Antioxidant Activity of Stryphnodendron rotundifolium Mart. Stem Bark Fraction in an Iron Overload Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Botanical Material and Extraction Procedure
2.2. Phytochemical Prospecting
2.3. Quantification of Phenolic Compounds
2.3.1. Total Phenolic Content
2.3.2. Determination of Total Condensed Tannins
2.3.3. Quantification of Total Hydrolyzable Tannins
2.4. UPLC–ESI-qTOF-MS/MS
2.5. In Vitro Antioxidant Activity Analysis
2.5.1. Fe2+-Chelating Activity and Fe3+-Reducing Power as a Function of Time
2.5.2. Deoxyribose Oxidative Degradation Assay
2.5.3. Analysis of the Mechanism Underlying the Inhibition of 2-DR Degradation
2.6. In Vivo Toxicity Analysis
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira Macedo, J.G.; Menezes, I.R.A.D.; Alves Ribeiro, D.; de Oliveira Santos, M.; Gonçalves de Mâcedo, D.; Ferreira Macedo, M.J.; Vilar de Almeida, B.; Souza de Oliveira, L.G.; Pereira Leite, C.; de Almeida Souza, M.M. Analysis of the variability of therapeutic indications of medicinal species in the Northeast of Brazil: Comparative study. Evid.-Based Complement. Altern. Med. 2018, 2018, 6769193. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, D.A.; de Oliveira, L.G.S.; de Macêdo, D.G.; de Menezes, I.R.A.; da Costa, J.G.M.; da Silva, M.A.P.; Lacerda, S.R.; de Almeida Souza, M.M. Promising medicinal plants for bioprospection in a Cerrado area of Chapada do Araripe, Northeastern Brazil. J. Ethnopharmacol. 2014, 155, 1522–1533. [Google Scholar] [CrossRef]
- Corrêa, M. Dicionário de Plantas Úteis do Brasil e Das Exóticas e Cultivadas; Impressa Nacional: Rio de Janeiro, Brazil, 1978. [Google Scholar]
- Costa, J.G.M.D.; Leite, G.D.O.; Dubois, A.F.; Seeger, R.L.; Boligon, A.A.; Athayde, M.L.; Campos, A.R.; Rocha, J.B.T.D. Antioxidant effect of Stryphnodendron rotundifolium Martius extracts from Cariri-Ceará state (Brazil): Potential involvement in its therapeutic use. Molecules 2012, 17, 934–950. [Google Scholar] [CrossRef]
- Baldivia, D.D.S.; Leite, D.F.; Castro, D.T.H.D.; Campos, J.F.; Santos, U.P.D.; Paredes-Gamero, E.J.; Carollo, C.A.; Silva, D.B.; de Picoli Souza, K.; Dos Santos, E.L. Evaluation of In Vitro Antioxidant and Anticancer Properties of the Aqueous Extract from the Stem Bark of Stryphnodendron adstringens. Int. J. Mol. Sci. 2018, 19, 2432. [Google Scholar] [CrossRef] [Green Version]
- da Silveira, G.D.; Motta, M.J.; Müller, L.S.; Lameira, O.; Athayde, M.L.; Piana, M.; da Rosa, M.B.; Viana, C.; de Carvalho, L.M. Determination of phenolic antioxidants in Amazonian medicinal plants by HPLC with pulsed amperometric detection. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1259–1266. [Google Scholar] [CrossRef]
- Fu, Y.; Qiao, L.; Cao, Y.; Zhou, X.; Liu, Y.; Ye, X. Structural elucidation and antioxidant activities of proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves. PLoS ONE 2014, 9, e96162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushner, J.P.; Porter, J.P.; Olivieri, N.F. Secondary iron overload. Hematol. Am. Soc. Hematol. Educ. Program. 2001, 47–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phatak, P.; Brissot, P.; Wurster, M.; Adams, P.C.; Bonkovsky, H.L.; Gross, J.; Malfertheiner, P.; McLaren, G.D.; Niederau, C.; Piperno, A.; et al. A phase 1/2, dose-escalation trial of deferasirox for the treatment of iron overload in HFE-related hereditary hemochromatosis. Hepatology 2010, 52, 1671–1779. [Google Scholar] [CrossRef] [Green Version]
- Nardi, G.; Cadiz, C.; Lachman, J.; Cornelio, C. Advances in hereditary hemochromatosis. Acta Gastroenterol. Latinoam. 2003, 33, 103–107, PMID. 14708503. [Google Scholar]
- Crawford, R.D. Proposed role for a combination of citric acid and ascorbic acid in the production of dietary iron overload: A fundamental cause of disease. Biochem. Mol. Med. 1995, 54, 1–11. [Google Scholar] [CrossRef]
- Weinberg, E.D. Cellular iron metabolism in health and disease. Drug Metab. Rev. 1990, 22, 531–579. [Google Scholar] [CrossRef]
- Beaumont, C. Vailont, Iron homeostasis. In Disorders of iron Homeostasis, Erythrocytes, Erythropoiesis; Beaumont, C., Beris, P., Beuzard, Y., Brugnara, C., Eds.; Forum Service Editore: Genova, Italy, 2006; pp. 393–406. [Google Scholar]
- Donovan, A.; Roy, C.N.; Andrews, N.C. The ins and outs of iron homeostasis. Physiology 2006, 21, 115–123. [Google Scholar] [CrossRef]
- Anderson, R.L.; Wolf, W.J. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J. Nutr. 1995, 125 (Suppl. 3), 581S–588S. [Google Scholar] [PubMed]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Sun, T.; Ho, C.-T. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- Cadahía, E.; Conde, E.; García-Vallejo, M.; Fernández de Simón, B. High pressure liquid chromatographic analysis of polyphenols in leaves of Eucalyptus camaldulensis, E. globulus and E. rudis: Proanthocyanidins, ellagitannins and flavonol glycosides. Phytochem. Anal. 1997, 8, 78–83. [Google Scholar] [CrossRef]
- Cork, S.J.; Krockenberger, A.K. Methods and pitfalls of extracting condensed tannins and other phenolics from plants: Insights from investigations on Eucalyptus leaves. J. Chem. Ecol. 1991, 17, 123–134. [Google Scholar] [CrossRef]
- Matos, F. Introduction to Experimental Phytochemical; Editions UFC: Fortaleza, Brazil, 1997. [Google Scholar]
- Nuutila, A.M.; Puupponen-Pimiä, R.; Aarni, M.; Oksman-Caldentey, K.-M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Bossu, C.M.; Ferreira, E.C.; Chaves, F.S.; Menezes, E.A.; Nogueira, A.R.A. Flow injection system for hydrolysable tannin determination. Microchem. J. 2006, 84, 88–92. [Google Scholar] [CrossRef]
- Andrews, N.C. Disorders of iron metabolism. N. Eng. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef]
- Burtis, C.A.; Ashwood, E.R.; Bruns, D.E. (Eds.) Tietz Textbook of Clinical Chemistry and Molecular Diagnostic, 4th ed.; Elsevier & Saunders: St. Louis, MI, USA, 2006; pp. 797–835. [Google Scholar]
- Worwood, M. The laboratory assessment of iron states–An update. Clin. Chim. Acta 1997, 259, 3–23. [Google Scholar] [CrossRef]
- Zhang, D.J.; Elswick, R.K.; Miller, W.G.; Bailey, J.L. Effect of serum-clot contact time on clinical chemistry laboratory results. Clin. Chem. 1998, 44, 1325–1333. [Google Scholar] [CrossRef] [Green Version]
- Minotti, G.; Aust, S.D. An investigation into thee mechanism of citrate FE2+-dependent lipid peroxidation. Free. Radic. Biol. Med. 1987, 3, 379–387. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Thiobarbituric acid-reactivity following iron-dependent free-radical damage to amino acids and carbohydrates. Febs Lett. 1981, 128, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Torres Salazar, G.J.; Duavy, S.M.; Adefegha, S.A.; Boligon, A.A.; Morel, A.F.; da Rocha, J.B.; Barbosa, N.B.; Ecker, A. Comparative antioxidant potential of aqueous and acetone/water extracts of grains and brans of Graniferous sorghum [Sorghum bicolor (L.) Moench] hybrid guanipa 71 in vitro. Int. J. Pharm. Sci. Res. 2018, 9, 932–944. [Google Scholar]
- McLaughlin, J. Brine shrimp: A convenient general bioassay for active constituents. Planta Med. 1982, 45, 31–32. [Google Scholar]
- Henriques, B.O.; Corrêa, O.; Azevedo, E.P.C.; Pádua, R.M.; Oliveira, V.L.S.D.; Oliveira, T.H.C.; Boff, D.; Dias, A.C.F.; Souza, D.G.D.; Amaral, F.A. In vitro TNF-inhibitory activity of brazilian plants and anti-inflammatory effect of Stryphnodendron adstringens in an acute arthritis model. Evid. Based Complement. Altern. Med. 2016, 2016, 9872598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mello, J.P.; Petereit, F.; Nahrstedt, A. Flavan-3-ols and prodelphinidins from Stryphnodendron adstringens. Phytochemistry 1996, 41, 807–813. [Google Scholar] [CrossRef]
- Pinto, S.C.G.; Bueno, F.G.; Panizzon, G.P.; Morais, G.; Dos Santos, P.V.P.; Baesso, M.L.; de Souza Leite-Mello, E.V.; de Mello, J.C.P. Stryphnodendron adstringens: Clarifying wound healing in streptozotocin-induced diabetic rats. Planta Med. 2015, 81, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Demarque, D.P.; Callejon, D.R.; de Oliveira, G.G.; Silva, D.B.; Carollo, C.A.; Lopes, N.P. The role of tannins as antiulcer agents: A fluorescence-imaging based study. Rev. Bras. Farmacogn. 2018, 28, 425–432. [Google Scholar] [CrossRef]
- Li, H.-J.; Deinzer, M.L. Tandem mass spectrometry for sequencing proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef]
- Robeson, D.J.; Ingham, J.L.; Harborne, J.B. Identification of two chromone phytoalexins in the sweet pea, Lathyrus odoratus. Phtochemistry 1980, 19, 2171–2173. [Google Scholar]
- Bladé, C.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B.; Bravo, F.I.; Salvadó, M.J.; Arola, L.; Suárez, M. Proanthocyanidins in health and disease. Biofactors 2016, 42, 5–12. [Google Scholar] [PubMed]
- Shriver, D.F.; Atkins, P.W. Inorganic Chemistry, 3rd ed.; Bookman: Porto Alegre, Brazil, 2003. [Google Scholar]
- Li, J.; Cao, F.; Yin, H.-L.; Huang, Z.-J.; Lin, Z.-T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Castro, L.; Freeman, B.A. Reactive oxygen species in human health and disease. Nutrition 2001, 17, 161–165. [Google Scholar] [CrossRef]
- Macáková, K.; Kolečkář, V.; Cahlíková, L.; Chlebek, J.; Hošt’álková, A.; Kuča, K.; Jun, D.; Opletal, L. Tannins and their influence on health. In Recent Advances in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 159–208. [Google Scholar]
- Zhao, X.; Sun, H.; Hou, A.; Zhao, Q.; Wei, T.; Xin, W. Antioxidant properties of two gallotannins isolated from the leaves of Pistacia weinmannifolia. Biochim. Biophys. Acta 2005, 1725, 103–110. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, B.; Shen, S.; Hou, J.; Hu, J.; Xin, W. ESR study on the structure–antioxidant activity relationship of tea catechins and their epimers. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1999, 1427, 13–23. [Google Scholar] [CrossRef]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Manjunatha, H.; Srinivasan, K. Protective effect of dietary curcumin and capsacin on induced oxidation of low-density lipoprotein, iron-induced hepatotoxicity and carrageenan-induced inflammation in experimental rats. FEBS J. 2006, 273, 4528–4537. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.C.; Cançado, R.D.; Terada, C.T.; Guerra-Shinohara, E.M. Alterações moleculares associadas à hemocromatose hereditária. Rev. Bras. Hematol. Hemoter. 2009, 31, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Birch, N.; Wang, X.; Chong, H.-S. Iron chelators as therapeutic iron depletion agents. Expert Opin. Ther. Pat. 2006, 16, 1533–1556. [Google Scholar] [CrossRef]
- Lopes, R.H.O.; Macorini, L.F.B.; Antunes, K.Á.; Espindola, P.P.D.T.; Alfredo, T.M.; Rocha, P.D.S.D.; Pereira, Z.V.; Santos, E.L.D.; de Picoli Souza, K. Antioxidant and hypolipidemic activity of the hydroethanolic extract of Curatella americana L. leaves. Oxidative Med. Cell. Longev. 2016, 2016, 9681425. [Google Scholar] [CrossRef] [Green Version]
- Kaplum, V.; Ramos, A.C.; Consolaro, M.E.; Fernandez, M.A.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Silva, S.D.O.; de Mello, J.C.; Nakamura, C.V. Proanthocyanidin polymer-rich fraction of Stryphnodendron adstringens promotes in vitro and in vivo cancer cell death via oxidative stress. Front. Pharmacol. 2018, 9, 694. [Google Scholar] [CrossRef] [Green Version]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
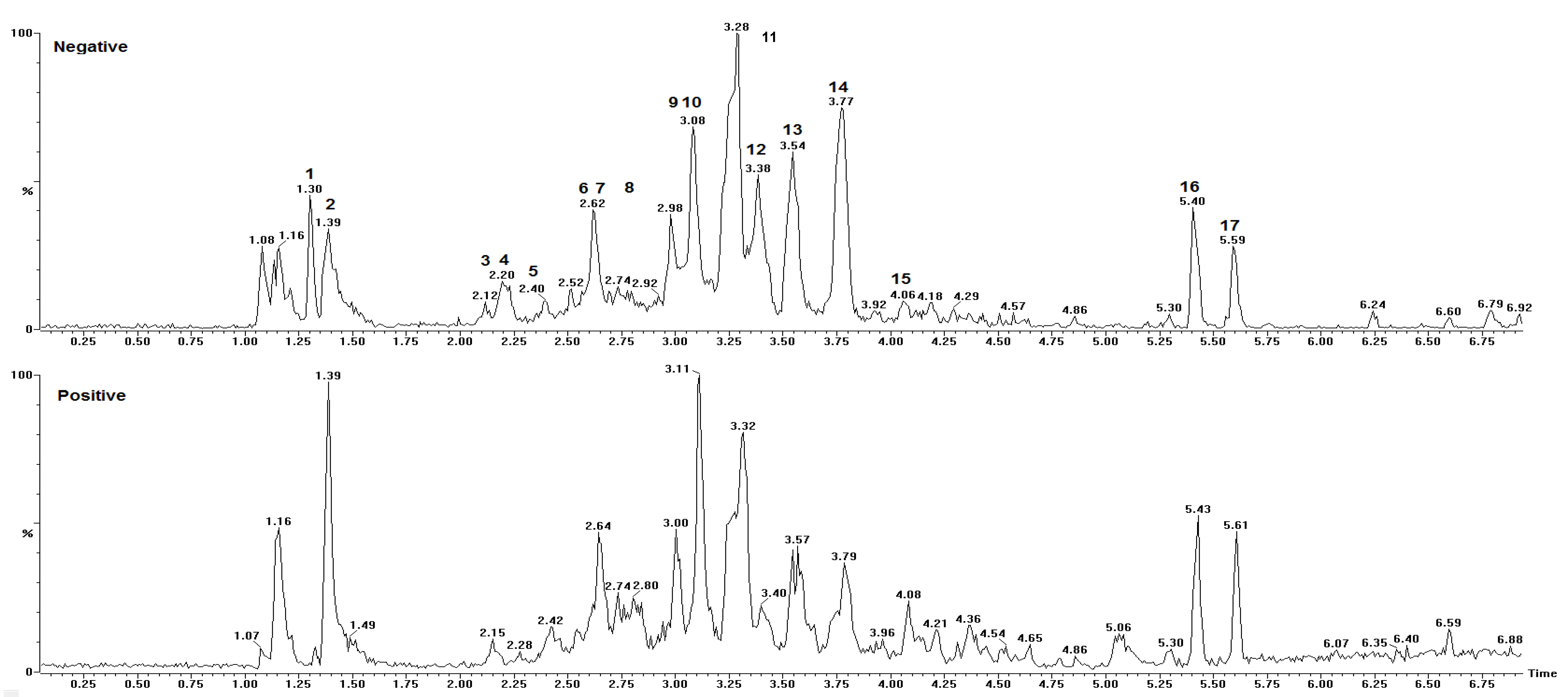
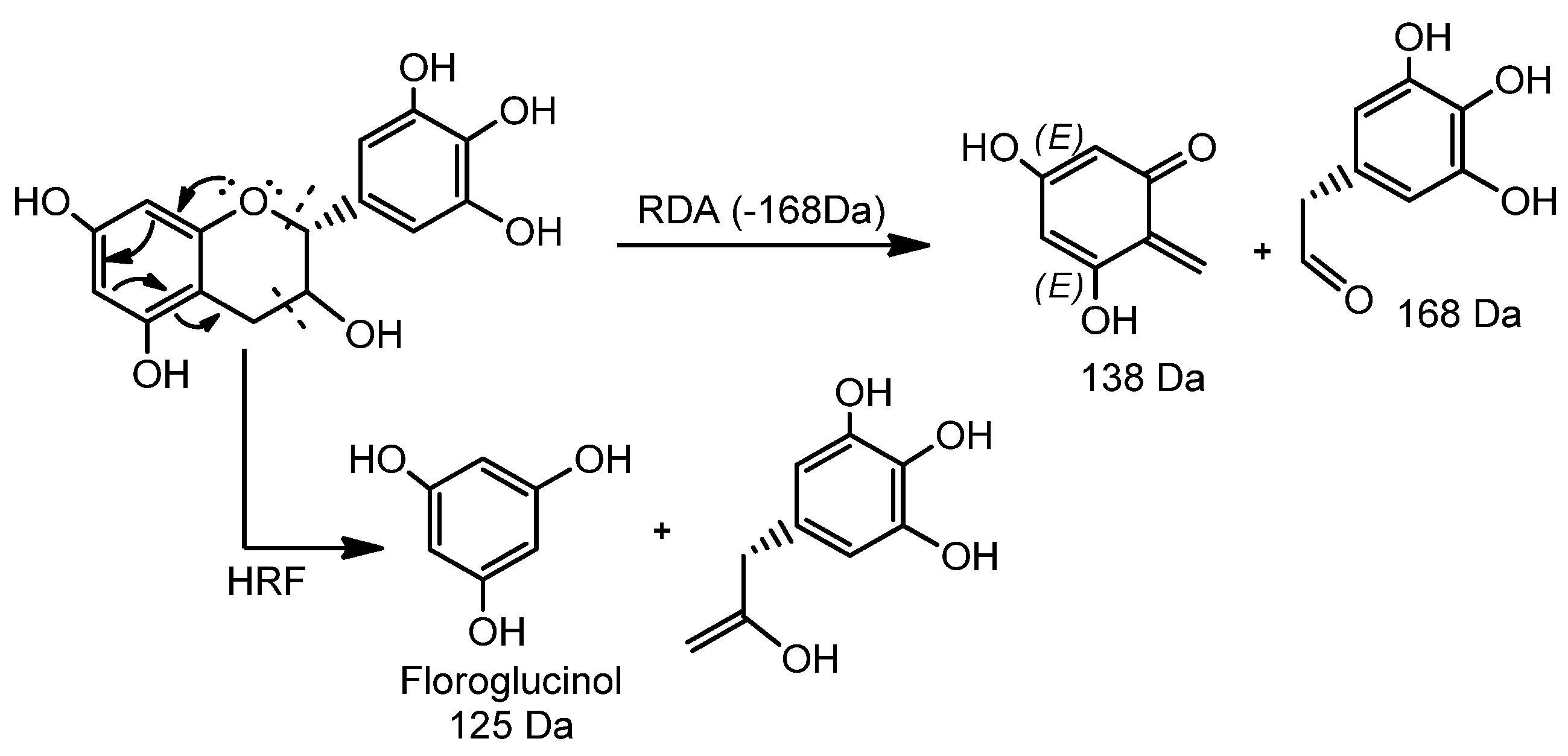
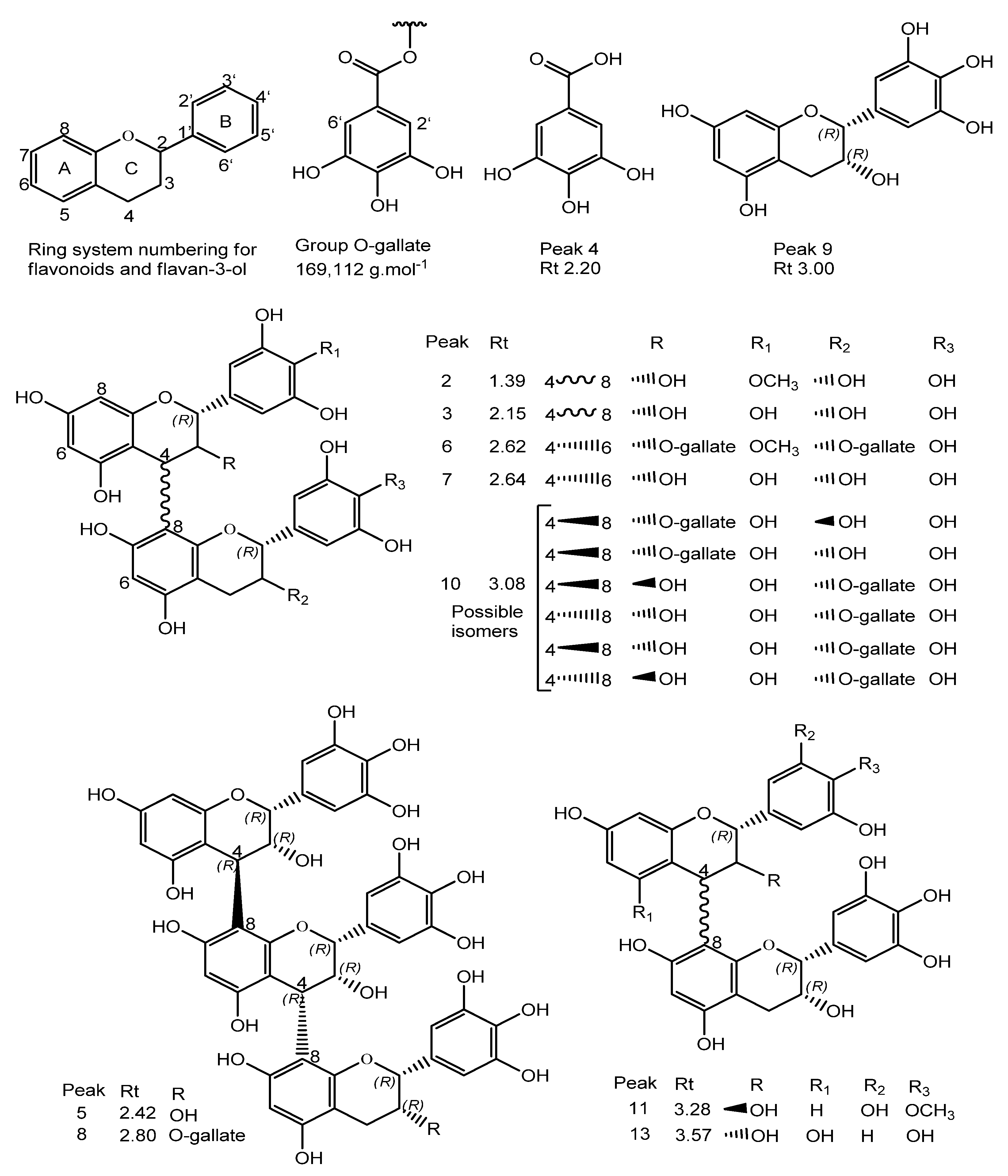

| Peak No. | Rt (min) | [M + H]+ Obs | [M − H]− Obs | Empirical Formula [M − H]− | Ion Products (MS/MS) | Empirical Formula [M + H]+ | Empirical Formula M/MM (g·mol−1) | ∆ 1 (Error) | Structure Name | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.30 | 383.1159 | no 2 | - | 203.0517 | C21H19O7 | −2.3 | Unknown | - | |
| 2 | 1.39 | 625.5280 | 623.1370 | C31H27O14 | 125.0226, 169.0148, 305.0661 | C31H29O14 | C31H28O14/624.5603 | 3.7 | 4’-O-methyl-epigallocatechin (4→8) epigallocatechin | [33] |
| 3 | 2.15 | 611.1428 | 609.1245 | C30H25O14 | 425.0808, 299.0632, 287.0573, 263.0517, 179.0361 | C30H27O14 | C30H26O14/610.494 | 4.4 | Epigallocatechin (4β→8) epigallocatechin | [5,33,34,35] |
| 4 | 2.20 | 171.0300 | 169.0138 | C7H5O5 | - | C7H7O5 | C7H6O5/170.113 | 4.1 | Gallic acid 3 | [5,35,36] |
| 5 | 2.42 | 915.2008 | 913.1827 | C45H37O21 | 611.1431, 425.0869, 287.0597, 263.0562, 179.0362 | C45H39O21 | C45H38O21/914.733 | 2.6 | Epigallocatechin (4→8) epigallocatechin (4→8) epigallocatechin | [5] |
| 6 | 2.62 | 929.7240 | 927.2041 | C45H35O22 | 761.1112, 423.0788, 305.0630, 125.0252 | - | C45H36O22/928.311 | 0.2 | 4’-O-methyl-epigallocatechin-3-O-gallate (4→6) epigallocatechin 3-O-gallate | [33] |
| 7 | 2.64 | 611.1406 | 609.1224 | C30H25O14 | 425.0943, 287.0575, 263.0571, 179.0337 | C30H27O14 | C30H26O14/610.494 | 0.8 | Epigallocatechin (4β→6) epigallocatechin | [5,34,35] |
| 8 | 2.80 | 1067.2070 | 1065.1937 | C52H41O25 | 915.2032, 611.1549, 425.0953, 287.0670, 263.0620, 179.0453 | C52H43O25 | C52H42O25/1066.831 | −2.2 | Epigallocatechin (4→8) epigallocatechin (4→8) epigallocatechin-3-O-gallate | [7] |
| 9 | 3.00 | 307.0824 | 305.0664 | C15H13O7 | 195.0573, 177.0488, 163.0406 | C15H15O7 | C15H14O7/306.255 | 2.0 | Epigallocatechin | [5,33,35] |
| 10 | 3.08 | 763.1538 | 761.1367 | C37H29O18 | 593.1270, 485.1284, 319.0786, 305.0662, 125.0253 | C37H31O18 | C37H30O18/762.6221 | 1.7 | Epigallocatechin (4→8) epigallocatechin-3-O-gallate/Epigallocatechin-3-O-gallate (4→8) epigallocatechin | [7,33,34,35] |
| 11 | 3.28 | 609.5290 | 607.1412 | C31H27O13 | 485.1264, 319.0786, 287.0568 | C31H29O13 | C31H28O13/608.5451 | 1.4 | Robinetinidol-4’-O-methyl (4→8) epigallocatechin | [34,35] |
| 12 | 3.32 | 487.1447 | 485.1295 | C21H25O13 | 355.1012, 319.0728, 289.0634, 259.0592 | C21H27O13 | C21H26O13/ 486.405 | −1.0 | C-hexosyl-O-pentosyl-5,7-dihydroxychromone isomer | [5] |
| 13 | 3.57 | 595.1460 | 593.1277 | C30H25O13 | 427.0926, 307.0875, 289.0704 | C30H27O13 | C30H26O13/594.495 | 1.3 | Procyanidin prodelphinidin type B | [5] |
| 14 | 3.79 | 623.1767 | no 2 | - | 423.1066, 287.0533 | C32H31O13 | - | 0.3 | Unknown | - |
| 15 | 4.08 | 623.1779 | no 2 | - | 423.1018, 287.0552 | C32H31O13 | - | 2.2 | Unknown | - |
| 16 | 5.43 | 677.2097 | no 2 | - | 545.1690, 367.1169, 235.0579, 191.0706 | C32H37O16 | - | 2.2 | Unknown | [5] |
| 17 | 5.60 | 707.2196 | no 2 | - | 575.1804, 367.0898, 236.0611, 221.0834 | C33H39O17 | - | 1.3 | Unknown |
| [TFSR] mg/mL | (%)Fe2+-Chelating Activity/Time (min) | Asc. ac. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 2.5 | 5 | 10 | 20 | 30 | 45 | 60 | 75 | 90 | 105 | |
| 0.200 | 87.88 ± 2.40 | 93.61 ± 1.78 | 93.29 ± 1.93 | 92.32 ± 1.75 | 95.37 ± 2.96 | 93.37 ± 3.29 | 97.82 ± 0.91 | 95.24 ± 2.69 | 93.15 ± 3.37 | 91.86 ± 3.08 | 91.94 ± 5.04 |
| 0.100 | 86.77 ± 4.25 | 84.55 ± 2.87 | 83.62 ± 2.62 | 85.77 ± 2.81 | 85.81 ± 1.77 | 84.44 ± 4.32 | 93.68 ± 3.47 | 86.32 ± 4.11 | 82.66 # ± 4.58 | 79.50 # ± 2.65 | 77.92 # ± 5.24 |
| 0.050 | 76.50 # ± 3.50 | 80.92 # ± 3.39 | 75.52 # ± 5.88 | 75.90 # ± 4.84 | 80.17 # ± 6.11 | 77.49 # ± 3.73 | 83.23 # ± 2.19 | 76.14 # ± 3.42 | 71.70 # ± 4.28 | 70.85 # ± 3.02 | 67.95 # ± 3.29 |
| [TFSR] mg/mL | (%)Fe3+-Reducing Activity/Time (min) | Asc. ac. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 2.5 | 5 | 10 | 20 | 30 | 45 | 60 | 75 | 90 | 105 | |
| 0.200 | 81.43 ±2.62 | 79.65 ±4.14 | 87.38 ±1.62 | 82.24 ±4.80 | 82.84 ±5.10 | 81.24 ±4.52 | 87.26 ±3.78 | 86.15 ±2.87 | 86.35 ±3.15 | 85.44 ±4.44 | 90.19 ± 3.99 |
| 0.100 | 64.70 # ± 2.09 | 64.18 # ± 5.40 | 70.34 # ± 4.84 | 66.10 # ± 2.53 | 65.01 # ± 3.32 | 62.91 # ± 4.19 | 70.31 # ± 5.17 | 68.58 # ± 3.39 | 69.80 # ± 3;06 | 71.66 # ± 4.49 | 77.69 #* ± 3.82 |
| 0.050 | 61.80 # ± 2.04 | 62.09 # ± 5.53 | 71.83 # ± 2.39 | 63.67 # ± 4.80 | 65.28 # ± 4.47 | 63.39 # ± 3.20 | 66.65 # ± 5.06 | 63.84 # ± 3.59 | 63.85 # ± 2.01 | 64.88 # ± 4.03 | 69.01 # ± 2.79 |
| TFSR Protective Activity (%) ± SEM | |||
|---|---|---|---|
| [2-DR] mM | 0.20 mg/mL | 0.10 mg/mL | 0.075 mg/mL |
| 1.50 | 27.22 ± 1.67 | 21.40 ± 1.95 | 17.37 ± 1.80 |
| 1.75 | 25.57 ± 1.87 | 21.09 ± 3.12 | 16.32 ± 1.90 |
| 2.00 | 24.53 ± 1.11 | 19.74 ± 0.49 | 15.46 ± 3.95 |
| Statistical Comparison | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, G.J.T.; Dias, F.J.; Ribeiro, P.R.V.; de Brito, E.S.; Canuto, K.M.; Coutinho, H.D.M.; Ribeiro-Filho, J.; Gallo, M.; Montesano, D.; Naviglio, D.; et al. Antioxidant Activity of Stryphnodendron rotundifolium Mart. Stem Bark Fraction in an Iron Overload Model. Foods 2021, 10, 2683. https://doi.org/10.3390/foods10112683
Salazar GJT, Dias FJ, Ribeiro PRV, de Brito ES, Canuto KM, Coutinho HDM, Ribeiro-Filho J, Gallo M, Montesano D, Naviglio D, et al. Antioxidant Activity of Stryphnodendron rotundifolium Mart. Stem Bark Fraction in an Iron Overload Model. Foods. 2021; 10(11):2683. https://doi.org/10.3390/foods10112683
Chicago/Turabian StyleSalazar, Gerson Javier Torres, Francisco Junio Dias, Paulo Riceli Vasconcelos Ribeiro, Edy Sousa de Brito, Kirley Marques Canuto, Henrique Douglas Melo Coutinho, Jaime Ribeiro-Filho, Monica Gallo, Domenico Montesano, Daniele Naviglio, and et al. 2021. "Antioxidant Activity of Stryphnodendron rotundifolium Mart. Stem Bark Fraction in an Iron Overload Model" Foods 10, no. 11: 2683. https://doi.org/10.3390/foods10112683










