1. Introduction
Snap beans (
Phaseolus vulgaris L. var.
chinensis Hort.) are soft-pod variants of common beans (
Phaseolus vulgaris L.) [
1]. These are mainly eaten by pods and have good taste and high nutritional value. Due to strict requirements of geographical environment and climatic conditions for the growth of snap beans, certain good-quality snap beans are only suitable for growth in the cold regions of China, such as Heilongjiang, Jilin, and the northeast region of Inner Mongolia [
1].
Unfortunately, snap beans are extremely perishable. The production loss of fruits and vegetables in industrialized and developing countries is up to 25% and 50%, respectively, during the postharvest stage due to fungal infection [
2]. Diseases that cause field and postharvest losses of snap beans include common bean rust, wilt, anthracnose, botrytis, bacterial blight, and bacterial halo [
3,
4]. Gray mold, caused by
Botrytis cinerea, is a well-known disease that causes severe yield losses of snap beans worldwide [
5,
6]. Fungal spores usually occur on the surface of fruits, and in the process of postharvest treatment the pods of snap beans can provide a suitable environment for spore germination (mainly damaged fruits) [
7]. In addition, an infection may occur during storage, sales, or even after customers purchase them [
6]. Because of its wide host range and huge economic losses, extensive studies have been carried out to control
B. cinerea effectively [
8]. Although fungicides have greatly reduced the incidence of gray mold, they have several disadvantages, such as environmental pollution, frequently occurring fungicide resistance, and chemical residues in food [
9,
10]. Therefore, there is a requirement to develop safer and more environmentally friendly disease control alternatives [
11]. An alternative method is the biological control that uses biological processes to decrease the inoculation density of pathogens to limit the disease, thereby reducing the crop losses [
12,
13].
Recently, the use of antagonistic microorganisms to control postharvest diseases has been widely implemented. Antagonists that inhibit postharvest diseases in nature are widely used. They have many sources, including leaves, fruits, soils, and existing antagonists (including bacteria, fungi, and actinomycetes). Biocontrol yeasts use two mechanisms to inhibit the growth of target pathogens. Direct inhibition involves the secretion of antimicrobial substances, the formation of biofilms on the inner surface of wounds, and parasitic effects on pathogens. As reported, in the
Saccharomyces cerevisiae flor strain, biofilm cells colonized the inner surface of apple wounds more effectively than planktonic cells; thereby, the development of blue mold caused by
Penicillium expansum was controlled [
14]. Indirect inhibition includes competition with pathogens for nutrient and growth space and induction of host resistance [
15,
16]. For example, the
Metschnikowia pulcherrima mutant (not pigment) exhibits reduced antifungal activity [
17]. The iron deficiency by fungal pathogens was believed to be one of the mechanisms by which the yeast antagonizes phytopathogenic fungi [
17].
In recent years, combining biological and safe chemical substances such as food additives to prevent and control plant diseases has effectively controlled diseases and reduced chemical fungicides [
18,
19,
20,
21]. A large number of studies have shown that the combination of biocontrol agents (yeast,
Pseudomonas syringae,
Pantoea agglomerans,
Bacillus subtilis, etc.) and food additives (such as chitosan, D-2-deoxyglucose, sodium bicarbonate, ascorbic acid, konjac powder, etc.) can improve the control effect of postharvest diseases of citrus, apple, lemon, tomato, carrot, pepper, cucumber, pomegranate, strawberry, cherry, kiwi fruit, loquat and other fruits and vegetables [
20,
22,
23,
24,
25,
26]. NaHCO
3 is a commonly used food additive, and it is also one of the permitted ingredients in international organic food. The enhancement of the synergistic inhibitory effect of NaHCO
3 on biocontrol agents is related to its direct inhibitory effect on pathogens. Earlier studies showed that NaHCO
3 can inhibit the germination of pathogenic spores and the elongation of germ tubes [
27]. The addition of NaHCO
3 changes the pH and other conditions at the fruit wound and pathogens are more sensitive to these changes than biocontrol agents, so their competitiveness with biocontrol agents is weakened. This, in turn, enhances the antagonistic ability of biocontrol agents [
26]. Many postharvest diseases are caused by wound pathogens, and the complete control of these diseases is achieved by using a rapidly growing and environmentally friendly agent. One of these strategies relies on the application of edible films and coatings as food-quality preservers using biopolymers (e.g., chitosan) [
23,
28]. Chitosan is tasteless, nontoxic, harmless, and slightly viscous, and can easily form a uniform and continuous film. It has a good preservation effect on fruits and can improve the storage quality of fruits and vegetables. Chitosan can quickly solidify into a film after being adsorbed on the surface of an object. It does not fall off or stick and has a slight permeability. Applying it to the surface of the harvested vegetables can restrict the entry of abundant O
2 in the air and prevent the emission of CO
2. Therefore, the respiration of the fruit is reduced; the production of other harmful substances is slowed down. The senescence of tissue cells is delayed to a certain extent, and the storage quality of fruits and vegetables is improved [
18]. Secondly, the protective layer formed by chitosan on the surface of fruits and vegetables can effectively prevent the invasion of pathogens and reduce the disease infection rate in fruits and vegetables [
18]. Ascorbic acid (VC) is an important water-soluble antioxidant and one of the most common food additives and endogenous substances. It can act as a scavenger of reactive oxygen species (ROS), thereby potentially protecting cells from the harmful effects of oxidation products [
21]. Konjac powder has many advantages, such as high viscosity, strong water absorption, and good film-forming properties, and is often used as the active ingredient of preservatives. The antibacterial film formed by konjac powder has a barrier effect, which can delay the entry of O
2 into fruits and vegetables and reduce the respiratory intensity, and it can be used as a carrier of antiseptic and antibacterial agents to achieve antiseptic and fresh-keeping effects [
19].
In the present study, three biocontrol yeasts (Ca63, Ca64, and Yett 1006) were used. The biocontrol mechanism of these strains in controlling B. cinerea in the postharvest stage of snap beans was studied by in vivo and in vitro experiments. The effects of three yeast single-agent, two combinations, and fresh-keeping additives (chitosan, sodium bicarbonate, ascorbic acid, and konjac powder) in preserving snap beans were compared. The results provide a theoretical basis for the application of three biocontrol yeasts for the postharvest preservation of snap beans in a better way.
2. Materials and Methods
2.1. Plant Materials
The physiologically mature fruit of test snap beans “Zihuajiayoudou” was harvested from an experimental farm in Harbin, Heilongjiang Province, China, in August 2018. These snap beans were put in a fresh-keeping box and immediately transported back to the laboratory in a car. The snap beans selected for the test were without any physical injury or infection and had uniform size and color, and firm texture. The selected snap beans were soaked in 1000 ppm sodium hypochlorite for 1 min for disinfection, washed with tap water, and subsequently air-dried at room temperature.
2.2. Microorganisms and Media
The pathogen,
B. cinerea, was isolated from infected snap beans, confirmed by the 5.8S ribosomal DNA (rDNA)-ITS and 18S rDNA sequences, and conserved in our laboratory [
29].
Three strains of yeast Cryptococcus albidus (Ca63), Cryptococcus albidus (Ca64), and Candida parapsilosis (Yett 1006) are preserved by China General Microbiological Culture Collection Center (CGMCC). The CGMCC numbers of Ca63, Ca64 and Yett 1006 are 18,014, 1976, 18,013, respectively. Ca63, Ca64, and Yett 1006 were named by us after we isolated these strains. The numbers 18,014, 1976, 18,013 were given after identification by CGMCC. These strains were cultured on potato dextrose agar (PDA) plates. Single colony biomass was subjected to in vitro biocontrol assay or inoculated into yeast peptone dextrose (YPD) liquid media (Glucose 20 g, protein 20 g, yeast extract 10 g, distilled water 1000 mL) and incubated at 28 °C for 24 h on a rotary shaker at 200 rpm (constant temperature shaker, LKYC-1102C, Ningbo Life Technology Co., Ltd., Ningbo, China). The cell pellets were centrifuged at 8000× g for 10 min at 4 °C (64R, Beckman Coulter, lnc., Shanghai, China). Subsequently, the cells were resuspended in distilled water, recentrifuged, and washed twice to remove the residual culture media thoroughly. The yeast cells were resuspended in sterilized distilled water, diluted to a concentration of 108 CFU mL−1 and used as an inoculum in in vivo assays.
2.3. Evaluation of Postharvest Biocontrol Ability of Three Yeast Strains and Their Combinations against Botrytis cinerea in Snap Beans
2.3.1. In Vitro Biocontrol Assay
The three biocontrol yeasts were inoculated in 50 mL of yeast extract peptone dextrose (YPD) media (inoculation concentration was 2% (v/v)), and cultured on a rotary shaker at 200 rpm for 36 h at 28 °C. The cell pellets were obtained by centrifugation at 8000× g for 10 min at 4 °C. Next, the yeast cells were resuspended in sterile physiological saline (0.9%), recentrifuged, and washed twice to remove the residual culture media thoroughly. The yeast cells were resuspended in sterile physiological saline (0.9%), diluted to a concentration of 108 CFU mL−1, and used as an inoculum in in vitro assays.
B. cinerea, confirmed and conserved in our laboratory, was cultured on potato dextrose agar (PDA) medium at 26 °C for 10 days. Next, 2 mL of sterile water was added to the Petri dish, the cultured spores and cells were gently scraped with a sterile blade, the cells were rinsed with sterile water, shaken well, and the hyphae were filtered with four layers of gauze to obtain pathogen spores. Spore concentrations were determined using a hemocytometer (79 mm × 39 mm × 13 mm, Shanghai Qiujing Biochemical Reagent Instrument Co., Ltd., Shanghai, China) and adjusted to 1 × 106 spores per mL with sterile water.
Filter paper method [
30]: on the PDA Petri dish, equidistant from the midpoint, a 5 mm diameter
B. cinerea plug was placed on one side and a piece of filter paper with a diameter of 1 cm was placed on the other side. The distance between
B. cinerea plug and filter paper was 20 mm. Next, 15 µL of 3 yeast suspensions at a concentration of 10
8 CFU mL
−1 was added dropwise to the filter paper separately. Dual cultures were incubated at 25 °C for 7 days. A Petri dish inoculated with
B. cinerea and sterile water was used as a control, and each test was repeated three times. At the end of the incubation period, the growth of
B. cinerea was observed.
Plate coating method [
31]: the three biocontrol yeast suspensions (100 µL, in 0.9% sterile physiological saline) at a concentration of 10
8 CFU mL
−1 were added into a PDA solid media Petri dish and uniformly spread with a spreader. After drying,
B. cinerea colonies, with a diameter of 5 mm, were inoculated on the media and cultured at 25 °C for 7 days. A PDA solid media Petri dish covered with sterile water and inoculated with
B. cinerea was used as a control. The growth of
B. cinerea was observed, and each group was treated thrice.
B. cinerea, with a diameter of 5 mm, was inoculated into 250 mL conical flasks containing 50 mL of potato dextrose broth (PDB) media. Next, the 3 biocontrol yeast suspensions were separately inoculated with B. cinerea at a concentration of 2% (v/v). The concentrations of the 3 yeast strains were 102, 104, 106 and 108 CFU mL−1, respectively. The inoculated flasks were incubated on a mini-roundabout shaker at 200 rpm at 25 °C for 7 days. The culture medium without the yeast inoculation was used as a control, and the hyphal growth of B. cinerea was recorded. The hyphae were filtered through gauze, dried, and weighed. The degree of growth inhibition of B. cinerea using the three strains was determined by the difference in dry weight of hyphae. Each treated group included three repetitions.
2.3.2. In Vivo Biocontrol Assay
Antagonistic Activity of Three Yeasts and Their Combinations against Botrytis cinerea
In order to assess the efficacy of three yeasts as biocontrol agents, the method described by Czarnecka et al. [
13] and Kharchoufi et al. [
9], with modifications, was used. Artificial wounds were created using a sterile needle to make 5 mm deep and 5 mm wide wounds on the pods of snap beans (2 wounds per fruit). The wounds were inoculated with a single yeast agent, 2 combined yeast agents, single yeast agent + 0.2% (
m/v) NaHCO
3 (Tianjin Yongda Chemical Reagent Co., Ltd., Tianjin, China), single yeast agent + 0.5% (
m/v) chitosan (Bozhou Baofeng Biological Technology Co., Ltd., Bozhou, China), single yeast agent + 1% (
m/v) ascorbic acid (Tianjin Komiou Chemical Reagent Co., Ltd., Tianjin, China), and single yeast agent + 0.1% (
m/v) konjac powder (Bozhou Baofeng Biological Technology Co., Ltd., Bozhou, China), wherein the yeast concentration was 10
8 CFU mL
−1 and the volume was 10 µL. The treated snap beans were naturally dried for 12 h, then divided into 2 groups: one group was inoculated with
B. cinerea with a spore concentration of 1 × 10
6 spores per mL and a volume of 10 µL; the other group was not inoculated with
B. cinerea [
32]. Then these snap beans were placed into plastic bags containing 90% relative humidity (RH) at room temperature 25 °C for 12 d. Snap beans treated with two wounds and sterilized water were used as the control group. The disease index was measured on the 12th day of storage. The other incidence of pods was recorded from the next day and counted every 3 days. The number of snap beans used in each treatment was 20, and each treatment was repeated 3 times.
Snap beans disease level [
32,
33]:
Level 0, the fruit does not develop.
Level 1, the disease area is less than 10% of the fruit length.
Level 2, the disease area accounts for 10% to 30% of the fruit length.
Level 3, the disease area is greater than 30% of the fruit length.
The Growth Dynamics of Three Yeasts on the Surface of Snap Beans
Snap beans of same size, no disease, and no mechanical damage were selected and processed as described in
Section 2.1. The 3 kinds of biocontrol yeast suspensions (100 µL, 10
8 CFU mL
−1, in 0.9% sterile NaCl solution) were evenly sprayed on the surface of the pods. Then these snap beans were placed into plastic bags containing 90% RH at room temperature for 96 h. The number of yeasts was determined every 12 h. The method for determining the number of yeasts cells on the surface of the pods was as follows: The processed pods were soaked in 500 mL sterile water, cleaned ultrasonically for 5 min, and then the number of yeasts in the cleaning solution was detected by the plate coating method using a microscope [
34]. The test was repeated three times.
Storage Tests of Postharvest Snap Beans
Snap beans with same size, no disease, and no mechanical damage were selected. The postharvest fruit storage test was conducted after processing as described in
Section 2.1, following the method of Wang et al. [
35] with slight modifications. Single yeast agent, two combined yeast agents, single yeast agent + 0.2% (
m/v) NaHCO3, single yeast agent + 0.5% (
m/v) chitosan, single yeast agent + 1% (
m/v) ascorbic acid, and single yeast agent + 0.1% (
m/v) konjac powder with a concentration of 10
8 CFU mL
−1 and a volume of 3 mL were sprayed evenly on snap beans. The amount of snap beans used in each treatment was 300 ± 5 g. The snap beans with different treatments were dried naturally at room temperature. Then these were placed into plastic bags containing 90% RH at room temperature. Snap beans treated with sterile water were used as the control group. The decay period of the snap beans in different treated groups was observed every 3 days for 12 days. The other quality indexes of the fruits were determined every 3 days for 12 days. Each treatment was repeated thrice.
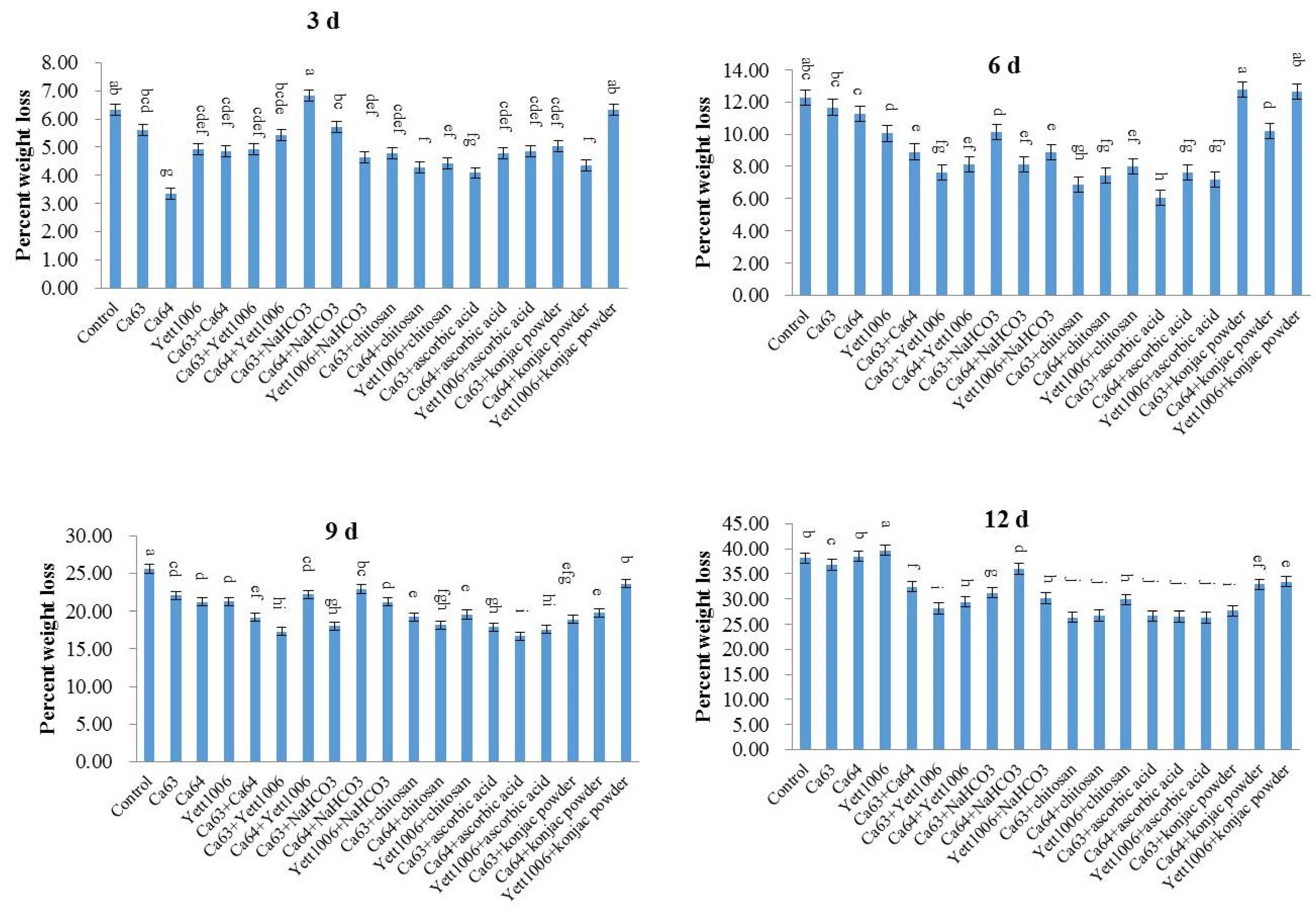
(1) Mass loss: Mass loss of snap beans was detected by the weighing method with balance instruments. The mass loss of the snap beans was measured from the beginning of storage and measured every 3 days [
32,
35].
The following formula was used to calculate the mass loss:
(2) Rate of decay: The size of the rot area on snap beans was divided into four levels:
Level 0, no decay.
Level 1, the decayed area is less than 20% of the whole area.
Level 2, the decayed area accounts for 20% to 50% of the whole area.
Level 3, the decayed area accounts for 50% to 70% of the whole area.
The following formula was used to calculate the decay index:
(3) Rust spots are a common phenomenon in the storage of snap beans, related to cold damage, relative humidity in the storage environment, and gas composition and concentration [
36]. The smaller the rust spots index, the lighter is the occurrence of rust spots. The rust spots index was divided into four levels according to the degree of rust occurrence on the surface of snap beans [
36].
Level 0, no rust spots.
Level 1, less rust with commodity value.
Level 2, more rust spots, no commodity value.
Level 3, serious rust spots, loss of food quality.
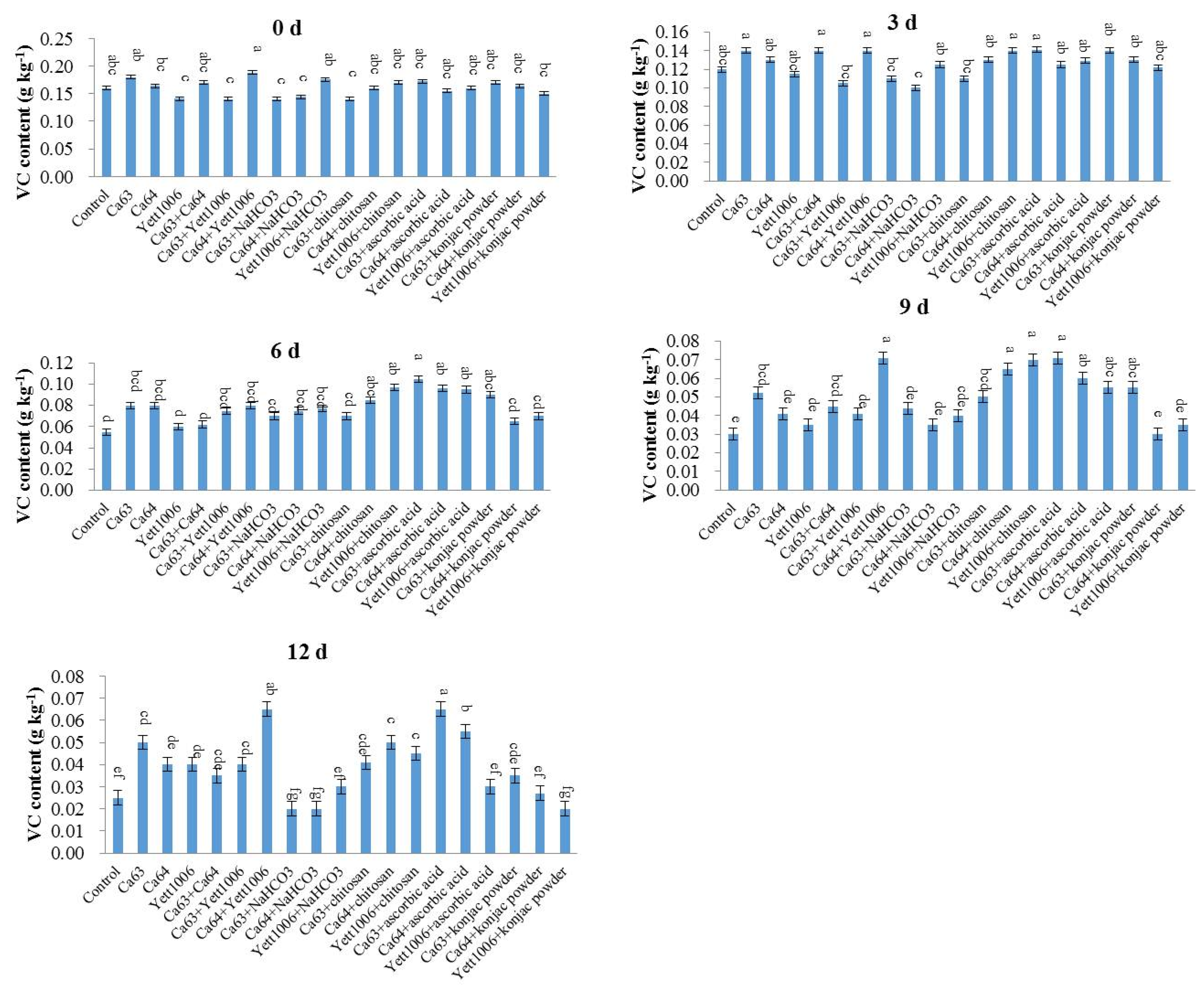
(4) Ascorbic acid (Vitamin C, VC) content
The VC content was determined according to trade standards of agricultural products of the People’s Republic of China: GB5009.86–2016, with modifications. The specific steps are as follows:
The sample of snap beans was appropriately weighed, and a small amount of 1% oxalic acid was then added, ground into slurry with a mortar, and filtered with a gauze. The filtrate was transferred to a 50 mL volumetric flask and diluted with 2% oxalic acid. Next, 1 mL of standard ascorbic acid solution (0.1 mg mL
−1) was taken, 9 mL of oxalic acid solution (1%,
v/v) was added and titrated with 0.1% 2, 6-dichlorophenol indole in a micro-burette. The endpoint of titration was that the light red color did not fade within 15 s. The T value (average value) was calculated from the volume of the dye used. Next, the amount of VC (mg, fresh weight) equivalent to 1 mL of dye was calculated. Two more samples were treated in the same manner.
where
m: 100 g sample contains the mass of VC (g).
V: dye volume used in titration (L).
T: mass of VC per milligram of dye can be oxidized (g L−1).
m0: 10 mL sample containing sample mass (kg).
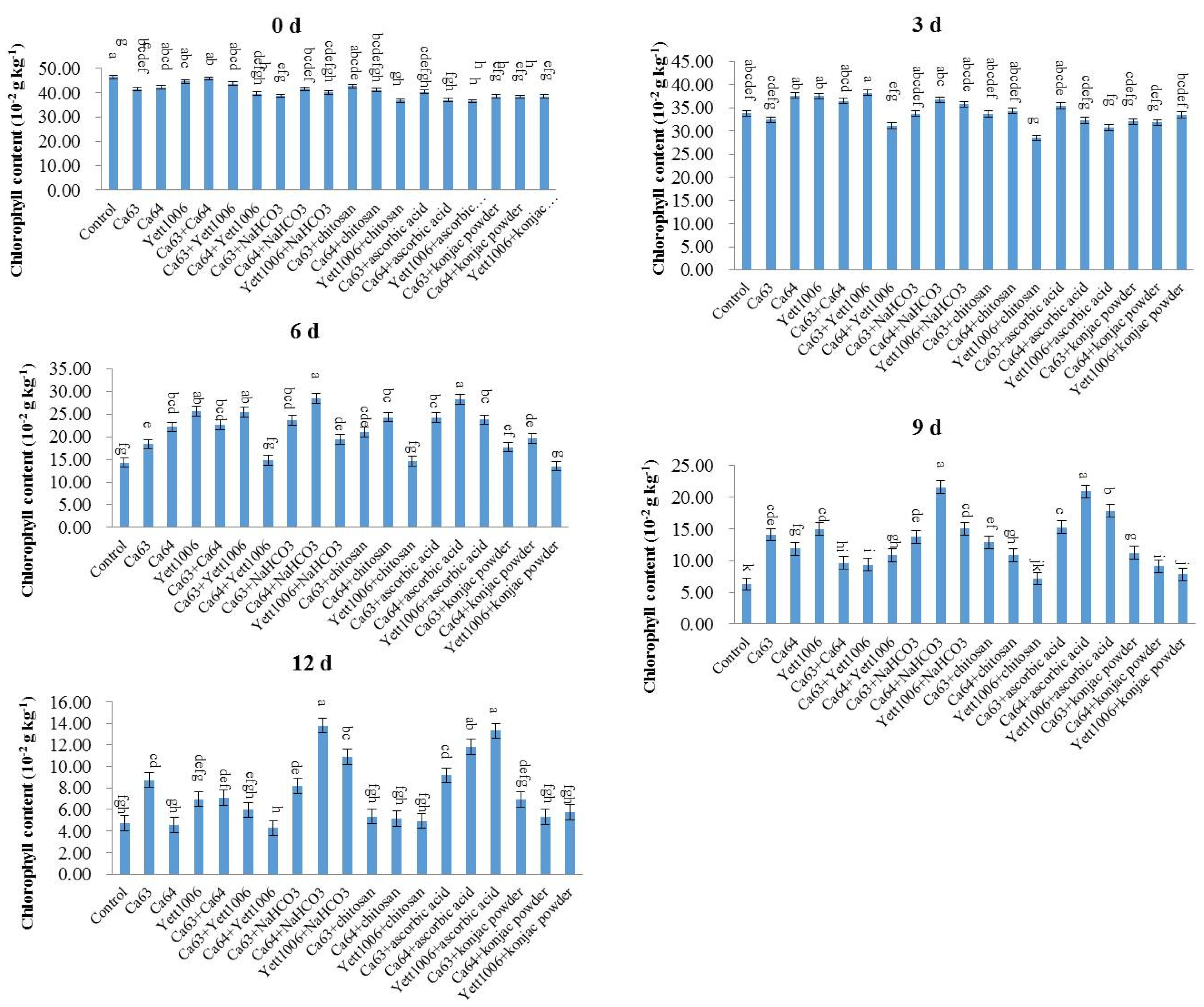
(5) Chlorophyll content and soluble protein content
Snap beans (0.5 g) were collected and cut into pieces. Distilled water (1 mL) and a small amount of calcium carbonate were added for grinding. Then, 10 mL of extract solution (absolute ethanol:acetone = 1:2 (
v/v)) was used and extracted for 3 h in the dark. The chlorophyll content (fresh weight) was determined according to the instructions provided in the kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) once the residue turned white [
37].
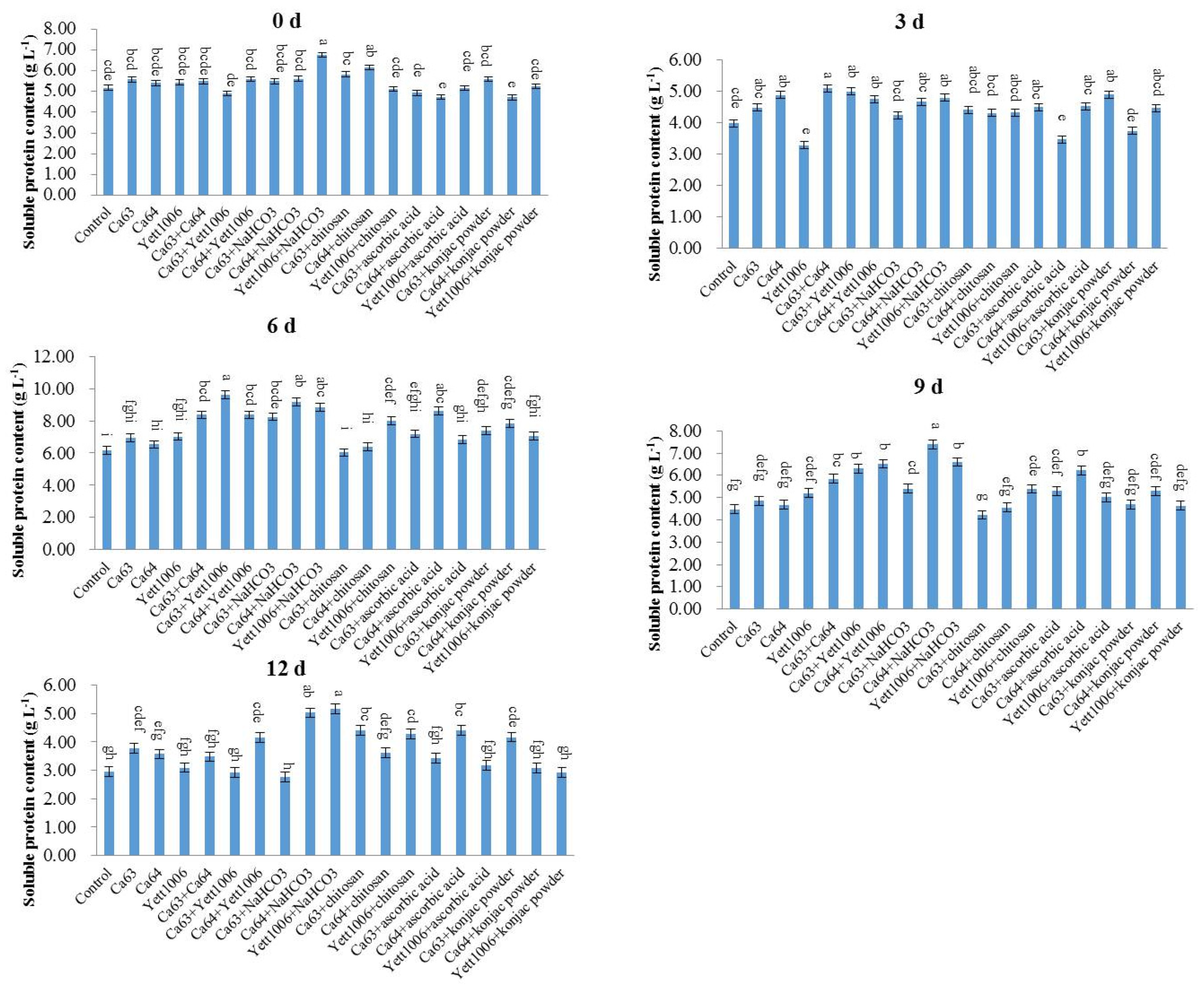
Snap beans (0.5 g) were collected, and physiological saline was added to them according to the weight (g):volume (mL) = 1:9 to prepare a 10% tissue homogenate in an ice-water bath. The supernatant was collected after centrifugation at 1000×
g for 10 min and mixed with physiological saline at a ratio of 1:4 (
v/v). The soluble protein content was measured according to the instructions provided in the kit (Nanjing Jiancheng Technology Co., Ltd., Nanjing, China) [
37].
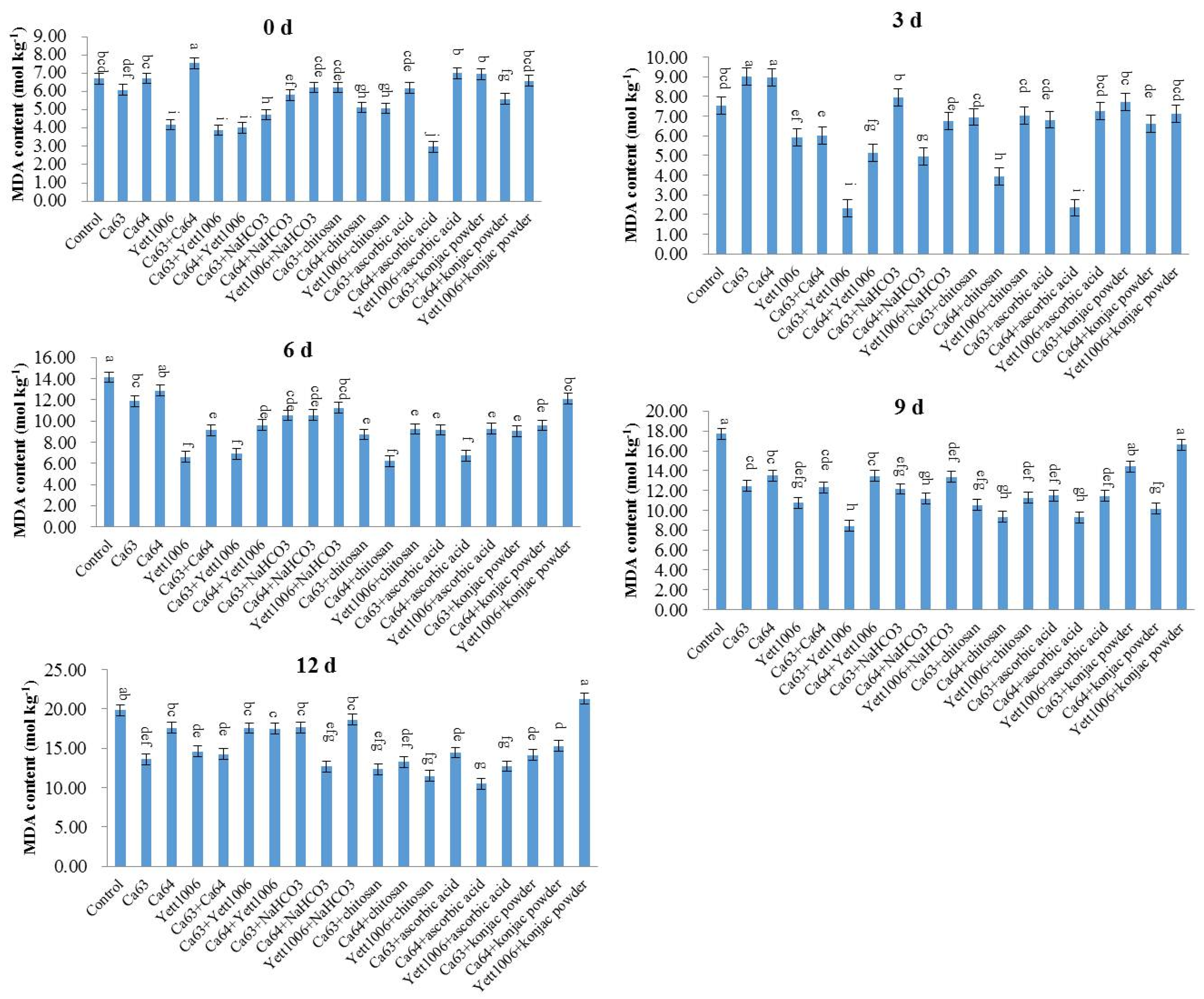
Assay of Defense-Related Mechanisms
The content of malondialdehyde (MDA) was detected by the thiobarbituric acid (TBA) method. Snap beans (0.5 g) were collected, and 0.9% NaCl solution was added according to the weight (g):volume (mL) = 1:9 to prepare a 10% tissue homogenate in an ice-water bath. The protein concentration (Cp) of the 10% tissue homogenate was measured. Then, we operated according to the
Table 1.
We covered the centrifuge tubes and tied a small hole with a needle on each cover. We mixed the tubes with a vortex mixer. After having them in a 95 °C water bath for 40 min, we took them out and cooled them with running water. Then, they were centrifuged at 2000× g for 10 min. The supernatant was used to measure the optical density (OD) value at 532 nm. For the preparation method of reagents I, II and III, see the kit manual (Nanjing Jiancheng Technology Co., Ltd., Nanjing, China).
The calculation formula of MDA content in the sample is as follows:
where
ODt: OD value of test tube.
ODc: OD value of control tube.
ODs: OD value of standard tube.
ODb: OD value of blank tube.
Cp: protein concentration (mg mL−1).
Standard concentration: 10 nmol mL−1.
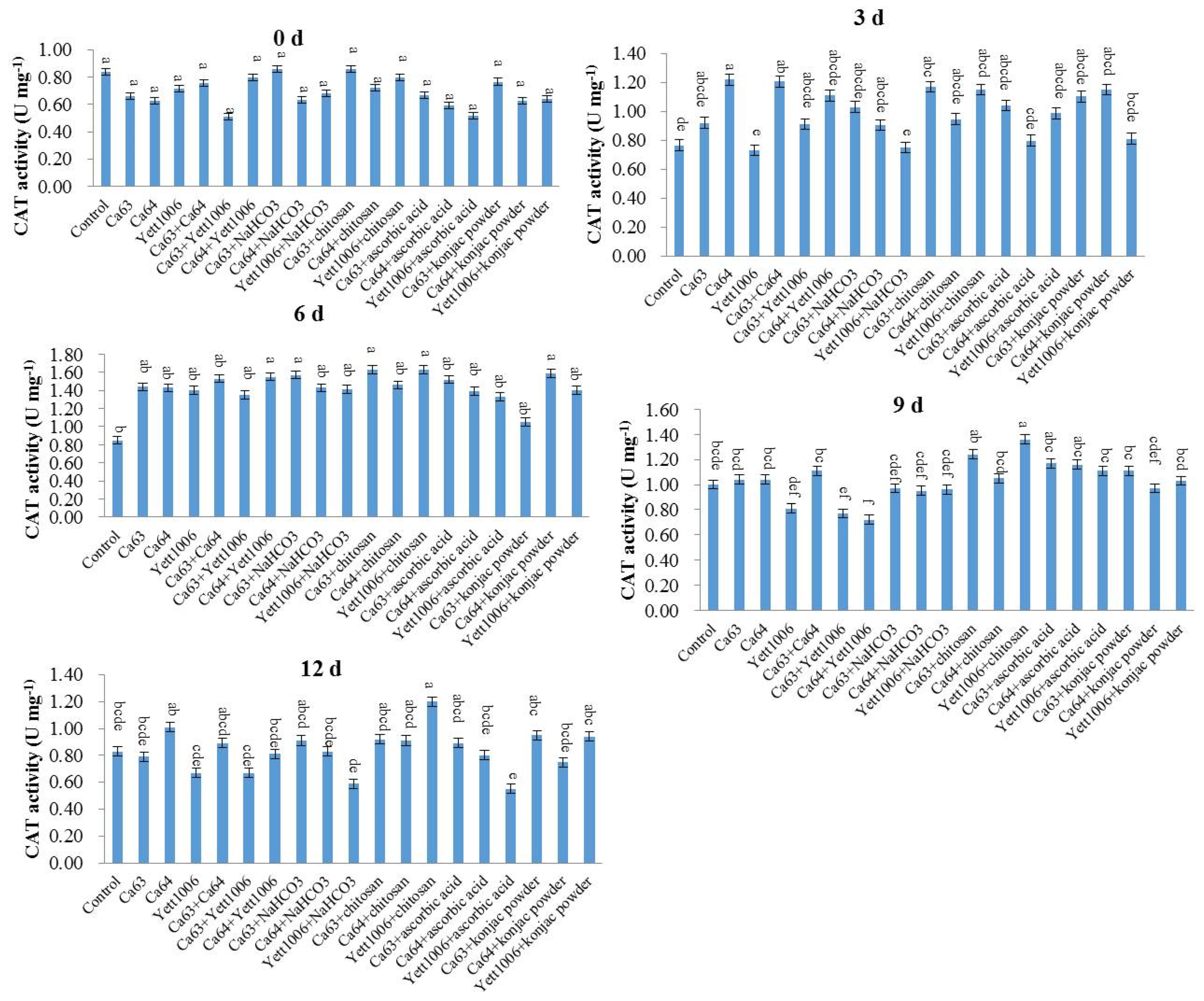
Catalase (CAT) activity was determined by the ammonium molybdate method. Snap beans (0.5 g) were collected and 0.9% NaCl solution was added according to the weight (g):volume (mL) = 1:9 to prepare a 10% tissue homogenate in an ice-water bath. The 10% tissue homogenate was centrifuged at 1000×
g for 10 min. We took the supernatant, added 0.9% NaCl solution at 1:4 to dilute it, and determined the protein concentration (CP). Then, we operated according to the
Table 2.
We mixed the supernatant well and measured the OD value of the supernatant at 405 nm. For the preparation method of reagents I, II, III and Ⅳ, see the kit manual (Nanjing Jiancheng Technology Co., Ltd., Nanjing, China).
Unit definition: the amount of 1 μmol H2O2 decomposed per second per milligram of tissue protein is a unit of activity.
The calculation formula of CAT activity in the sample is as follows:
where
ODt: OD value of test tube.
ODc: OD value of control tube.
Cp: protein concentration (mg mL−1).
Note: 271 is the reciprocal of the slope.
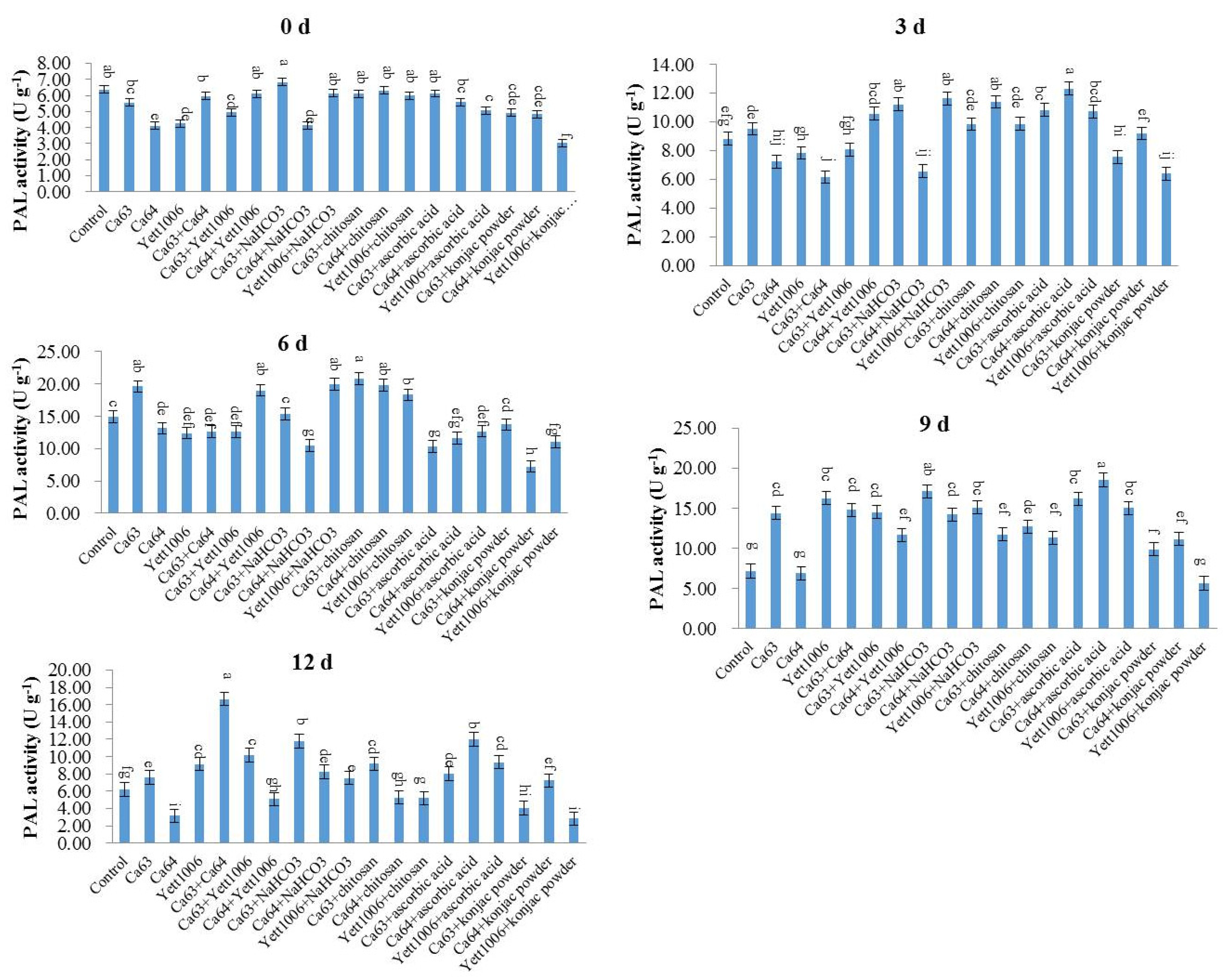
Phenylalnine ammonialyase (PAL) catalyzes the cleavage of L-phenylalanine into trans cinnamic acid and ammonia. Trans cinnamic acid has the maximum OD value at 290 nm. The PAL activity is calculated by measuring the change of OD value. Snap beans (0.5 g) were collected, and extract solution was added to weight (g):Volume (mL) = 1:9 to prepare a 10% tissue homogenate in an ice-water bath. The 10% tissue homogenate was centrifuged at 8000×
g for 10 min. We took the supernatant, then operated according to the
Table 3.
Mixed the supernatant well, stood for 10 min, and measured the OD value of the supernatant at 290 nm. For the preparation method of reagents R2, R3, R4 and crude enzyme solution, see the kit manual (Nanjing Jiancheng Technology Co., Ltd., Nanjing, China) [
35].
Unit definition: 0.1 change in 290 nm OD value per minute per g of tissue in each mL reaction system is a unit of enzyme activity.
The calculation formula of PAL activity in the sample is as follows:
where
The activity of superoxide dismutase (SOD) was determined by the 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2 H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) method. Snap beans (0.5 g) were collected and 0.9% NaCl solution was added according to the weight (g):volume (mL) = 1:4 to prepare a 20% tissue homogenate in an ice-water bath. The 20% tissue homogenate was centrifuged at 2000×
g for 10 min. We took the supernatant, then operated according to the
Table 4.
The supernatant was mixed well, incubated at 37 °C for 20 min, and the absorbance value of the supernatant was determined at 450 nm by microplate reader. For the preparation method of the enzyme working solution, enzyme diluent solution and substrate application solution, see the kit manual (Nanjing Jiancheng Technology Co., Ltd., Nanjing, China) [
35].
Unit definition: in this reaction system, when the SOD inhibition rate reaches 50%, the corresponding enzyme amount is a SOD activity unit (U).
The calculation formula of SOD activity in the sample is as follows:
where
At: absorbance value of test hole.
Atb: absorbance value of test blank hole.
Ac: absorbance value of control hole.
Acb: absorbance value of control blank hole.
2.4. Statistical Analysis
The data obtained were subjected to a one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test with statistical product and service solutions (SPSS) 20.0 software. Data are presented as mean ± SD with three replicates per treatment. Significant differences were considered at p < 0.05 and respective significant differences were marked using different letters.
5. Conclusions
In conclusion, the three yeast strains, namely Ca63, Ca64, and Yett 1006, including single yeast agent, two combined yeast strains, single yeast agent + NaHCO3, single yeast agent + chitosan, single yeast agent + ascorbic acid, and single yeast agent + konjac powder, showed high potential as biocontrol agents to control postharvest disease of snap beans caused by Botrytis cinerea. The optimal combination of the lowest disease index was Ca63 + Ca64, with a preventing effect of 75%. In addition, the decay rate and rust spots index of Ca64 + ascorbic acid combination were 25% and 20%, respectively, which were the lowest. Potential action modes of three yeasts include competition for space and nutrients with pathogens; ability to activate defense-related enzymes, such as SOD, PAL, and CAT; reduce the MDA content, enhance plants’ resistance, and delay plants’ senescence.