Google Scholar] [CrossRef]
- Alghamdi, S.; Asif, M. Role of pyridazine analogs as acetylcholinesterase inhibitor: An approach for management of alzheimer’s disease. Eurasian Chem. Commun. 2021, 3, 435–442. [Google Scholar] [CrossRef]
- Mella, M.; Moraga-Nicolas, F.; Machuca, J.; Quiroz, A.; Mutis, A.; Becerra, J.; Astudillo, A.; Hormazabal, E. Acetylcholinesterase inhibitory activity from Amaryllis belladonna growing in Chile: Enzymatic and molecular docking studies. Nat. Prod. Res. 2021, 1–5. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Liu, H.; Suo, R.; Lu, C. Naringin protects endothelial cells from apoptosis and inflammation by regulating the Hippo-YAP Pathway. Biosci. Rep. 2020, 40, BSR20193431. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Niu, X.; Yu, J.; Xiao, X.; Li, W.; Zang, L.; Hu, Z.; Siu-Po Ip, P.; Li, W. Poria cocos polysaccharides attenuated ox-LDL-induced inflammation and oxidative stress via ERK activated Nrf2/HO-1 signaling pathway and inhibited foam cell formation in VSMCs. Int. Immunopharmacol 2020, 80, 106173. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, J.; Zhang, X. Astragaloside IV Protects Against Oxidized Low-Density Lipoprotein (ox-LDL)-Induced Endothelial Cell Injury by Reducing Oxidative Stress and Inflammation. Med. Sci. Monit. 2019, 25, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Angleman, S.; Melis, R.; Mangialasche, F.; Karp, A.; Garmen, A.; Meinow, B.; Fratiglioni, L. Aging with multimorbidity: A systematic review of the literature. A Ageing Res. Rev. 2011, 10, 430–439. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Di Domenico, F.; Barone, E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim. Biophys Acta 2014, 1842, 1693–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baunthiyal, M.; Singh, V.; Dwivedi, S. Insights of Antioxidants as Molecules for Drug Discovery. Int. J. Pharmacol. 2017, 13, 874–889. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.K. Antioxidants: New-generation therapeutic base for treatment of polygenic disorders. Curr. Sci. 2004, 86, 1092–1102. [Google Scholar]
- Gendel, S.M. Potential functional food ingredients: Insufficient ingredient descriptions. J. Funct Foods 2021, 86, 104721. [Google Scholar] [CrossRef]
- Singh, P.; Bajpai, V.; Gupta, A.; Gaikwad, A.N.; Maurya, R.; Kumar, B. Identification and quantification of secondary metabolites of Pterocarpus marsupium by LC-MS techniques and its in-vitro lipid lowering activity. Ind. Crop. Prod. 2019, 127, 26–35. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Yu, Y.; Zhao, Y.P.; Jiang, L.Y.; Zhou, Y.; Huang, D.J.; Xu, L. Interrelation of cholesterol-lowering, antioxidant activity and DNA damage protection to the different solvent extracts of mulberry (Morus alba L.). J. Food Process. Pres. 2020, 45, e14891. [Google Scholar] [CrossRef]
- Liu, C.; Xiang, W.; Yu, Y.; Shi, Z.-Q.; Huang, X.-Z.; Xu, L. Comparative analysis of 1-deoxynojirimycin contribution degree to α-glucosidase inhibitory activity and physiological distribution in Morus alba L. Ind. Crop. Prod. 2015, 70, 309–315. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.L.; Fu, T.M.; Li, W.; Xu, X.L.; Sun, H.P. Discovery of new acetylcholinesterase inhibitors with small core structures through shape-based virtual screening. Bioorg. Med. Chem. Lett. 2015, 25, 3442–3446. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.M.; Bar, F.M.A.; Baraka, H.N.; Gohar, A.A.; Lahloub, M.F. A new antioxidant stilbene and other constituents from the stem bark of Morus nigra L. Nat. Prod. Res. 2014, 28, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Aelenei, P.; Luca, S.V.; Horhogea, C.E.; Rimbu, C.M.; Dimitriu, G.; Macovei, I.; Silion, M.; Aprotosoaie, A.C.; Miron, A. Morus alba leaf extract: Metabolite profiling and interactions with antibiotics against Staphylococcus spp. including MRSA. Phytochem. Lett. 2019, 31, 217–224. [Google Scholar] [CrossRef]
- Liu, J.; Mu, Y.; Xiong, S.; Sun, P.; Deng, Z. A UPLC-MS/MS method for comparative pharmacokinetics study of morusin and morin in normal and diabetic rats. Biomed. Chromatogr. 2019, 33, e4516. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, C.; Xiang, W.; Chen, H.; Qin, X.L.; Huang, X.Z. Advances in the Study of Oxyresveratrol. Int. J. Pharmacol. 2014, 10, 44–54. [Google Scholar] [CrossRef]
- Wu, S.C.; Han, F.; Song, M.R.; Chen, S.; Li, Q.; Zhang, Q.; Zhu, K.; Shen, J.Z. Natural Flavones from Morus alba against Methicillin-Resistant Staphylococcus aureus via Targeting the Proton Motive Force and Membrane Permeability. J. Agr. Food Chem. 2019, 67, 10222–10234. [Google Scholar] [CrossRef] [PubMed]
- Mascarello, A.; Menegatti, A.C.O.; Calcaterra, A.; Martins, P.G.A.; Chiaradia-Delatorre, L.D.; D’Acquarica, I.; Ferrari, F.; Pau, V.; Sanna, A.; De Logu, A.; et al. Naturally occurring Diels-Alder-type adducts from Morus nigra as potent inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatase B. Eur. J. Med. Chem. 2018, 144, 277–288. [Google Scholar] [CrossRef]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.L.; Aligiannis, N. Anti-Melanogenic Properties of Greek Plants. A Novel Depigmenting Agent from Morus alba Wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef] [Green Version]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, Productions, Biological Activities, Pharmacokinetics, and Delivery Systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef] [PubMed]
- Nastic, N.; Borras-Linares, I.; Lozano-Sanchez, J.; Svarc-Gajic, J.; Segura-Carretero, A. Optimization of the extraction of phytochemicals from black mulberry (Morus nigra L.) leaves. J. Ind. Eng. Chem. 2018, 68, 282–292. [Google Scholar] [CrossRef]
- Sanchez-Salcedo, E.M.; Tassotti, M.; Del Rio, D.; Hernandez, F.; Martinez, J.J.; Mena, P. (Poly)phenolic fingerprint and chemometric analysis of white (Morus alba L.) and black (Morus nigra L.) mulberry leaves by using a non-targeted UHPLC-MS approach. Food Chem. 2016, 212, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ou, X.; Zhang, X.; Zhou, Z.; Ma, L. Effect of Different Solvents on the Measurement of Phenolics and the Antioxidant Activity of Mulberry (Morus atropurpurea Roxb.) with Accelerated Solvent Extraction. J. Food Sci. 2017, 82, 605–612. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, J.F.; Ma, L.Y.; Wen, D.W.; Chen, F.; Li, J.M. Identification of polyphenols in mulberry (genus Morus) cultivars by liquid chromatography with time-of-flight mass spectrometer. J. Food Compos. Anal. 2017, 63, 55–64. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Arranz, S.; Tabernero, M.; Diaz-Rubio, M.E.; Serrano, J.; Goni, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Jin, J.; Shi, L.G. Antioxidant Properties of Ethanolic Extract from Ramulus mori (Sangzhi). Food Sci. Technol. Int. 2009, 15, 435–444. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Du, X.; Yang, Y.Y.; Cui, X.M.; Zhang, Z.J.; Li, Y. Comparative study of chemical composition and active components against alpha-glucosidase of various medicinal parts of Morus alba L. Biomed. Chromatogr. 2018, 32, e4328. [Google Scholar] [CrossRef]
- Bond, M.; Rogers, G.; Peters, J.; Anderson, R.; Hoyle, M.; Miners, A.; Moxham, T.; Davis, S.; Thokala, P.; Wailoo, A.; et al. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease (review of Technology Appraisal No. 111): A systematic review and economic model. Health Technol. Assess. 2012, 16, 1–470. [Google Scholar] [CrossRef]
- Cao, T.Q.; Ngo, Q.M.T.; Seong, S.H.; Youn, U.J.; Kim, J.A.; Kim, J.; Kim, J.C.; Woo, M.H.; Choi, J.S.; Min, B.S. Cholinesterase inhibitory alkaloids from the rhizomes of Coptis chinensis. Bioorg. Chem. 2018, 77, 625–632. [Google Scholar] [CrossRef]
- Can, M.V.; Tran, A.H.; Pham, D.M.; Dinh, B.Q.; Le, Q.V.; Nguyen, B.V.; Nguyen, M.T.T.; Nguyen, H.X.; Nguyen, N.T.; Nishijo, H. Willughbeia cochinchinensis prevents scopolamine-induced deficits in memory, spatial learning, and object recognition in rodents. J. Ethnopharmacol 2018, 214, 99–105. [Google Scholar] [CrossRef]
- Yuan, N.N.; Cai, C.Z.; Wu, M.Y.; Su, H.X.; Li, M.; Lu, J.H. Neuroprotective effects of berberine in animal models of Alzheimer’s disease: A systematic review of pre-clinical studies. BMC Complementary Altern. Med. 2019, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Kuk, E.B.; Jo, A.R.; Oh, S.I.; Sohn, H.S.; Seong, S.H.; Roy, A.; Choi, J.S.; Jung, H.A. Anti-Alzheimer’s disease activity of compounds from the root bark of Morus alba L. Arch. Pharm Res. 2017, 40, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Namdaung, U.; Athipornchai, A.; Khammee, T.; Kuno, M.; Suksamrarn, S. 2-Arylbenzofurans from Artocarpus lakoocha and methyl ether analogs with potent cholinesterase inhibitory activity. Eur. J. Med. Chem 2018, 143, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Profumo, E.; Buttari, B.; D’Arcangelo, D.; Tinaburri, L.; Dettori, M.A.; Fabbri, D.; Delogu, G.; Rigano, R. The Nutraceutical Dehydrozingerone and Its Dimer Counteract Inflammation- and Oxidative Stress-Induced Dysfunction of In Vitro Cultured Human Endothelial Cells: A Novel Perspective for the Prevention and Therapy of Atherosclerosis. Oxid. Med. Cell Longev. 2016, 2016, 1246485. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Zhang, X.W.; Wang, K.; Wang, X.Y.; Ma, W.L.; Cao, W.; Mo, D.; Sun, Y.; Li, X.Q. Kuwanon G attenuates atherosclerosis by upregulation of LXR alpha-ABCA1/ABCG1 and inhibition of NF kappa B activity in macrophages. Toxicol Appl. Pharm 2018, 341, 56–63. [Google Scholar] [CrossRef]
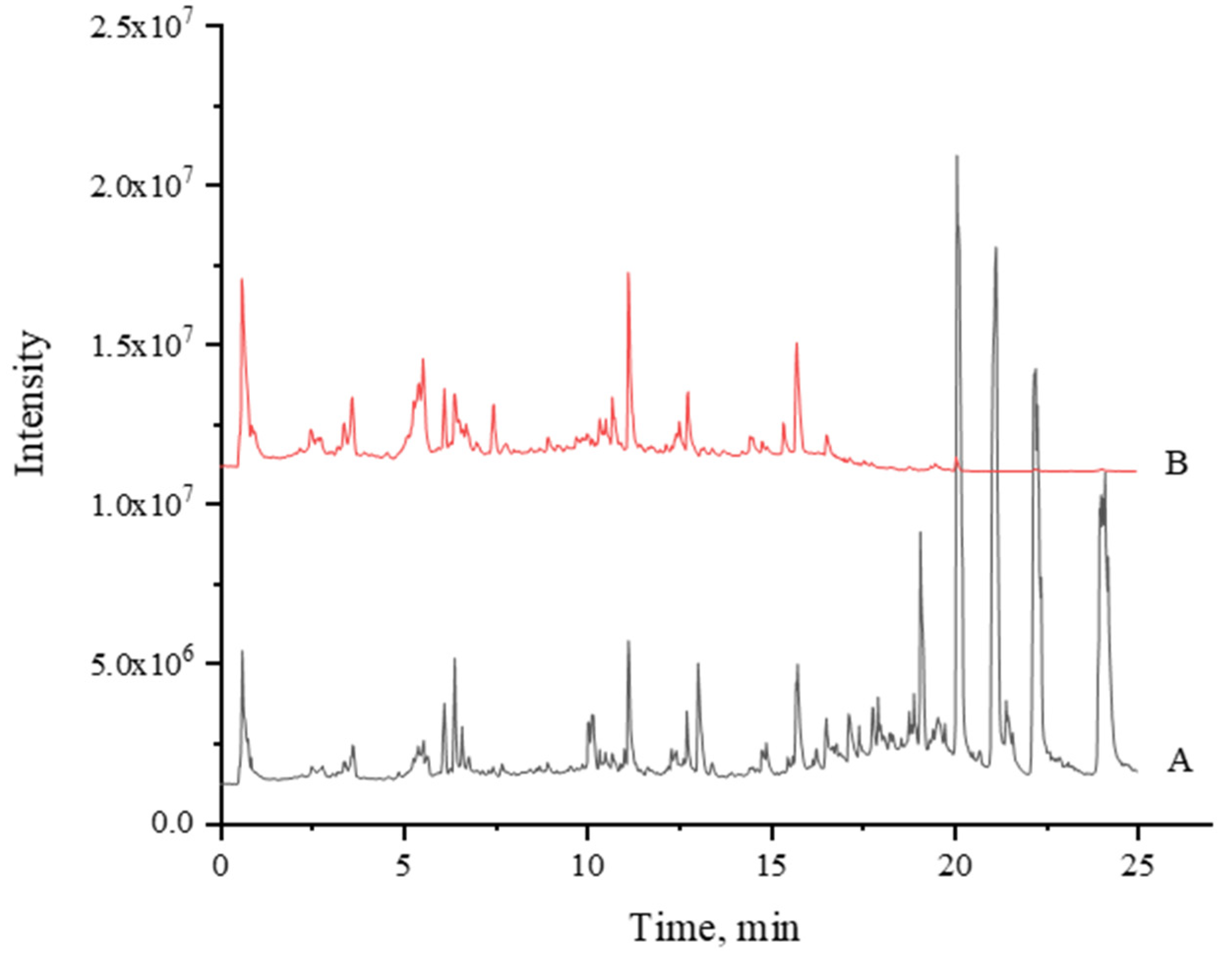

| No. | RT | Formula | m/z | MS/MS | Name | Adducts | Mass Error (ppm) | Raw Abundance | Fragmentation Score | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.57 | C6H13NO4 | 164.0916 | 80.0493, 69.0331, 146.0814 | 1-Deoxynojirimycin | M+H | −0.67 | 546.60 | 92.3 | TCM |
| 2 | 1.93 | C16H18O9 | 353.0880 | 191.0559, 179.0355 | Neochlorogenic acid | M−H | 0.47 | 216.73 | 82.3 | TCM |
| 3 | 2.21 | C15H16O9 | 339.0718 | 177.0190, 133.0290 | Esculin | M−H | −1.04 | 4822.19 | 85.2 | TCM |
| C15H16O9 | 341.0865 | 179.0331, 133.0274 | M+H | −1.06 | 1025.21 | 90.6 | TCM | |||
| 4 | 2.49 | C26H32O14 | 567.1732 | 243.0663, 405.1193 | Mulberroside A | M−H | 0.31 | 9166.15 | 93.0 | TCM |
| C26H32O14 | 569.1839 | 245.0798, 227.0692, 407.1312 | M+H | −4.48 | 252.79 | 80.5 | TCM | |||
| 5 | 2.68 | C16H18O9 | 353.0879 | 191.0575 | Chlorogenic acid | M−H | 0.14 | 850.31 | 94.5 | TCM |
| 6 | 2.8 | C15H16O8 | 325.0916 | 163.0384 | Skimmin | M+H | −0.75 | 58.25 | 99.7 | TCM |
| 7 | 2.85 | C16H18O9 | 399.0928 | 191.0351, 176.0112,148.0174 | Scopolin | M+FA−H | −1.29 | 722.21 | 94.0 | TCM |
| C16H18O9 | 355.1014 | 193.0491, 133.0272, 178.0245 | M+H | −2.57 | 167.72 | 94.5 | TCM | |||
| 8 | 2.94 | C9H6O4 | 177.0196 | 133.0287, 149.0240 | 7,8-Dihydroxycoumarin | M−H | 1.36 | 310.70 | 86.8 | TCM |
| 9 | 2.97 | C7H6O4 | 153.0193 | 67.0188, 109.0303, 65.0398 | 2,4-Dihydroxybenzoic acid | M−H | 0.01 | 453.74 | 99.2 | TCM |
| 10 | 4.08 | C9H8O3 | 163.0401 | 119.0502, 93.0345, 117.0332 | 2-Hydroxycinnamic acid | M−H | −0.09 | 101.52 | 94.1 | TCM |
| 11 | 4.44 | C9H6O3 | 161.0243 | 133.0295 | 7-Hydroxycoumarin | M−H | −0.97 | 284.98 | 77.0 | TCM |
| C9H6O3 | 163.0391 | 77.0389, 107.0513 | M+H | 1.06 | 42.80 | 88.6 | TCM | |||
| 12 | 4.65 | C10H8O4 | 191.0350 | 176.0117, 148.0172 | 6-Hydroxy-7-methoxycoumarin | M−H | 0.17 | 218.22 | 82.7 | TCM |
| 13 | 4.66 | C10H8O4 | 193.0502 | 178.0268, 133.0294, 150.0320 | Scopoletin | M+H | 1.70 | 646.40 | 78.7 | TCM |
| 14 | 4.83 | C20H22O8 | 435.1292 | 227.0709, 185.0603, 389.1201 | Polydatin | M+FA−H | −1.18 | 71.60 | 70.8 | TCM |
| 15 | 5.08 | C15H12O7 | 303.0507 | 125.0241, 177.0193, 217.0508 | 3,5,7,3′,4′-Pentahydroxyflavanone | M−H | −1.01 | 8497.43 | 67.9 | TCM |
| C15H12O7 | 305.0659 | 153.0176, 123.0435 | M+H | −0.95 | 847.04 | 77.0 | TCM | |||
| 16 | 5.14 | C21H20O12 | 463.0869 | 301.0356, 151.0052 | Spiraeoside | M−H | −2.78 | 180.28 | 89.9 | TCM |
| 17 | 5.16 | C21H20O12 | 465.1012 | 303.0495, 257.0413 | Hyperoside | M+H | −3.29 | 91.81 | 82.1 | TCM |
| 18 | 5.54 | C14H12O4 | 243.0662 | 175.0760, 159.0448, 199.0762 | Oxyresveratrol | M−H | −0.44 | 41,040.20 | 93.0 | TCM |
| C14H12O4 | 245.0811 | 107.0486, 161.0588, 181.0642 | M+H | −0.19 | 12,078.74 | 81.1 | TCM | |||
| 19 | 5.94 | C21H22O10 | 433.1139 | 271.0621, 119.0513, 365.0850 | Naringenin-7-O-glucoside | M−H | −0.26 | 30.86 | 74.3 | TCM |
| 20 | 5.94 | C21H20O11 | 447.0929 | 285.0400, 257.0459 | Kaempferol-7-O-β-D-glucopyranoside | M−H | −0.89 | 171.05 | 75.5 | TCM |
| 21 | 6.36 | C14H12O4 | 243.0664 | 159.0458, 201.0558 | Piceatannol | M−H | 0.55 | 324.46 | 75.6 | TCM |
| 22 | 7.12 | C15H10O6 | 285.0404 | 151.0048, 241.0509, 267.0360 | Luteolin | M−H | −0.04 | 245.55 | 67.2 | TCM |
| 23 | 7.18 | C10H18O4 | 201.1139 | 139.1137, 183.1048 | Sebacic acid | M−H | 3.21 | 27.21 | 95.5 | TCM |
| 24 | 7.27 | C10H10O3 | 177.0561 | 117.0350, 145.0304 | Methyl 4-hydroxycinnamate | M−H | 2.30 | 19.37 | 78.2 | TCM |
| 25 | 7.33 | C14H12O3 | 273.0770 | 227.0720, 185.0611, 209.0604 | Resveratrol | M+FA−H | 0.86 | 31.51 | 89.4 | TCM |
| 26 | 10.68 | C40H36O11 | 693.2291 | 137.0227, 203.0701, 365.1010 | Kuwanon G | M+H | −4.77 | 5434.08 | 68.5 | TCM |
| C40H36O11 | 691.2192 | 581.1812, 353.1023 | M−H | 0.98 | 10,494.42 | 90.2 | TCM | |||
| 27 | 11.06 | C18H34O4 | 313.2385 | 183.1371, 295.2283 | 12,13-Dihydroxy-9Z-octadecenoic acid | M−H | 0.29 | 58.35 | 92.7 | TCM |
| 28 | 11.13 | C25H26O6 | 421.1635 | 299.1276, 309.0390 | Mulberrin | M−H | 0.17 | 16,531.24 | - | [34] |
| 29 | 11.32 | C18H39NO3 | 318.2998 | 60.0440, 300.2894 | Phytosphingosine | M+H, M+Na | −2.45 | 999.00 | 86.8 | TCM |
| 30 | 11.46 | C45H44O11 | 759.2827 | 581.1841, 353.1017 | Kuwanon H | M−H | 0.28 | 3452.38 | - | [35] |
| 31 | 12.15 | C15H12O | 191.0854 | 165.0040 | Chalcone | M+H−H2O | −0.81 | 128.30 | 84.2 | TCM |
| 32 | 12.41 | C18H32O3 | 295.2277 | 277.2154, 183.1409 | 9(10)-Epoxy-12Z-octadecenoic acid | M−H | −0.55 | 2353.73 | 77.5 | TCM |
| 33 | 12.73 | C25H24O6 | 419.1494 | 297.1126, 309.1122, 217.0504, 350.0788 | Morusin | M−H | 0.15 | 13,245.32 | - | [36] |
| 34 | 13.94 | C21H36O4 | 353.2674 | 261.2201 | Monolinolenin (9c,12c,15c) | M+H | −3.15 | 1235.93 | 91.6 | TCM |
| 35 | 14.9 | C21H38O4 | 355.2844 | 263.2374, 245.2272, 337.2738 | 1-Monolinoleoyl-rac-glycerol | M+H | −1.28 | 1494.67 | 87.9 | TCM |
| 36 | 14.91 | C16H32O3 | 271.2278 | 225.2246 | 2-Hydroxypalmitic acid | M−H | −0.13 | 169.52 | 82.8 | TCM |
| 37 | 15.47 | C19H38O4 | 331.2840 | 57.0695, 313.2730, 239.2370 | 1-Palmitoylglycerol | M+H | −0.72 | 183.92 | 96.5 | TCM |
| 38 | 15.69 | C18H32O2 | 325.2373 | 279.2323 | Linoleic acid | M+FA−H | −3.96 | 147.72 | 82.8 | TCM |
| 39 | 15.7 | C18H32O2 | 281.2474 | 55.0544, 69.0701, 83.0856, 97.1007 | 10E,12Z-Octadecadienoic acid | M+H | −0.55 | 1828.19 | 81.1 | TCM |
| 40 | 17.15 | C18H34O | 267.2676 | 67.0545, 81.0685, 95.0854, 109.0993 | cis,cis-9,12-Octadecadien-1-ol | M+H | −2.38 | 163.02 | 84.2 | TCM |
| 41 | 17.5 | C19H36O3 | 295.2624 | 263.2372, 245.2260, 55.0551, 69.0706, 81.0700, 95.0867, | Ricinoleic acid methyl ester | M+H−H2O | −2.48 | 378.47 | 93.0 | TCM |
| 42 | 18.78 | C29H50O | 397.3830 | 147.1182, 161.1323 | β-Sitosterol | M+H−H2O | 0.31 | 792.17 | 77.3 | TCM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, W.; Xia, Z.; Xu, L. UPLC-MS/MS Profiling, Antioxidant, α-Glucosidase Inhibitory, Cholinesterase Inhibitory, and Cardiovascular Protection Potentials of Jialing 20 (Morus multicaulis Perr.) Mulberry Branch Extract. Foods 2021, 10, 2659. https://doi.org/10.3390/foods10112659
Xiang W, Xia Z, Xu L. UPLC-MS/MS Profiling, Antioxidant, α-Glucosidase Inhibitory, Cholinesterase Inhibitory, and Cardiovascular Protection Potentials of Jialing 20 (Morus multicaulis Perr.) Mulberry Branch Extract. Foods. 2021; 10(11):2659. https://doi.org/10.3390/foods10112659
Chicago/Turabian StyleXiang, Wei, Zhining Xia, and Li Xu. 2021. "UPLC-MS/MS Profiling, Antioxidant, α-Glucosidase Inhibitory, Cholinesterase Inhibitory, and Cardiovascular Protection Potentials of Jialing 20 (Morus multicaulis Perr.) Mulberry Branch Extract" Foods 10, no. 11: 2659. https://doi.org/10.3390/foods10112659
APA StyleXiang, W., Xia, Z., & Xu, L. (2021). UPLC-MS/MS Profiling, Antioxidant, α-Glucosidase Inhibitory, Cholinesterase Inhibitory, and Cardiovascular Protection Potentials of Jialing 20 (Morus multicaulis Perr.) Mulberry Branch Extract. Foods, 10(11), 2659. https://doi.org/10.3390/foods10112659





