Reduced Washing Cycle for Sustainable Mackerel (Rastrelliger kanagurta) Surimi Production: Evaluation of Bio-Physico-Chemical, Rheological, and Gel-Forming Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fish Sample
2.3. Surimi and Surimi Gel Preparation
2.4. Determination of Bio-Physico-Chemical Properties of Unwashed Mince and Surimi
2.5. Determination of Rheological Properties
2.6. Determination of Gelling Properties
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yield
3.2. Bio-Physico-Chemical Properties
3.2.1. pH
3.2.2. Lipid Reduction
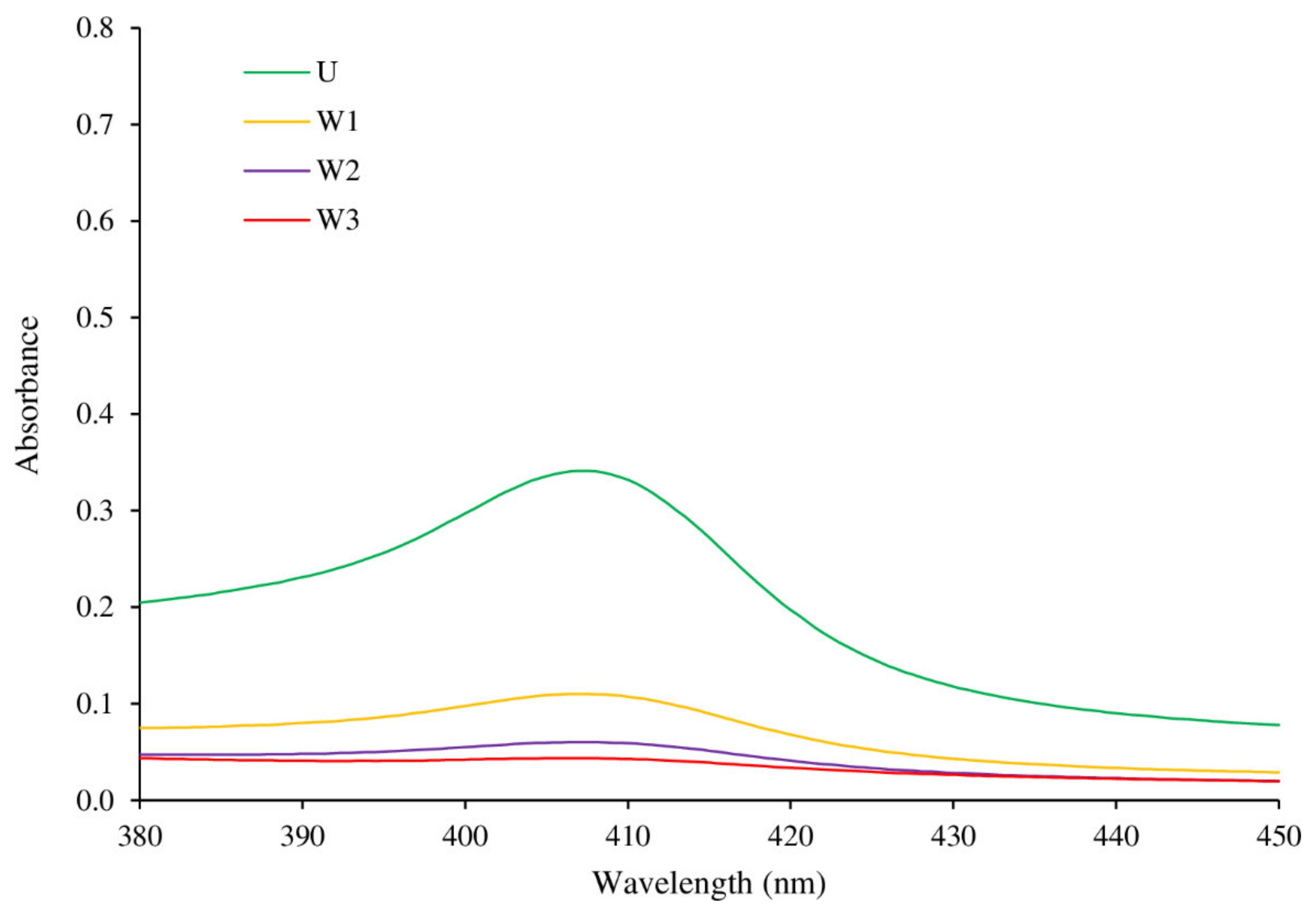
3.2.3. Myoglobin Content, Derivatives, and Absorption Spectra in the Soret Region
3.2.4. Ca2+-ATPase Activity, Reactive SH Content, and Surface Hydrophobicity
3.2.5. TCA-Soluble Peptide
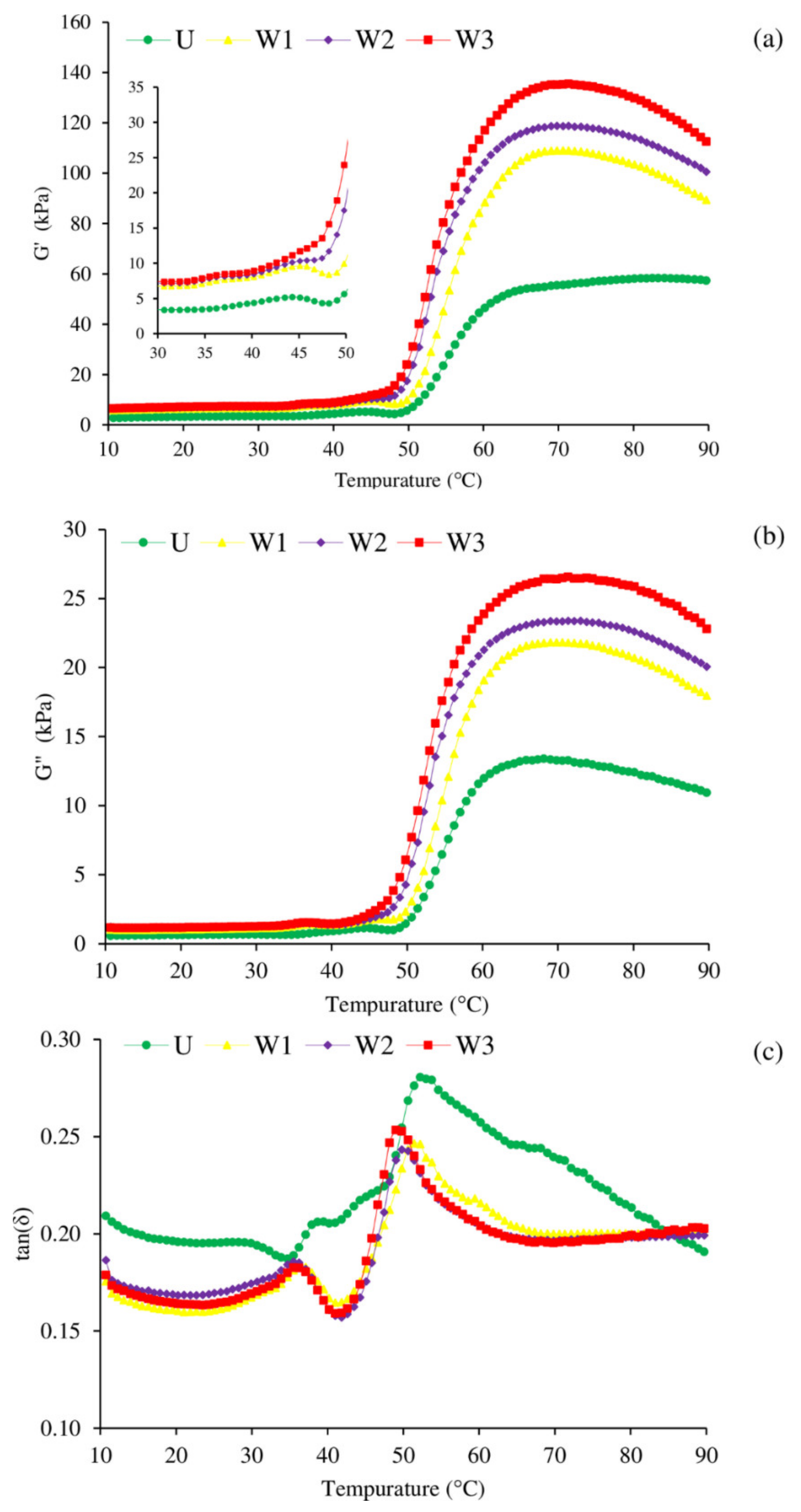
3.3. Oscillatory Dynamic Rheology
3.4. Gelling Characteristics
3.4.1. Breaking Force, Deformation, and Gel Strength
3.4.2. Expressible Drip
3.4.3. Whiteness
3.4.4. Lipid Oxidation and Fishy Odor
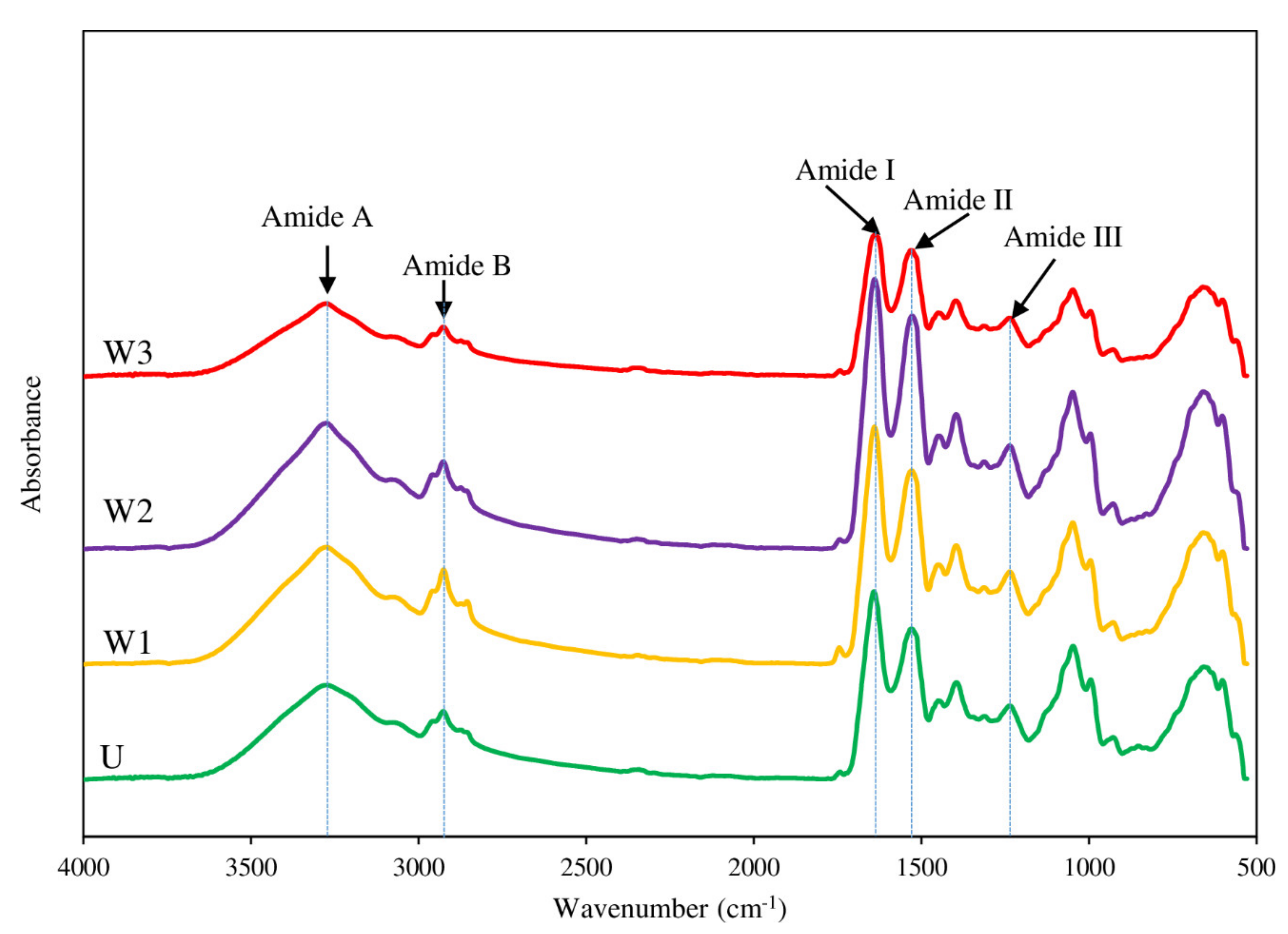
3.4.5. FTIR Spectra
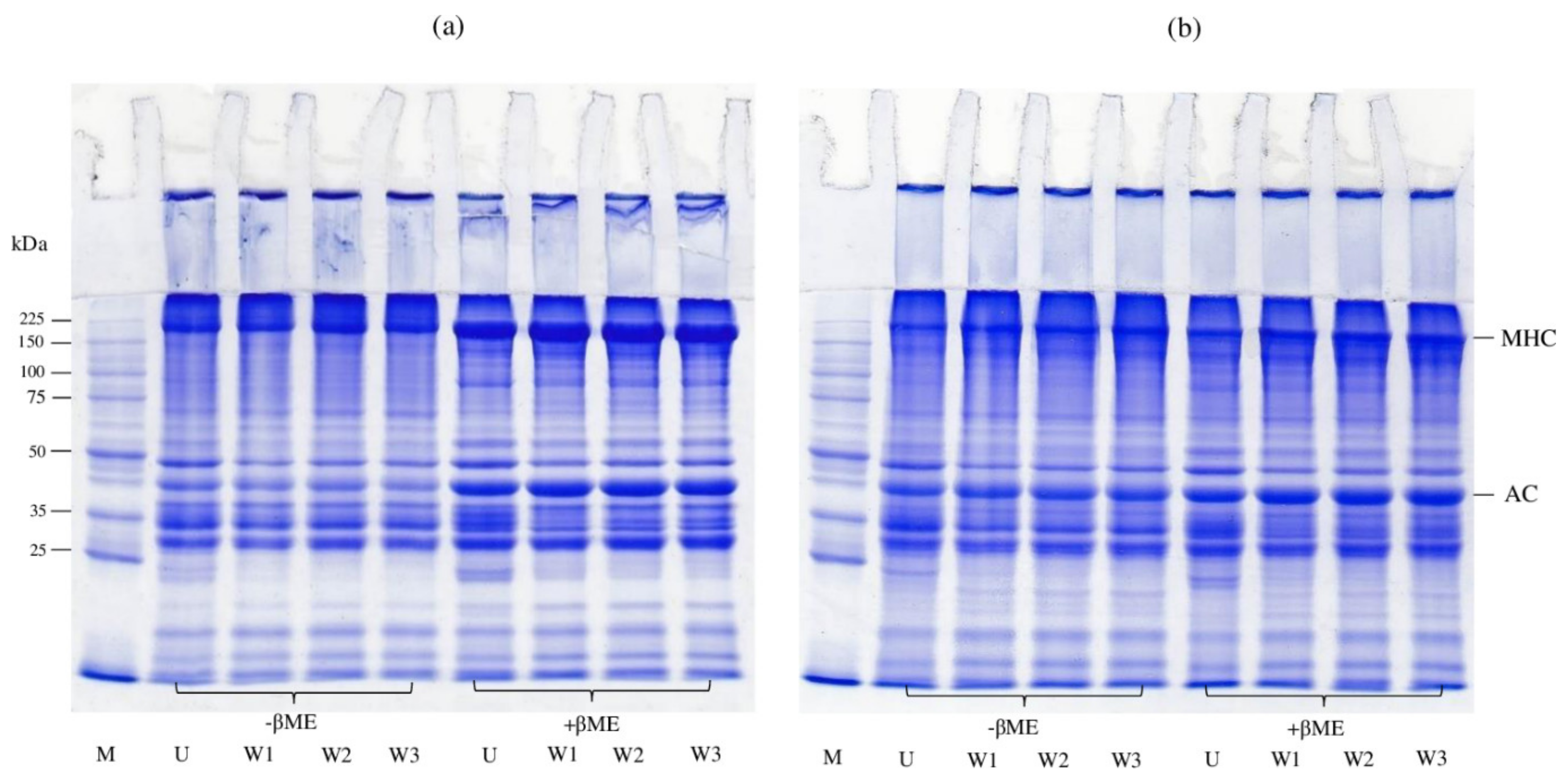
3.4.6. SDS-PAGE Protein Patterns
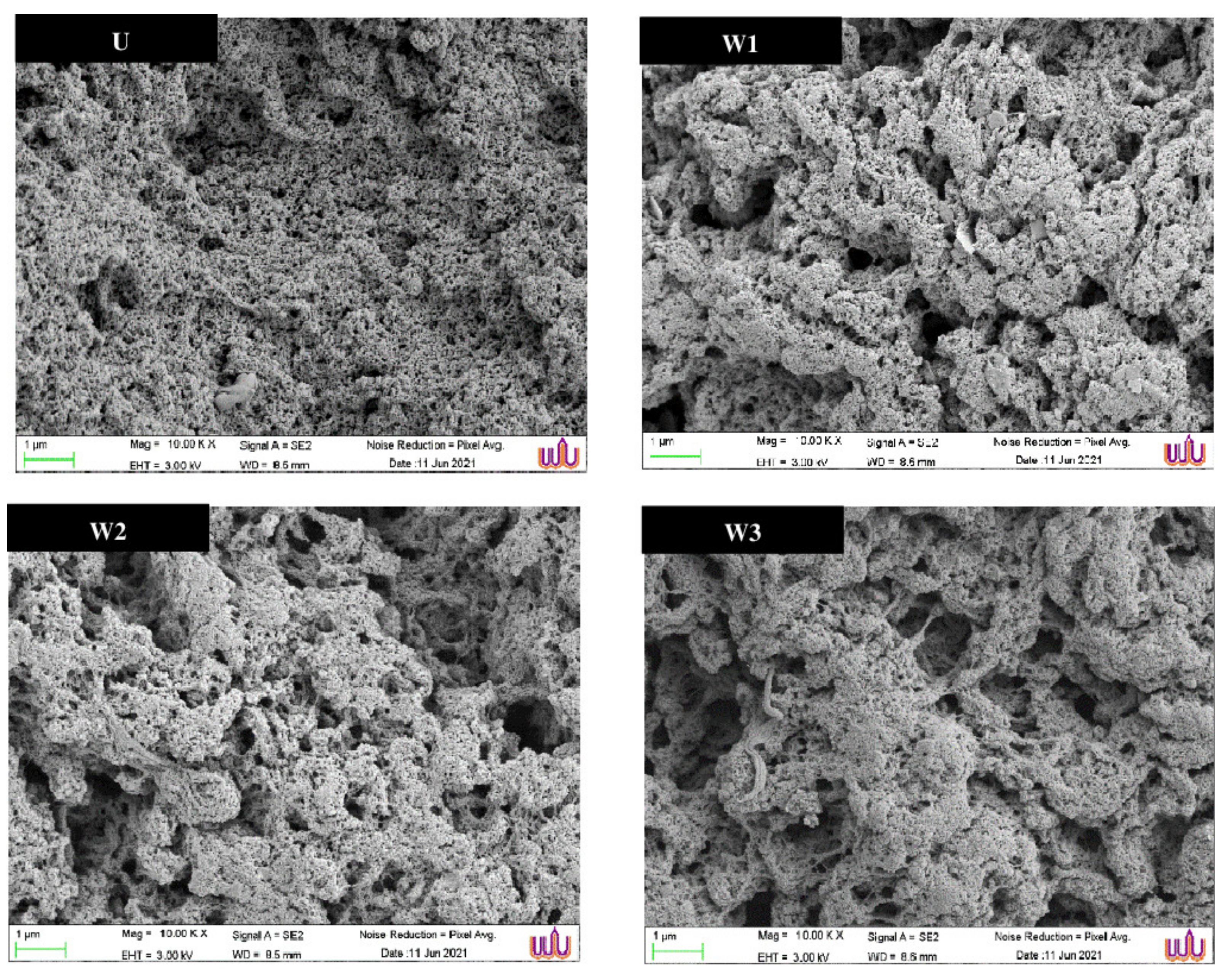
3.4.7. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.X.; Lin, H.H.; Zhu, S.C.; Xu, X.; Lyu, F.; Ding, Y.T. Textural, rheological and chemical properties of surimi nutritionally enhanced with lecithin. LWT 2020, 122, 108984. [Google Scholar] [CrossRef]
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Gelation characteristics of tropical surimi under water bath and ohmic heating. LWT 2012, 46, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Fishery Statistics Analysis and Research Group. Fisheries Statistics of Thailand; Fisheries Development Policy and Strategy Division, Department of Fisheries: Bangkok, Thailand, 2020. [Google Scholar]
- Panpipat, W.; Chaijan, M.; Benjakul, S. Gel properties of croaker-mackerel surimi blend. Food Chem. 2010, 122, 1122–1128. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Xavier, K.M.; Nayak, B.B.; Dhanapal, K.; Balange, A.K. Instrumental quality attributes of single washed surimi gels of tilapia: Effect of different washing media. LWT 2017, 86, 385–392. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W.; Benjakul, S. Physicochemical properties and gel-forming ability of surimi from three species of mackerel caught in Southern Thailand. Food Chem. 2010, 121, 85–92. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Characteristics and gel properties of muscles from sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) caught in Thailand. Food Res. Int. 2004, 37, 1021–1030. [Google Scholar] [CrossRef]
- Shimizu, Y.; Toyohara, H.; Lanier, T.C. Surimi production from fatty and dark-flesh species. In Surimi Technology, 1st ed.; Lanier, T.C., Lee, C.M., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1992; pp. 181–207. [Google Scholar]
- Somjid, P.; Panpipat, W.; Chaijan, M. Carbonated water as a novel washing medium for mackerel (Auxis thazard) surimi production. J. Food Sci. Technol. 2017, 54, 3979–3988. [Google Scholar] [CrossRef]
- Chen, H.H.; Chiu, E.M.; Huang, J.R. Color and gel-forming properties of horse mackerel (Trachurus japonicus) as related to washing conditions. J. Food Sci. 1997, 62, 985–991. [Google Scholar] [CrossRef]
- AOAC. Offcial Methods of Analysis, 16th ed.; Association of Offcial Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Tang, J.; Faustman, C.; Hoagland, T.A. Krzywicki revisited: Equations for spectrophotometric determination of myoglobin redox forms in aqueous meat extracts. J. Food Sci. 2004, 69, 717–720. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Fukushima, H.; Satoh, Y.; Nakaya, M.; Ishizaki, S.; Watabe, S. Thermal effects on fast skeletal myosins from Alaska pollock, white croaker, and rabbit in relation to gel formation. J. Food Sci. 2003, 68, 1573–1577. [Google Scholar] [CrossRef]
- Chaijan, M.; Srirattanachot, K.; Panpipat, W. Biochemical property and gel-forming ability of surimi-like material from goat meat. Int. J. Food Sci. Technol. 2021, 56, 988–998. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hassan, M.A.; Balange, A.K.; Senapati, S.R.; Xavier, K.A. Effect of different washing cycles on the quality of Pangasius hypophthalmus surimi. Fish. Technol. 2017, 54, 51–59. [Google Scholar]
- Foegeding, E.A.; Lanier, T.C.; Hultin, H.O. Characteristics of edible muscle tissues. In Food Chemistry, 3rd ed.; Fennema, O.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 880–942. [Google Scholar]
- Kelleher, S.D.; Hultin, H.O.; Wilhelm, K.A. Stability of mackerel surimi prepared under lipid-stabilizing processing conditions. J. Food Sci. 1994, 59, 269–271. [Google Scholar] [CrossRef]
- Das, N.; Khuntia, B.K.; Raychaudhuri, U.; Dora, K.C.; Ganguly, S. Effect of water washing on the functional properties of fish meat. Int. J. Med. Microbiol. Trop. Dis. 2015, 1, 8–12. [Google Scholar]
- Chaijan, M.; Undeland, I. Development of a new method for determination of total haem protein in fish muscle. Food Chem. 2015, 173, 1133–1141. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W. Mechanism of oxidation in foods of animal origin. In Natural Antioxidants, 1st ed.; Banerjee, R., Verma, A.K., Siddiqui, M.W., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 21–58. [Google Scholar]
- Priyadarshini, B.; Majumdar, R.K.; Parhi, J.; Maurya, P.K.; Roy, D.; Saha, A. Gel properties of sutchi catfish (Pangasius hypophthalmus) surimi as affected by selected washing process and number of washing cycles. Food Sci. Technol. Int. 2016, 22, 266–274. [Google Scholar] [CrossRef]
- Undeland, I.; Kristinsson, H.G.; Hultin, H.O. Hemoglobin-mediated oxidation of washed minced cod muscle phospholipids: Effect of pH and hemoglobin source. J. Agric. Food Chem. 2004, 52, 4444–4451. [Google Scholar] [CrossRef]
- Wongwichian, C.; Klomklao, S.; Panpipat, W.; Benjakul, S.; Chaijan, M. Interrelationship between myoglobin and lipid oxidations in oxeye scad (Selar boops) muscle during iced storage. Food Chem. 2015, 174, 279–285. [Google Scholar] [CrossRef]
- Anderson, A.B.; Robertson, C.R. Absorption spectra indicate conformational alteration of myoglobin adsorbed on polydimethylsiloxane. Biophys. J. 1995, 68, 2091–2097. [Google Scholar] [CrossRef] [Green Version]
- Panpipat, W.; Chaijan, M. Effect of atmospheric pressure cold plasma on biophysical properties and aggregation of natural actomyosin from threadfin bream (Nemipterus bleekeri). Food Bioproc. Technol. 2020, 13, 851–859. [Google Scholar] [CrossRef]
- Monahan, F.J.; German, J.B.; Kinsella, J.E. Effect of pH and temperature on protein unfolding and thiol/disulfide interchange reactions during heat-induced gelation of whey proteins. J. Agric. Food Chem. 1995, 43, 46–52. [Google Scholar] [CrossRef]
- Buttkus, H. Accelerated denaturation of myosin in frozen solution. J. Food Sci. 1970, 35, 558–562. [Google Scholar] [CrossRef]
- Benjakul, S.; Seymour, T.A.; Morrissey, M.T.; An, H. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J. Food Sci. 1997, 62, 729–733. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Yang, W.; Xue, C. Effects of ozone-induced oxidation on the physicochemical properties of myofibrillar proteins recovered from bighead carp (Hypophthalmichthys nobilis). Food Bioproc. Technol. 2015, 8, 181–190. [Google Scholar] [CrossRef]
- Careche, M.; Li-Chan, E.C.Y. Structural changes in cod myosin after modification with formaldehyde or frozen storage. J. Food Sci. 1997, 62, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Ye, B.; Wang, W.; Li, J.; Yi, S.; Li, X.; Mi, H. Incorporation effect of inulin and microbial transglutaminase on the gel properties of silver carp (Hypophthalmichthys molitrix) surimi. J. Food Meas. Charact. 2021, 15, 1–11. [Google Scholar] [CrossRef]
- Egelandsdal, B.; Martinsen, B.; Autio, K. Rheological parameters as predictors of protein functionality: A model study using myofibrils of different fiber-type composition. Meat Sci. 1995, 39, 97–111. [Google Scholar] [CrossRef]
- Park, J.D.; Yongsawatdigul, J.; Choi, Y.J.; Park, J.W. Biochemical and conformational changes of myosin purified from Pacific sardine at various pHs. J. Food Sci. 2008, 73, C191–C197. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S.; Konno, K. Improvement of gel quality of sardine surimi with low setting phenomenon by ethanolic coconut husk extract. J. Texture Stud. 2017, 48, 47–56. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Proteolysis and its control using protease inhibitors in fish and fish products: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef]
- Sano, T.; Noguchi, S.F.; Tsuchiya, T.; Matsumoto, J.J. Dynamic viscoelastic behavior of natural actomyosin and myosin during thermal gelation. J. Food Sci. 1988, 53, 924–928. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Benjakul, S.; Visessanguan, W.; Lanier, T.C. Rheological and textural properties of pacific whiting surimi gels as influenced by chicken plasma. Int. J. Food Prop. 2008, 11, 820–832. [Google Scholar] [CrossRef]
- Yuan, L.; Yu, J.; Mu, J.; Shi, T.; Sun, Q.; Jin, W.; Gao, R. Effects of deacetylation of konjac glucomannan on the physico-chemical properties of surimi gels from silver carp (Hypophthalmichthys molitrix). RSC Adv. 2019, 9, 19828–19836. [Google Scholar] [CrossRef] [Green Version]
- Buamard, N.; Benjakul, S. Improvement of gel properties of sardine (Sardinella albella) surimi using coconut husk extracts. Food Hydrocoll. 2015, 51, 146–155. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Chang, T.; Wang, C.; Yang, H.; Cui, M. Effects of vegetable oils on gel properties of surimi gels. LWT 2014, 57, 586–593. [Google Scholar] [CrossRef]
- Wang, S.F.; Smith, D.M. Dynamic rheological properties and secondary structure of chicken breast myosin as influenced by isothermal heating. J. Agric. Food Chem. 1994, 42, 1434–1439. [Google Scholar] [CrossRef]
- Samejima, K.; Ishioroshi, M.; Yasui, T. Relative roles of the head and tail portions of the molecule in heat-induced gelation of myosin. J. Food Sci. 1981, 46, 1412–1418. [Google Scholar] [CrossRef]
- Wu, C.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Effect of temperature, ionic strength and 11S ratio on the rheological properties of heat-induced soy protein gels in relation to network proteins content and aggregates size. Food Hydrocoll. 2017, 66, 389–395. [Google Scholar] [CrossRef]
- Yongsawatdigul, J.; Park, J.W. Thermal denaturation and aggregation of threadfin bream actomyosin. Food Chem. 2003, 83, 409–416. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Dileep, A.A.; Shamasundar, B.A. Effect of water washing on the functional and rheological properties of proteins from threadfin bream (Nemipterus japonicus) meat. Int. J. Food Sci. Technol. 2006, 41, 1002–1010. [Google Scholar] [CrossRef]
- Lee, M.G.; Yoon, W.B. Developing an effective method to determine the heat transfer model in fish myofibrillar protein paste with computer simulation considering the phase transition on various dimensions. Int. J. Food Eng. 2016, 12, 889–900. [Google Scholar] [CrossRef]
- Nowsad, A.A.; Huang, W.F.; Kanoh, S.; Niwa, E. Washing and cryoprotectant effects on frozen storage of spent hen surimi. Poultry Sci. 2000, 79, 913–920. [Google Scholar] [CrossRef]
- Hossain, M.I.; Morioka, K.; Shikha, F.H.; Itoh, Y. Effect of preheating temperature on the microstructure of walleye pollack surimi gels under the inhibition of the polymerization and degradation of myosin heavy chain. J. Sci. Food Agric. 2011, 91, 247–252. [Google Scholar] [CrossRef]
- Thai Industrial Standards Institute. TIS-935 Standard for Frozen Mince Fish (Surimi); Ministry of Industry: Bangkok, Thailand, 1990. [Google Scholar]
- Chaijan, M.; Klomklao, S.; Benjakul, S. Characterization of muscles from Frigate mackerel (Auxis thazard) and catfish (Clarias macrocephalus). Food Chem. 2013, 139, 414–419. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Lee, S.; Faustman, C. The effect of freezing and aldehydes on the interaction between fish myoglobin and myofibrillar proteins. J. Agric. Food Chem. 2007, 55, 4562–4568. [Google Scholar] [CrossRef]
- Karoui, R.; Blecker, C. Fluorescence spectroscopy measurement for quality assessment of food systems-a review. Food Bioproc. Technol. 2011, 4, 364–386. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Chitooligosaccharides from squid pen prepared using different enzymes: Characteristics and the effect on quality of surimi gel during refrigerated storage. Food Prod. Process. Nutr. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Murthy, L.N.; Phadke, G.G.; Jeyakumari, A.; Ravishankar, C.N. Effect of added calcium and heat setting on gel forming and functional properties of Sardinella fimbriata surimi. J. Food Sci. Technol. 2021, 58, 427–436. [Google Scholar] [CrossRef]
- Phetsang, H.; Panpipat, W.; Undeland, I.; Panya, A.; Phonsatta, N.; Chaijan, M. Comparative quality and volatilomic characterisation of unwashed mince, surimi, and pH-shift-processed protein isolates from farm-raised hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Food Chem. 2021, 364, 130365. [Google Scholar] [CrossRef] [PubMed]
- Moreno, H.M.; Bargiela, V.; Tovar, C.A.; Cando, D.; Borderias, A.J.; Herranz, B. High pressure applied to frozen flying fish (Parexocoetus brachyterus) surimi: Effect on physicochemical and rheological properties of gels. Food Hydrocoll. 2015, 48, 127–134. [Google Scholar] [CrossRef]
- Ahmad, S.; Rizawi, J.A.; Srivastava, P.K. Effect of soy protein isolate incorporation on quality characteristics and shelf-life of buffalo meat emulsion sausage. J. Food Sci. Technol. 2010, 47, 290–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. High pressure effects on protein structure and function. Proteins: Struct. Funct. Genet. 1996, 24, 81–91. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Blanchard, S.P.; Ooizumi, T.; Ma, Y. Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. J. Food Sci. 2010, 75, 215–221. [Google Scholar] [CrossRef]
- Benjakul, S.; Chantarasuwan, C.; Visessanguan, W. Effect of medium temperature setting on gelling characteristics of surimi from some tropical fish. Food Chem. 2003, 82, 567–574. [Google Scholar] [CrossRef]
- Balange, A.K.; Benjakul, S. Effect of oxidised tannic acid on the gel properties of mackerel (Rastrelliger kanagurta) mince and surimi prepared by different washing processes. Food Hydrocoll. 2009, 23, 1693–1701. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S.; H-Kittikun, A. Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish. J. Sci. Food Agric. 2012, 92, 844–852. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S. Gelling characteristics of surimi from yellow stripe trevally (Selaroides leptolepis). Int. Aquat. Res. 2012, 4, 1–13. [Google Scholar] [CrossRef]
- Bertram, H.C.; Meyer, R.L.; Wu, Z.; Zhou, X.; Andersen, H.J. Water distribution and microstructure in enhanced pork. J. Agric. Food Chem. 2008, 56, 7201–7207. [Google Scholar] [CrossRef]
| Sample | Unwashed | W1 | W2 | W3 |
|---|---|---|---|---|
| Yield (%) | - | 89.57 ± 4.32a | 83.43 ± 3.77b | 79.74 ± 2.46c |
| pH | 6.31 ± 0.01d | 6.45 ± 0.01c | 6.62 ± 0.01b | 6.75 ± 0.01a |
| Lipid reduction (%) | - | 52.76 ± 1.31b | 54.36 ± 0.76ab | 56.43 ± 1.24a |
| Myoglobin content (mg/g sample) | 6.77 ± 0.72a | 2.56 ± 0.15b | 1.63 ± 0.01c | 1.61 ± 0.06c |
| Myoglobin derivatives | ||||
| Deoxymyoglobin (%) | 17.59 ± 0.23d | 18.92 ± 0.33c | 20.96 ± 0.85b | 22.56 ± 0.32a |
| Oxymyoglobin (%) | 6.29 ± 0.09a | 5.23 ± 0.65a | 5.27 ± 0.65a | 4.61 ± 0.76a |
| Metmyoglobin (%) | 75.12 ± 0.13a | 75.85 ± 0.92a | 73.78 ± 0.78b | 72.83 ± 0.45b |
| Ca2+-ATPase activity (µmol/mg protein/min) | 2.27 ± 0.03a | 2.17 ± 0.04b | 2.12 ± 0.03b | 2.10 ± 0.02b |
| Reactive SH content (mol/108 g protein) | 6.14 ± 0.05b | 6.29 ± 0.02ab | 6.48 ± 0.09a | 6.49 ± 0.20a |
| Surface hydrophobicity (BPB bound (µg)) | 30.43 ± 1.42d | 41.42 ± 2.48c | 49.43 ± 2.86b | 57.61 ± 0.33a |
| TCA-soluble peptide content (µmol tyrosine/g sample) | 0.79 ± 0.03a | 0.43 ± 0.05b | 0.31 ± 0.03c | 0.19 ± 0.03d |
| Sample | Unwashed | W1 | W2 | W3 |
|---|---|---|---|---|
| Appearance |  |  |  |  |
| Breaking force (g) | 218.12 ± 0.44c | 353.60 ± 1.93b | 393.98 ± 1.53a | 393.09 ± 1.81a |
| Deformation (mm) | 10.06 ± 0.36b | 10.83 ± 0.34a | 11.20 ± 0.58a | 11.26 ± 0.31a |
| Gel strength (g.cm) | 219.32 ± 3.67c | 382.71 ± 17.04b | 436.32 ± 25.23a | 442.17 ± 23.97a |
| Expressible drip (%) | 8.14 ± 0.40c | 5.37 ± 0.04b | 4.63 ± 0.19a | 4.51 ± 0.20a |
| Whiteness | 65.11 ± 0.18c | 66.44 ± 0.14b | 68.43 ± 0.31a | 68.98 ± 0.42a |
| TBARS (mg MDA equivalent/kg) | 0.73 ± 0.04a | 0.67 ± 001b | 0.59 ± 0.01c | 0.57 ± 0.04c |
| Fishy odor score * | 9.03 ± 0.45a | 4.43 ± 1.12b | 2.27 ± 1.17c | 1.37 ± 0.98c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somjid, P.; Panpipat, W.; Cheong, L.-Z.; Chaijan, M. Reduced Washing Cycle for Sustainable Mackerel (Rastrelliger kanagurta) Surimi Production: Evaluation of Bio-Physico-Chemical, Rheological, and Gel-Forming Properties. Foods 2021, 10, 2717. https://doi.org/10.3390/foods10112717
Somjid P, Panpipat W, Cheong L-Z, Chaijan M. Reduced Washing Cycle for Sustainable Mackerel (Rastrelliger kanagurta) Surimi Production: Evaluation of Bio-Physico-Chemical, Rheological, and Gel-Forming Properties. Foods. 2021; 10(11):2717. https://doi.org/10.3390/foods10112717
Chicago/Turabian StyleSomjid, Panumas, Worawan Panpipat, Ling-Zhi Cheong, and Manat Chaijan. 2021. "Reduced Washing Cycle for Sustainable Mackerel (Rastrelliger kanagurta) Surimi Production: Evaluation of Bio-Physico-Chemical, Rheological, and Gel-Forming Properties" Foods 10, no. 11: 2717. https://doi.org/10.3390/foods10112717







