Optimization of Volatile Compounds Extraction from Industrial Celery (Apium graveolens) By-Products by Using Response Surface Methodology and Study of Their Potential as Antioxidant Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Celery By-Products Preparation
2.3. Response Surface Methodology and HS-SPME Extraction Procedure
2.4. GC–MS Conditions
2.5. Antioxidant Activity
2.5.1. ABTS
2.5.2. FRAP
2.6. Total Phenolic Content
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of HS-SPME Procedure
× C + 570, 835 × B2 − 379, 394 × B × C + 197, 762 × C2
3.2. Volatile Compounds
3.3. Quantification of Limonene and γ-Terpinene Compounds
3.4. Antioxidant Activity
3.5. Total Phenolic Content
3.6. Correlation of Total Phenolic Content with Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning Agri-Food Cooperative Vegetable Residues into Functional Powdered Ingredients for the Food Industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef] [Green Version]
- Scheel, C. Beyond sustainability. Transforming Industrial Zero-Valued Residues into Increasing Economic Returns. J. Clean. Prod. 2016, 131, 376–386. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Frezza, C.; Yücetepe, A.; Altuntaş, U.; Uluata, S.; Butnariu, M.; Sarac, I.; Shaheen, S.; Petropoulos, S.A.; et al. Apium Plants: Beyond Simple Food and Phytopharmacological Applications. Appl. Sci. 2019, 9, 3547. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.-K.; Xu, C.-C.; Zhang, L.; Ma, H.; Chen, X.-J.; Sui, Y.-C.; Zhang, H.-Z. Evaluation of Bioactive Components and Antioxidant Capacity of Four Celery (Apium graveolens L.) Leaves and Petioles. Int. J. Food Prop. 2020, 23, 1097–1109. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Özcan, M.M.; Alkaltham, M.S.; Uslu, N.; Hayat, K. Influence of Boiling on Total Phenol, Antioxidant Activity, and Phenolic Compounds of Celery (Apium graveolens L.) root. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Turner, L.; Lignou, S.; Gawthrop, F.; Wagstaff, C. Investigating the Factors that Influence the Aroma Profile of Apium graveolens: A Review. Food Chem. 2021, 345, 128673. [Google Scholar] [CrossRef]
- USDA. Database. Available online: https://fdc.nal.usda.gov/ (accessed on 15 September 2021).
- Guijarro-Real, C.; Rodríguez-Burruezo, A.; Prohens, J.; Raigón, M.D.; Fita, A. HS-SPME Analysis of the Volatiles Profile of Water Celery (Apium nodiflorum), a Wild Vegetable with Increasing Culinary Interest. Food Res. Int. 2019, 121, 765–775. [Google Scholar] [CrossRef]
- Deng, C.; Song, G.; Zheng, X.; Hu, Y.; Zhang, X. Analysis of the Volatile Constituents of Apium graveolens L. and Oenanthe L. by gas Chromatography-Mass Spectrometry, Using Headspace Solid-Phase Microextraction. Chromatographia 2003, 57, 805–809. [Google Scholar] [CrossRef]
- Tanasawet, S.; Boonruamkaew, P.; Sukketsiri, W.; Chonpathompikunlert, P. Anxiolytic and Free Radical Scavenging Potential of Chinese Celery (Apium graveolens) Extract in Mice. Asian Pac. J. Trop. Biomed. 2017, 7, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Al-Moubaraki, A.H.; Al-Howiti, A.A.; Al-Dailami, M.M.; Al-Ghamdi, E.A. Role of Aqueous Extract of Celery (Apium graveolens L.) Seeds Against the Corrosion of Aluminium/Sodium Hydroxide Systems. J. Environ. Chem. Eng. 2017, 5, 4194–4205. [Google Scholar] [CrossRef]
- Mencherini, T.; Cau, A.; Bianco, G.; Della Loggia, R.; Aquino, R.P.; Autore, G. An Extract of Apium graveolens var. dulce leaves: Structure of the Major Constituent, Apiin, and its Anti-Inflammatory Properties. J. Pharm. Pharmacol. 2007, 59, 891–897. [Google Scholar] [CrossRef]
- Bezerra, T.K.A.; Araújo, A.R.R.; Arcanjo, N.M.D.O.; Da Silva, F.L.H.; Queiroga, R.D.C.R.D.E.; Madruga, M.S. Optimization of the HS-SPME-GC/MS Technique for the Analysis of Volatile Compounds in Caprine Coalho Cheese Using Response Surface Methodology. Food Sci. Technol. 2016, 36, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Durant-Archibold, A.A.; Santana, A.; Murillo, E.; Abuín, C.M.F. Analysis of the Volatile Components of Pouteria sapota (Sapote Mamey) Fruit by HS-SPME-GC-MS. Nat. Prod. Commun. 2018, 13, 1934578X1801300826. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, Z.A.; Ebrahimi, A.; Salimi, M. Chemical Composition of Three Ecotypes of Wild Celery (Kelussia odoratissima). J. Herbs. Spices Med. Plants 2011, 17, 62–68. [Google Scholar] [CrossRef]
- Rożek, E.; Nurzyńska-Wierdak, R.; Sałata, A.; Gumiela, P. The Chemical Composition of the Essential Oil of Leaf Celery (Apium Graveolens L. Var. Secalinum Alef.) under the Plants’ Irrigation and Harvesting Method. Acta Sci. Pol. Hortorum Cultus 2016, 15, 147–157. [Google Scholar]
- Turner, L.; Lignou, S.; Gawthrop, F.; Wagstaff, C. Investigating the Relationship of Genotype and Climate Conditions on the Volatile Composition and Sensory Profile of Celery (Apium graveolens). Foods 2021, 10, 1335. [Google Scholar] [CrossRef] [PubMed]
- Sellami, I.H.; Bettaieb, I.; Bourgou, S.; Dahmani, R.; Limam, F.; Marzouk, B. Essential Oil and Aroma Composition of Leaves, Stalks and Roots of Celery (Apium graveolens var. dulce) from Tunisia. J. Essent. Oil Res. 2012, 24, 513–521. [Google Scholar] [CrossRef]
- Yao, Y.; Ren, G. Effect of Thermal Treatment on Phenolic Composition and Antioxidant Activities of Two Celery Cultivars. LWT 2011, 44, 181–185. [Google Scholar] [CrossRef]
- Nikolić, N.; Cvetković, D.; Todorović, Z. A Characterization of Content, Composition and Antioxidant Capacity of Phenolic Compounds in Celery Roots. Ital. J. Food Sci. 2011, 23, 214–219. [Google Scholar]
- Derouich, M.; Bouhlali, E.D.T.; Bammou, M.; Hmidani, A.; Sellam, K.; Alem, C. Bioactive Compounds and Antioxidant, Antiperoxidative, and Antihemolytic Properties Investigation of Three Apiaceae Species Grown in the Southeast of Morocco. Scientifica 2020, 2020, 3971041. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Phenolic Composition and Antioxidant Activities of 11 Celery Cultivars. J. Food Sci. 2010, 75, C9–C13. [Google Scholar] [CrossRef]
- Moreno, E.; Fita, A.; González-Mas, M.C.; Rodríguez-Burruezo, A. HS-SPME Study of the Volatile Fraction of Capsicum Accessions and Hybrids in Different Parts of the Fruit. Sci. Hortic. 2012, 135, 87–97. [Google Scholar] [CrossRef]
- Kebede, B.; Grauwet, T.; Palmers, S.; Vervoort, L.; Carle, R.; Hendrickx, M.E.; Van Loey, A. Effect of High Pressure High Temperature Processing on the Volatile Fraction of Differently Coloured Carrots. Food Chem. 2014, 153, 340–352. [Google Scholar] [CrossRef]
- Gaggiotti, S.; Mascini, M.; Pittia, P.; Della Pelle, F.; Compagnone, D. Headspace Volatile Evaluation of Carrot Samples—Comparison of GC/MS and AuNPs-hpDNA-Based E-Nose. Foods 2019, 8, 293. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.; Góis, A.; Mendes, F.; Perestrelo, R.; Medina, S.; Câmara, J.S. Typicality Assessment of Onions (Allium cepa) from Different Geographical Regions Based on the Volatile Signature and Chemometric Tools. Foods 2020, 9, 375. [Google Scholar] [CrossRef] [Green Version]
- Cecchi, L.; Ieri, F.; Vignolini, P.; Mulinacci, N.; Romani, A. Characterization of Volatile and Flavonoid Composition of Different Cuts of Dried Onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GC×GC-TOF and HPLC-DAD. Molecules 2020, 25, 408. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nićetin, M.; Pezo, L.; Loncar, B.; Filipovic, V.; Suput, D.; Knezevic, V.; Filipović, J. The Possibility of Increasing the Antioxidant Activity of Celery Root During Osmotic Treatment. J. Serbian Chem. Soc. 2017, 82, 253–265. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Wang, L.; Chen, S.; Xu, Y. Optimization and Validation of a Head Space Solid-Phase Microextraction-Arrow Gas Chromatography-Mass Spectrometry Method Using Central Composite Design for Determination of Aroma Compounds in Chinese Liquor (Baijiu). J. Chromatogr. A 2020, 1610, 460584. [Google Scholar] [CrossRef] [PubMed]
- Carasek, E.; Pawliszyn, J. Screening of Tropical Fruit Volatile Compounds Using Solid-Phase Microextraction (SPME) Fibers and Internally Cooled SPME Fiber. J. Agric. Food Chem. 2006, 54, 8688–8696. [Google Scholar] [CrossRef]
- Ma, Q.; Hamid, N.; Bekhit, A.E.-D.; Robertson, J.; Law, T. Optimization of Headspace Solid Phase Microextraction (HS-SPME) for Gas Chromatography Mass Spectrometry (GC–MS) Analysis of Aroma Compounds in Cooked Beef Using Response Surface Methodology. Microchem. J. 2013, 111, 16–24. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Panigrahi, S. Solid-Phase Microextraction (SPME) Techniques for Quality Characterization of Food Products: A Review. Food Bioprocess Technol. 2011, 4, 1–26. [Google Scholar] [CrossRef]
- De Santana, K.L.; Galvão, M.D.S.; De Jesus, M.S.; Nogueira, J.P.; Narain, N. HS-SPME Optimization and Extraction of Volatile Compounds from Soursop (Annona Muricata L.) Pulp with Emphasis on their Characteristic Impact Compounds. Food Sci. Technol. 2017, 37, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Robotti, E.; Campo, F.; Riviello, M.; Bobba, M.; Manfredi, M.; Mazzucco, E.; Gosetti, F.; Calabrese, G.; Sangiorgi, E.; Marengo, E. Optimization of the Extraction of the Volatile Fraction from Honey Samples by SPME-GC-MS, Experimental Design, and Multivariate Target Functions. J. Chem. 2017, 2017, 6437857. [Google Scholar] [CrossRef]
- Ferreira, L.; Perestrelo, R.; Câmara, J.S.; Ferreira, L.; Perestrelo, R.; Câmara, J.S. Comparative Analysis of the Volatile Fraction from Annona Cherimola Mill. Cultivars by Solid-Phase Microextraction and Gas Chromatography–Quadrupole Mass Spectrometry Detection. Talanta 2009, 77, 1087–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perestrelo, R.; Silva, C.L.; Silva, P.; Câmara, J.S. Establishment of the Volatile Signature of Wine-Based Aromatic Vinegars Subjected to Maceration. Molecules 2018, 23, 499. [Google Scholar] [CrossRef] [Green Version]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Comparison of the Proximate Composition, Vitamins (Ascorbic Acid, α-Tocopherol and Retinol), Anti-Nutrients (Phytate and Oxalate) and the GC-MS Analysis of the Essential Oil of the Root and Leaf of Rumex crispus L. Plants 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, J.; Wu, C.; Huang, J.; Zhou, R.; Jin, Y. Characteristics and Valorization Potential of Fermentation Waste of Greengage (Prunus mume). Appl. Sci. 2021, 11, 8296. [Google Scholar] [CrossRef]
- The Good Scents Company Information System Providing Information for the Flavor, Fragrance, Food and Cosmetic Industries. Available online: http://www.thegoodscentscompany.com (accessed on 15 September 2021).
- Ferreira, L.; Perestrelo, R.; Caldeira, M.; Câmara, J.S. Characterization of Volatile Substances in Apples from Rosaceae Family by Headspace Solid-Phase Microextraction Followed by GC-qMS. J. Sep. Sci. 2009, 32, 1875–1888. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Qi, M.; Shao, Q.; Zhou, S.; Fu, R. Analysis of the Volatile Compounds in Ligusticum chuanxiong Hort. using HS-SPME–GC-MS. J. Pharm. Biomed. Anal. 2007, 44, 464–470. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of Plant-Derived Flavor Compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Urbaneja-Bernat, P.; Cloonan, K.; Zhang, A.; Salazar-Mendoza, P.; Rodriguez-Saona, C. Fruit Volatiles Mediate Differential Attraction of Drosophila suzukii to Wild and Cultivated Blueberries. J. Pest Sci. 2021, 94, 1249–1263. [Google Scholar] [CrossRef]
- Machyňáková, A.; Khvalbota, L.; Špánik, I. Enantiomer Distribution of Major Chiral Volatile Organic Compounds in Botrytized Grapes and Wines. Eur. Food Res. Technol. 2021, 247, 2321–2331. [Google Scholar] [CrossRef]
- León, A.; Del-Ángel, M.; Ávila, J.L.; Delgado, G. Phthalides: Distribution in Nature, Chemical Reactivity, Synthesis, and Biological Activity. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Berlin, Germany, 2017; Volume 104, pp. 127–246. [Google Scholar] [CrossRef]
- Lin, G.; Chan, S.S.-K.; Chung, H.-S.; Li, S.-L. Chemistry and Biological Activities of Naturally Occurring Phthalides. Stud. Nat. Prod. Chem. 2005, 32, 611–669. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Barros, H.R.D.M.; Ferreira, T.A.P.D.C.; Genovese, M.I. Antioxidant Capacity and Mineral Content of Pulp and Peel from Commercial Cultivars of Citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef]
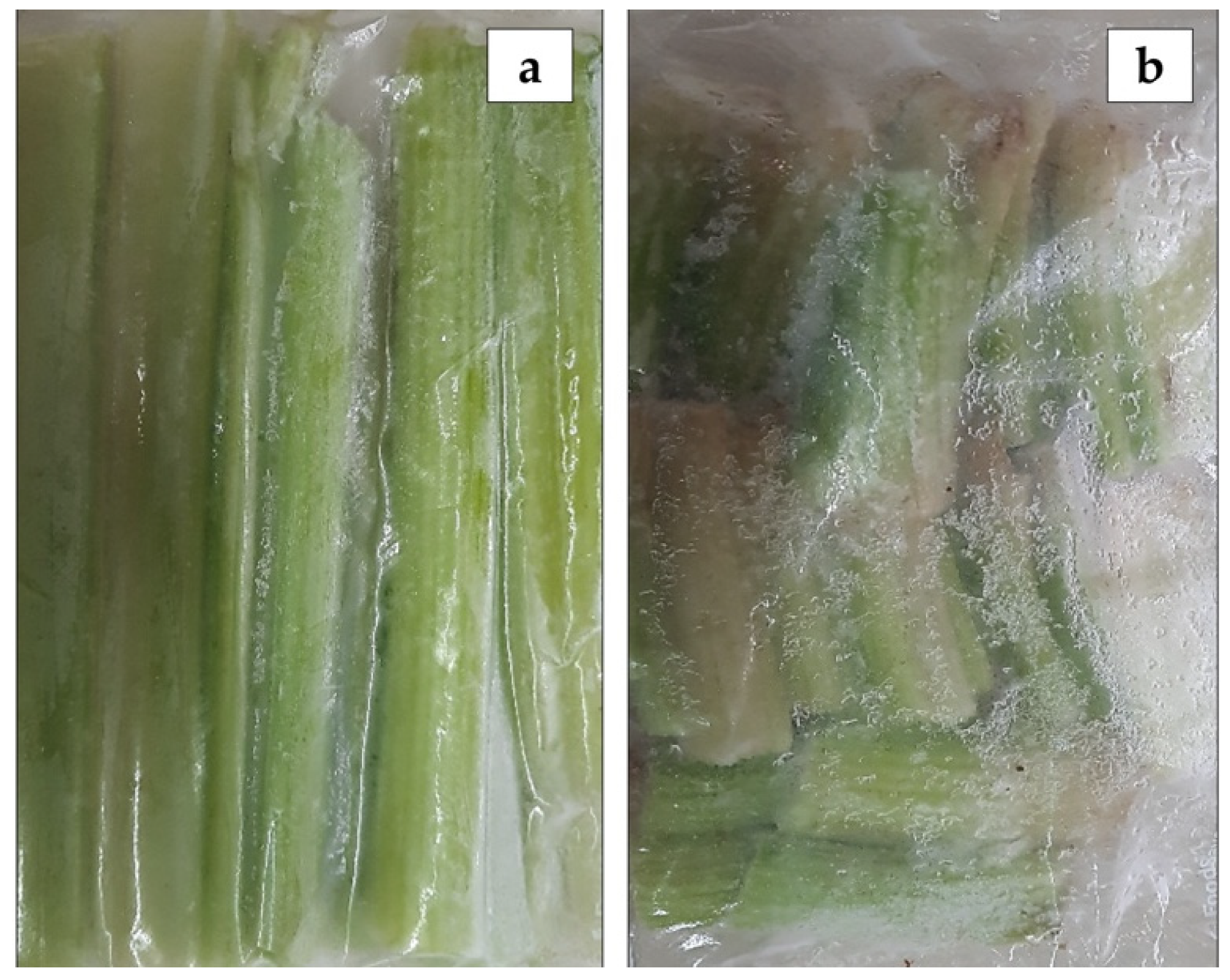

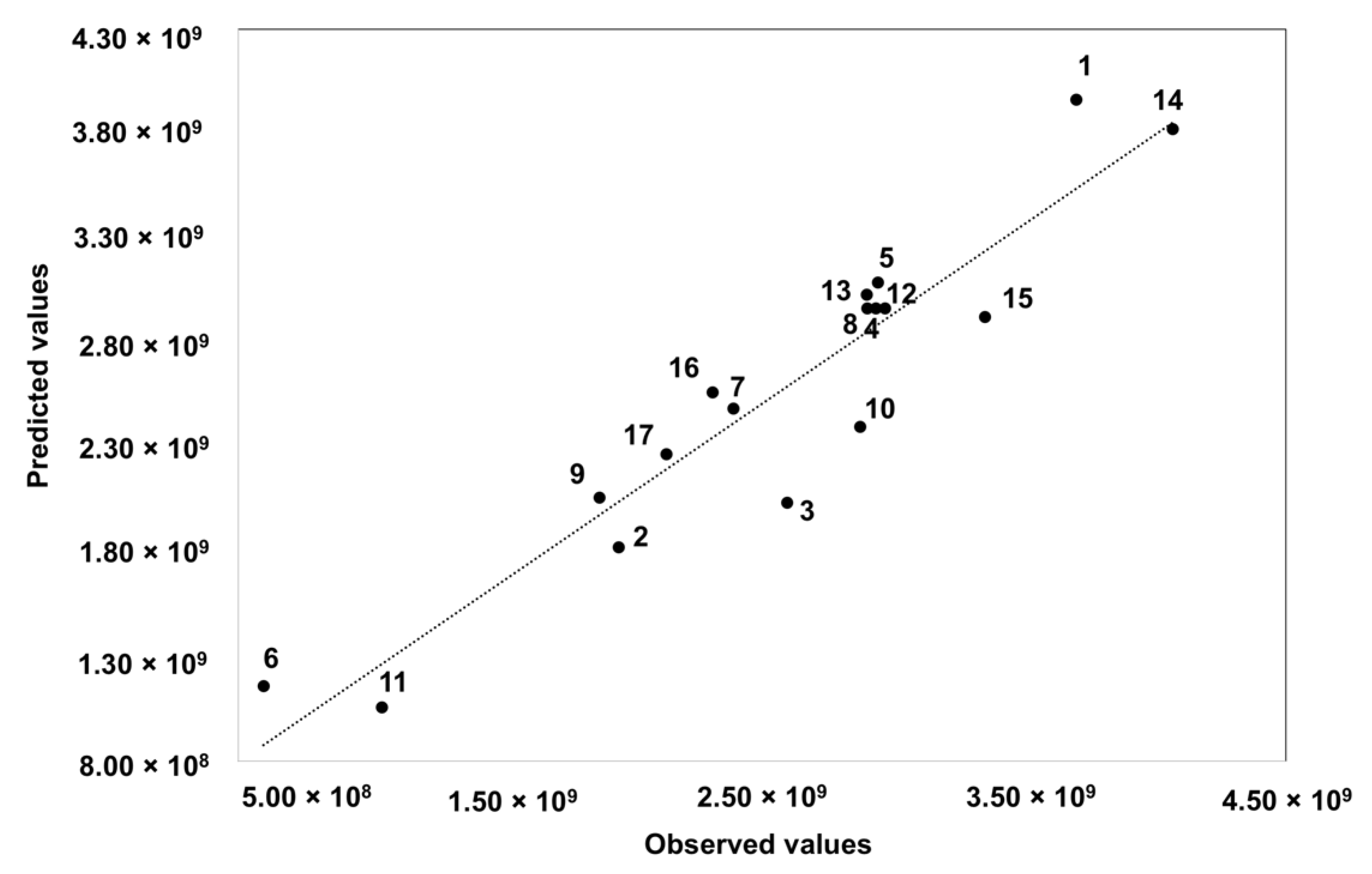
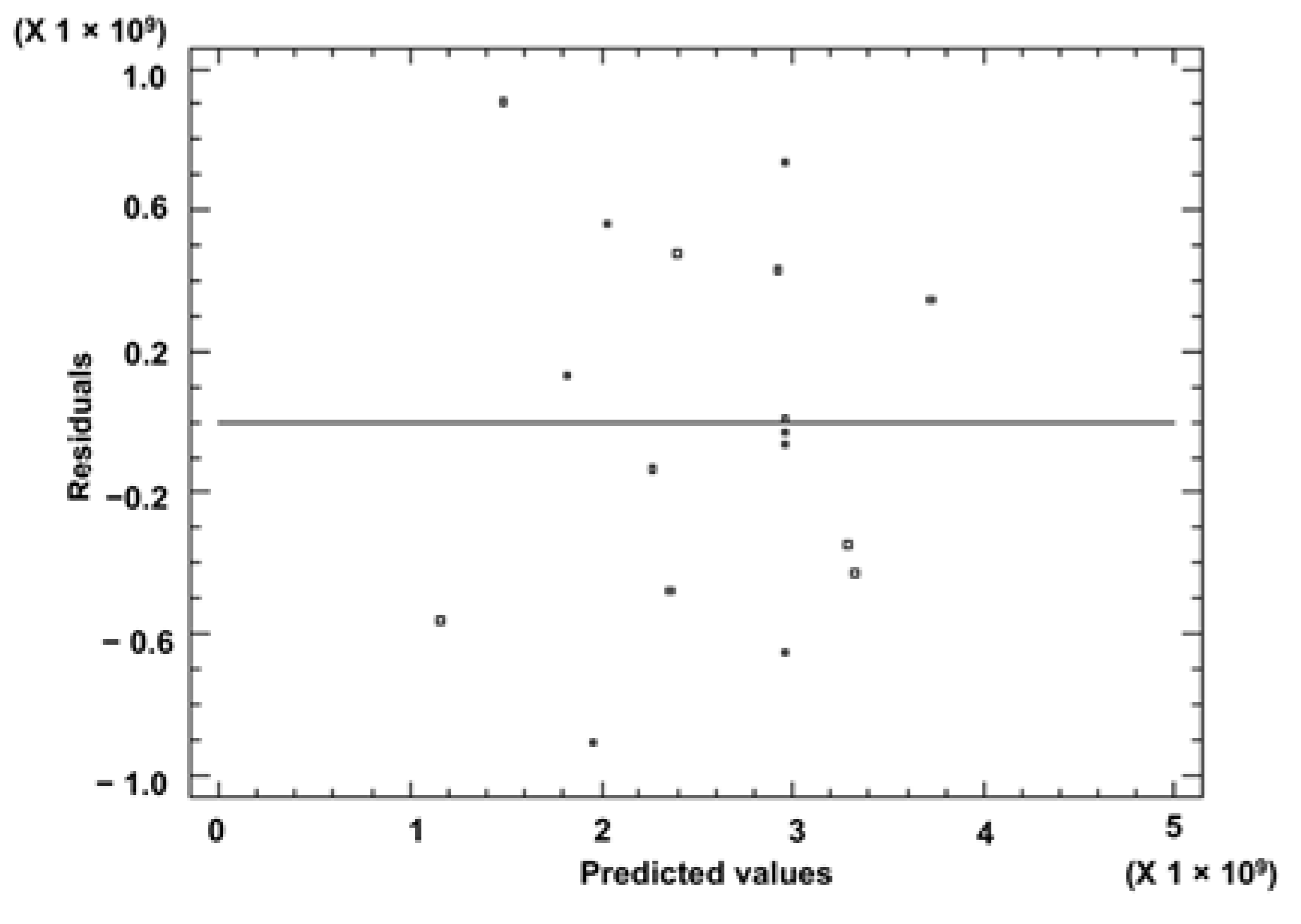



| Run | Sample Weight (g) | Temperature (°C) | Time (Min) | Sum of Areas |
|---|---|---|---|---|
| 1 | 1.75 | 50 | 35 | 3.70 × 109 |
| 2 | 1.75 | 50 | 35 | 2.90 × 109 |
| 3 | 3.0 | 50 | 60 | 1.05 × 109 |
| 4 | 1.75 | 70 | 10 | 2.94 × 109 |
| 5 | 1.75 | 30 | 10 | 2.90 × 109 |
| 6 | 0.5 | 50 | 10 | 2.39 × 109 |
| 7 | 1.75 | 70 | 60 | 3.35 × 109 |
| 8 | 3.0 | 50 | 10 | 2.14 × 109 |
| 9 | 0.5 | 30 | 35 | 1.88 × 109 |
| 10 | 3.0 | 70 | 35 | 2.88 × 109 |
| 11 | 1.75 | 30 | 60 | 4.07 × 109 |
| 12 | 1.75 | 50 | 35 | 2.94 × 109 |
| 13 | 0.5 | 70 | 35 | 5.97 × 108 |
| 14 | 1.75 | 50 | 35 | 2.97 × 109 |
| 15 | 3.0 | 30 | 35 | 2.60 × 109 |
| 16 | 1.75 | 50 | 35 | 2.31 × 109 |
| 17 | 0.5 | 50 | 60 | 1.95 × 109 |
| Compound | Retention Time (Min) | Chemical Group | Odour Descriptor a |
|---|---|---|---|
| 3-hydroxybutanone | 10.08 | Methyl ketone | Buttery, fatty |
| 2,3-butanediol | 14.00 | Alcohol | Creamy |
| M-xylene | 14.31 | Aromatic hydrocarbon | Plastic |
| α-pinene | 16.16 | Monoterpene | Herbal |
| Camphene | 16.85 | Monoterpene | Woody |
| Sabinene | 17.77 | Monoterpene | Woody |
| β-pinene | 17.89 | Monoterpene | Terpenic |
| 2-pentylfuran | 18.40 | Furan | Fruity, green, earthy |
| Limonene | 19.74 | Monoterpene | Lemon, orange, citrus |
| β-cymene | 19.82 | Monoterpene | Cumin, lemon |
| γ-terpinene | 20.60 | Monoterpene | Sweet, citrus |
| Terpinolene | 22.99 | Monoterpene | Herbal |
| 6-butyl-1,4-cycloheptadiene | 23.92 | Alkene | Not identified |
| Pentylbenzene | 24.12 | Aromatic hydrocarbon | Not identified |
| Dihydrocarvone | 26.80 | Monoterpene | Herbal, minty |
| (Z)-carveol | 27.07 | Alcohol | Minty |
| Cyclosativene | 30.33 | Sesquiterpene | Not identified |
| α-copaene | 30.63 | Sesquiterpene | Woody, spicy, honey |
| Caryophyllene | 32.01 | Sesquiterpene | Sweet, woody, spice |
| Humulene | 32.94 | Sesquiterpene | Woody |
| β-selinene | 33.74 | Sesquiterpene | Herbal |
| δ-cadinene | 34.21 | Sesquiterpene | Herbal |
| β-ionone | 34.48 | Ketone | Floral |
| Globulol | 34.89 | Alcohol | Floral, rose |
| 3-butylphthalide | 39.81 | Phthalide | Celery, herbal |
| 4-heptylphenol | 41.76 | Phenol | Not identified |
| Compound | M1 | M2 | M3 | M4 | M5 | M6 |
|---|---|---|---|---|---|---|
| Monoterpenes | ||||||
| α-pinene | 1.43 ± 0.03 a | 1.79 ± 0.03 b | 1.66 ± 0.02 c | 0.38 ± 0.01 d | 0.39 ± 0.01 d | 0.29 ± 0.02 e |
| Camphene | 0.26 ± 0.02 a | 0.28 ± 0.05 a | 0.27 ± 0.04 a | 0.10 ± 0.01 b | 0.05 ± 0.01 c | 0.03 ± 0.01 c |
| Sabinene | 0.06 ± 0.03 a | 0.18 ± 0.06 a | 0.10 ± 0.05 a | 1.76 ± 0.06 b | 1.34 ± 0.06 c | 0.54 ± 0.04 d |
| β-pinene | 3.43 ± 0.06 a | 1.74 ± 0.08 b | 2.11 ± 0.05 b | 6.71 ± 0.38 c | 5.16 ± 0.25 d | 5.18 ± 0.62 d |
| Limonene | 33.16 ± 2.46 a | 35.72 ± 1.51 a | 36.02 ± 0.48 a | 28.64 ± 0.74 b | 29.82 ± 1.20 b | 27.44 ± 0.70 b |
| β -cymene | 19.17 ± 1.93 a | 22.57 ± 0.74 b | 23.70 ± 0.54 b | 13.55 ± 0.55 c | 13.63 ± 0.55 c | 15.60 ± 0.69 c |
| γ-terpinene | 24.95 ± 0.89 a | 23.51 ± 0.91 a | 24.28 ± 0.76 a | 23.96 ± 1.07 a | 25.30 ± 0.54 ab | 26.59 ± 0.16 b |
| Terpinolene | 0.24 ± 0.05 a | 0.29 ± 0.07 a | 0.20 ± 0.09 a | 0.87 ± 0.07 b | 2.29 ± 0.10 c | 1.79 ± 0.16 d |
| Dihydrocarvone | 0.23 ± 0.02 a | 0.06 ± 0.01 b | 0.03 ± 0.02 b | 0.18 ± 0.03 c | 0.46 ± 0.04 d | 0.20 ± 0.02 ac |
| Sesquiterpenes | ||||||
| Cyclosativene | 0.25 ± 0.50 a | 0.28 ± 0.05 b | 0.20 ± 0.02 c | 0.02 ± 0.01 d | 0.02 ± 0.01 d | 0.03 ± 0.01 d |
| α-copaene | 1.32 ± 0.10 a | 0.02 ± 0.01 b | 0.03 ± 0.01 b | 0.24 ± 0.01 c | 0.12 ± 0.01 b | 0.07 ± 0.01 b |
| Caryophyllene | 8.57 ± 0.22 ac | 7.13 ± 0.39 b | 7.59 ± 0.43 ab | 9.97 ± 0.60 c | 6.58 ± 0.62 bd | 5.24 ± 0.52 d |
| Humulene | 0.55 ± 0.06 ad | 0.07 ± 0.02 b | 0.64 ± 0.02 a | 2.84 ± 0.12 c | 0.55 ± 0.03 ad | 0.45 ± 0.02 d |
| β-selinene | 3.06 ± 0.18 a | 1.96 ± 0.08 b | 1.15 ± 0.06 b | 4.57 ± 0.32 c | 7.57 ± 0.64 d | 5.01 ± 0.31 c |
| δ-cadinene | 0.18 ± 0.03 a | 0.27 ± 0.05 a | 0.20 ± 0.04 a | 0.34 ± 0.01 b | 0.98 ± 0.07 c | 1.00 ± 0.03 c |
| Ketones | ||||||
| 3-hydroxybutanone | 0.03 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.16 ± 0.05 b | 0.22 ± 0.09 b | 0.19 ± 0.08 b |
| β-ionone | 0.12 ± 0.03 a | 0.12 ± 0.02 a | 0.11 ± 0.02 a | 0.10 ± 0.04 ab | 0.05 ± 0.03 b | 0.04 ± 0.02 b |
| Alcohols | ||||||
| 2,3-butanediol | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.03 ± 0.01 a | 0.46 ± 0.04 b | 1.62 ± 0.51 c | 1.14 ± 0.15 c |
| (Z)-carveol | 0.15 ± 0.02 a | 0.15 ± 0.04 a | 0.28 ± 0.10 ab | 0.29 ± 0.01 b | 0.38 ± 0.03 c | 0.39 ± 0.02 c |
| Globulol | 0.15 ± 0.01 a | 0.15 ± 0.07 a | 0.29 ± 0.11 a | nd | nd | nd |
| Others | ||||||
| M-xylene | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.02 a | 0.32 ± 0.20 b | 0.05 ± 0.01 c | 0.05 ± 0.02 c |
| 2-pentylfuran | 0.31 ± 0.05 a | 0.30 ± 0.07 a | 0.28 ± 0.03 a | 0.21 ± 0.01 b | 0.09 ± 0.01 c | 0.10 ± 0.01 c |
| 6-butyl-1,4-cyclo Heptadiene | 3.74 ± 0.09 a | 1.51 ± 0.09 b | 0.29 ± 0.12 c | 3.94 ± 0.04 a | 3.97 ± 0.17 a | 3.11 ± 0.55 a |
| 3-butylphthalide | 0.49 ± 0.10 a | 0.58 ± 0.04 a | 0.54 ± 0.12 a | nd | nd | nd |
| 4-heptylphenol | 0.73 ± 0.32 a | 0.07 ± 0.02 b | 0.06 ± 0.03 b | nd | nd | nd |
| Pentylbenzene | 1.50 ± 0.12 a | 1.23 ± 0.09 b | 0.06 ± 0.05 c | 0.28 ± 0.01 d | 0.37 ± 0.03 de | 0.52 ± 0.03 e |
| Sample | FRAP (μM Trolox 100 g−1 FW) | ABTS (μM Trolox 100 g−1 FW) | TPC (mg GAE 100 g−1 FW) |
|---|---|---|---|
| M1 | 328.3 ± 10.5 a | 63.1 ± 0.2 a | 16.3 ± 0.5 a |
| M2 | 368.7 ± 39.9 a | 232.2 ± 25.0 b | 49.0 ± 5.4 b |
| M3 | 355.7 ± 21.8 a | 144.4 ± 31.3 cd | 32.5 ± 3.7 c |
| M4 | 172.4 ± 2.7 b | 85.8 ± 1.9 a | 14.6 ± 0.5 a |
| M5 | 389.9 ± 24.8 a | 196.7 ± 25.1 bc | 33.2 ± 4.7 c |
| M6 | 340.5 ± 28.5 a | 129.6 ± 11.5 d | 21.8 ± 2.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán Sanahuja, A.; Ponce Landete, M.; Domingo Martínez, M.I.; Prats Moya, M.S.; Valdés García, A. Optimization of Volatile Compounds Extraction from Industrial Celery (Apium graveolens) By-Products by Using Response Surface Methodology and Study of Their Potential as Antioxidant Sources. Foods 2021, 10, 2664. https://doi.org/10.3390/foods10112664
Beltrán Sanahuja A, Ponce Landete M, Domingo Martínez MI, Prats Moya MS, Valdés García A. Optimization of Volatile Compounds Extraction from Industrial Celery (Apium graveolens) By-Products by Using Response Surface Methodology and Study of Their Potential as Antioxidant Sources. Foods. 2021; 10(11):2664. https://doi.org/10.3390/foods10112664
Chicago/Turabian StyleBeltrán Sanahuja, Ana, Mercedes Ponce Landete, María Isabel Domingo Martínez, María Soledad Prats Moya, and Arantzazu Valdés García. 2021. "Optimization of Volatile Compounds Extraction from Industrial Celery (Apium graveolens) By-Products by Using Response Surface Methodology and Study of Their Potential as Antioxidant Sources" Foods 10, no. 11: 2664. https://doi.org/10.3390/foods10112664
APA StyleBeltrán Sanahuja, A., Ponce Landete, M., Domingo Martínez, M. I., Prats Moya, M. S., & Valdés García, A. (2021). Optimization of Volatile Compounds Extraction from Industrial Celery (Apium graveolens) By-Products by Using Response Surface Methodology and Study of Their Potential as Antioxidant Sources. Foods, 10(11), 2664. https://doi.org/10.3390/foods10112664









