Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences
Abstract
:1. Introduction
2. Materials and Methods
2.1. Industrial Processing of Oilseeds, Milling of Oilseed Cake and Preparation of Flour Fractions
2.2. Proximate Composition
2.3. Water Solubility and Water Holding Capacity
2.4. Fat-Holding Capacity
2.5. Colour Analysis
2.6. Total Phenolic Content
2.7. Antioxidant Activity
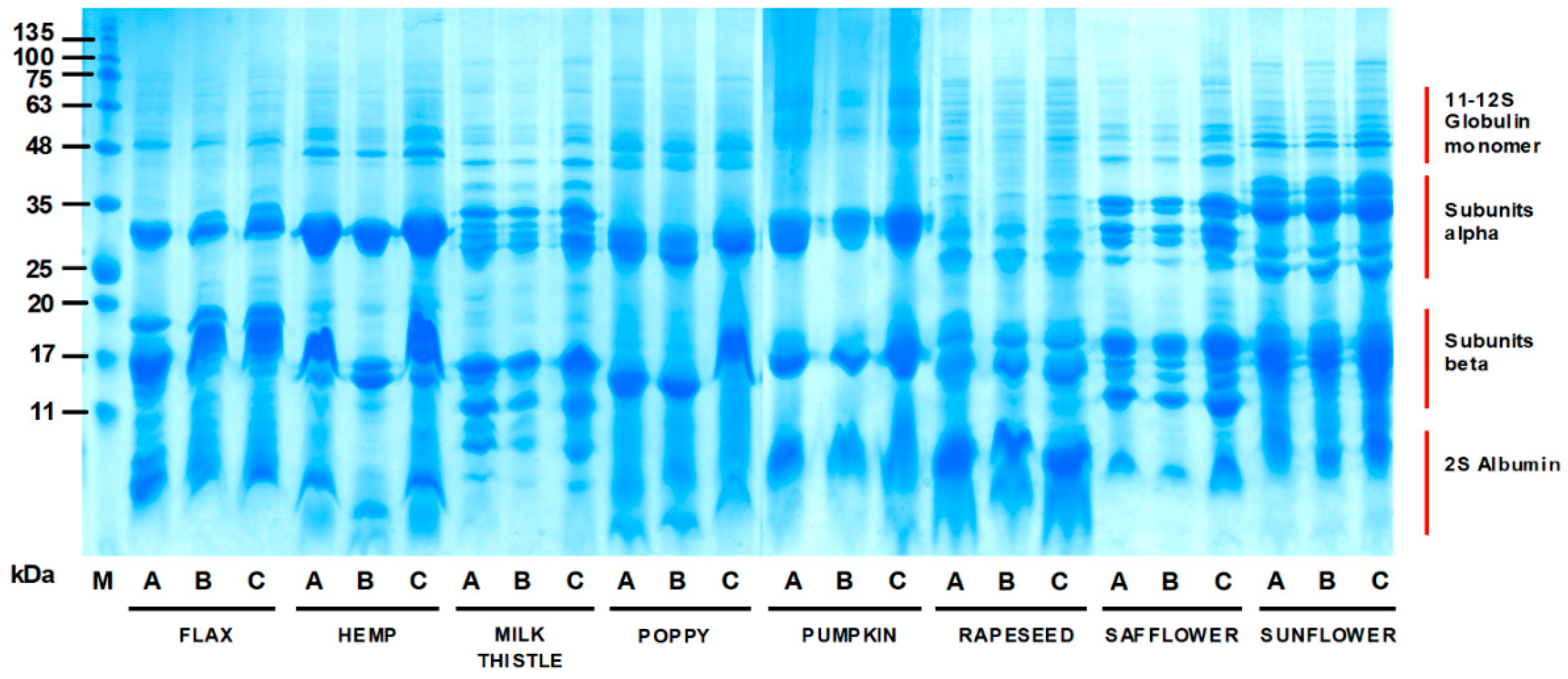
2.8. Electrophoretic Analysis of Oilseed Cake Protein Profiles
2.9. Statistics
3. Results and Discussion
3.1. Proximate Analysis of Oilseed Cake Flours
3.2. Electrophoretic Characteristics of Oilseed Cake Flour Proteins
3.3. Functional Characteristics of Oilseed Cake Flours
3.4. Total Polyphenols Content and Antioxidant Activities of Oilseed Cake Flours
3.5. Colour Characteristics of Oilseed Cake Flours
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Gupta, S.K.; Mondal, K. Production and trade of major world oil crops. In Technological Innovations in Major World Oil Crops; Gupta, S.K., Ed.; Springer: New York, NY, USA, 2012; Volume 1, pp. 1–15. [Google Scholar]
- OECD-FAO. Oilseeds and oilseed products. In OECD-FAO Agricultural Outlook 2019–2028; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Parry, J.; Su, L.; Moore, J.; Cheng, Z.; Luther, M.; Rao, J.; Wang, J.Y.; Yu, L. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected fruit seed flours. J. Agric. Food Chem. 2006, 54, 3773–3778. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.; Cheng, Z.; Moore, J.; Yu, L.L. Fatty acid composition, antioxidant properties, and antiproliferative capacity of selected cold-pressed seed flours. J. Am. Oil Chem. Soc. 2008, 85, 457–464. [Google Scholar] [CrossRef]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, A.; Pathak, R.; Kumar, A.; Sharma, S. Solid state fermentation of non-edible oil seed cakes for production of proteases and cellulases and degradation of anti-nutritional factors. J. Food Biotechnol. Res. 2018, 1, 3–8. [Google Scholar]
- Teh, S.S.; Bekhit, A.E. Utilization of oilseed cakes for human nutrition and health benefits. In Agricultural Biomass Based Potential Materials; Hakeem, K.R., Jawaid, M., Alothman, O.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 191–229. [Google Scholar]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.-M.; Hellström, J.; Pihlanto, A. Nutritional value of commercial protein-rich plant products. Plant Foods Hum. Nutr. 2018, 73, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zając, M.; Guzik, P.; Kulawik, P.; Tkaczewska, J.; Florkiewicz, A.; Migdal, W. The quality of pork loaves with the addition of hemp seeds, de-hulled hemp seeds, hemp protein and hemp flour. LWT-Food Sci. Technol. 2019, 105, 190–199. [Google Scholar] [CrossRef]
- Elsorady, M.E. Characterization and functional properties of proteins isolated from flaxseed cake and sesame cake. Croat. J. Food Sci. Technol. 2020, 12, 77–83. [Google Scholar] [CrossRef]
- Pojić, M.; Hadnađev, T.D.; Hadnađev, M.; Rakita, S.; Brlek, T. Bread supplementation with hemp seed cake: A by-product of hemp oil processing. J. Food Qual. 2015, 38, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Mejicanos, G.A.; Rogiewicz, A.; Nyachoti, C.M.; Slominski, B.A. Fractionation of canola meal using sieving technology. Can. J. Anim. Sci. 2017, 97, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Murru, M.; Calvo, C.L. Sunflower protein enrichment. Methods and potential applications. OCL-Oilseeds Fats Crop. Lipids 2020, 27, 17. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Oilseed proteins—properties and application as a food ingredient. Trends Food Sci. Technol. 2020, 106, 160–170. [Google Scholar] [CrossRef]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed Cake as a Tool for the Improvement of Nutraceutical and Sensorial Features of Sourdough Bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lamo, B.; Gómez, M. Bread Enrichment with Oilseeds. A Review. Foods 2018, 7, 191. [Google Scholar] [CrossRef] [Green Version]
- American Association of Cereal Chemists. AOAC Approved Methods of the AAAC, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2006. [Google Scholar]
- Lachman, J.; Hamouz, K.; Čepl, J.; Pivec, V.; Šulc, M.; Dvořák, P. The effect of selected factors on polyphenol content and antioxidant activity in potato tubers. Chem. Listy 2006, 100, 522–527. [Google Scholar]
- Šulc, M.; Lachman, J.; Hamouz, K.; Orsák, M.; Dvořák, P.; Horáčková, V. Selection and evaluation of methods for determination of antioxidant activity of purple- and red-fleshed potato varieties. Chem. Listy 2007, 101, 584–591. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Lan, Y.; Zha, F.; Peckrul, A.; Hanson, B.; Johnson, B.; Rao, J.; Chen, B. Genotype x environmental effects on yielding ability and seed chemical composition of industrial hemp (Cannabis sativa L.) varieties grown in North Dakota, USA. J. Am. Oil Chem. Soc. 2019, 96, 1417–1425. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Xu, M.; Ohm, J.B.; Rao, J.; Chen, B. The impact of hempseed dehulling on chemical composition, structure properties and aromatic profile of hemp protein isolate. Food Hydrocoll. 2020, 106, 105889. [Google Scholar] [CrossRef]
- Dotto, J.M.; Chacha, J.S. The potential of pumpkin seeds as a functional food ingredient: A review. Sci. Afr. 2020, 10, e00575. [Google Scholar] [CrossRef]
- Amin, A.Z.; Islam, T.; Uddin, M.R.; Uddin, M.J.; Rahman, M.M.; Satter, M.A. Comparative study on nutrient contents in the different parts of indigenous and hybrid varieties of pumpkin (Cucurbita maxima Linn.). Heliyon 2019, 5, e02462. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, I.; Dokić, L.; Rakić, D.; Tomović, V.; Maravić, N.; Vidosavljević, S.; Šereš, Z.; Šoronja-Simović, D. The role of two types of continuous phases bases on cellulose during textural, color, and sensory characterization of novel food spread with pumpkin seed flour. J. Food Process. Preserv. 2018, 42, e13684. [Google Scholar] [CrossRef]
- Kreft, I.; Stibilj, V.; Trkov, Z. Iodine and selenium contents in pumpkin (Cucurbita pepo L.) oil and oil-cake. Eur. Food Res. Technol. 2002, 215, 279–281. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis sativa subsp. sativa) flour and protein preparation as natural nutrients and structure forming agents in starch based gluten-free bread. LWT-Food Sci. Technol. 2017, 84, 143–150. [Google Scholar] [CrossRef]
- Bochkarev, B.; Egorova, E.; Poznyakovkiy, V. Reasons for the ways of using oilcakes in food industry. Foods Raw Mater. 2016, 4, 4–12. [Google Scholar] [CrossRef]
- Vonapartis, E.; Aubin, M.P.; Seguin, P.; Mustafa, A.F.; Charron, J.B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Compost. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Bucsella, B.; Molnár, D.; Harasztos, A.H.; Tömösközi, S. Comparison of the rheological and end-productproperties of an industrial aleurone-rich wheat flour, whole grain wheat and rye flour. J. Cereal Sci. 2016, 69, 40–48. [Google Scholar] [CrossRef]
- Yilmaz, E.; Emir, D.D. Compositional and functional characterisation of poppy seed (Papaver somniferum L.) press cake meals. Qual. Assur. Saf. Crops Foods 2017, 9, 141–151. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.Y.; Zhao, T.; Li, F.; Zhao, J.L.; Yang, L.Q. Extraction, physicochemical, and functional properties of proteins from milk thistle Silybum marianum L. gaernt seeds. Int. J. Food Prop. 2013, 16, 1750–1763. [Google Scholar] [CrossRef]
- Guo, X.F.; Tian, S.; Small, D.M. Generation of meat-like flavourings from enzymatic hydrolysates of proteins from Brassica spp. Food Chem. 2010, 119, 167–172. [Google Scholar] [CrossRef]
- Žilić, S.; Barać, M.; Pešić, M.; Crevar, M.; Stanojević, S.; Nišavić, A.; Saratlić, G.; Tolimir, M. Characterization of sunflower seed and kernel proteins. Helia 2010, 33, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Bučko, S.; Katona, J.; Popović, L.; Petrović, L.; Milinković, J. Influence of enzymatic hydrolysis on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. Food Hydrocoll. 2016, 60, 271–278. [Google Scholar] [CrossRef]
- Hara, I.; Wada, K.; Wakabayashi, S.; Matsubara, H. Pumpkin (Cucurbita sp.) seed globulin I. Purification, characterization, and subunit structure. Plant Cell Physiol. 1976, 17, 799–814. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D.; McIntosh, T.C.; Perera, S.P.; Withana-Gamage, T.S. Canola/rapeseed protein-functionality and nutrition. OCL-Oilseeds Fats Crop. Lipids 2016, 23, D407. [Google Scholar] [CrossRef] [Green Version]
- Kasprzak, M.M.; Houdijk, J.G.M.; Liddell, S.; Davis, K.; Olukosi, O.A.; Kightley, S.; White, G.A.; Wiseman, J. Rapeseed napin and cruciferin are readily digested by poultry. J. Anim. Physiol. Anim. Nutr. 2017, 101, 658–666. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Y.L. Processing, nutrition, and functionality of hempseed protein: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 936–952. [Google Scholar] [CrossRef] [Green Version]
- Malomo, S.A.; Aluko, R.E. A comparative study of the structural and functional properties of isolated hemp seed (Cannabis sativa L.) albumin and globulin fractions. Food Hydrocoll. 2015, 43, 43–752. [Google Scholar] [CrossRef]
- Malomo, S.A.; He, R.; Aluko, R.E. Structural and functional properties of hemp seed protein products. J. Food Sci. 2014, 79, C1512–C1521. [Google Scholar] [CrossRef]
- Hu, Y.; Shim, Y.Y.; Reaney, M.J.T. Flaxseed gum solution functional properties. Foods 2020, 9, 681. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Xu, X.; Zhou, G. Influence of various levels of flaxseed gum addition on the water-holding capacities of heat-induced porcine myofibrillar protein. J. Food Sci. 2011, 76, C472–C478. [Google Scholar] [CrossRef]
- Nguyen, D.; Mounir, S.; Allaf, K. Functional properties of water holding capacity, oil holding capacity, wettability, and sedimentation of swell-dried soybean powder. Sch. J. Eng. Tech. 2015, 3, 402–412. [Google Scholar]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of functional ingredients from flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, T.; Potenza, E.; Moschella, A.; Zaccheria, F.; Benedettelli, S.; Andrzejewska, J. Phenotypic evaluation of milk thistle germplasm collection: Fruit morphology and chemical composition. Crop Sci. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Viktorova, J.; Stranska-Zachariasova, M.; Fenclova, M.; Vitek, L.; Hajslova, J.; Kren, V.; Ruml, T. Complex evaluation of antioxidant capacity of milk thistle dietary supplements. Antioxidants 2019, 8, 317. [Google Scholar] [CrossRef] [Green Version]
- Cappelletti, E.M.; Caniato, R. Silymarin localization in the fruit and seed of Silybum marianum L. Gaertn. Herb. Hung. 1984, 23, 53–66. [Google Scholar]
- Venglat, P.; Xiang, D.; Qiu, S.; Stone, S.L.; Tibiche, C.; Cram, D.; Alting-Mees, M.; Nowak, J.; Cloutier, S.; Deyholos, M.; et al. Gene expression analysis of flax seed development. BMC Plant Biol. 2011, 11, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickardt, C.; Weisz, G.M.; Eisner, P.; Kammerer, D.R.; Neidhart, S.; Carle, R. Processing of low polyphenol protein isolates from residues of sunflower seed oil production. Procedia Food Sci. 2011, 1, 1417–1424. [Google Scholar] [CrossRef]
- Xu, L.; Diosady, L.L. Removal of phenolic compounds in the production of high-quality canola protein isolates. Food Res. Int. 2002, 35, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.; Pinelli, P.; Moschini, V.; Heimler, D. Seeds and oil polyphenol content of sunflower (Helianthus annuus L.) grown with different agricultural management. Adv. Hortic. Sci. 2017, 31, 85–88. [Google Scholar]
- Serçe, A.; Toptanci, Ç.B.; Tanrikut, S.E.; Altaş, S.; Kizil, G.; Kizil, S.; Kizil, M. Assessment of the antioxidant activity of Silybum marianum seed extract and its protective effect against DNA oxidation, protein damage and lipid peroxidation. Food Technol. Biotechnol. 2016, 54, 455–461. [Google Scholar] [CrossRef]
- Bortíková, V.; Kolarič, L.; Šimko, P. Application of milk thistle (Silybum marianum) in functional biscuits formulation. Acta Chim. Slovaca 2019, 12, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Apostol, L.; Lorga, S.; Moşoiu, C.; Racovita, R.C.; Niculae, O. The effects of partially defatted milk thistle (Silybum marianum) seed flour on wheat flour. J. Int. Sci. Publ. Agric. Food. 2017, 5, 74–84. [Google Scholar]
- Schorno, A.L.; Manthey, F.A.; Hall, C.A. Effect of particle size and sample size on lipid stability of milled flaxseed (Linum usitatissimum L.). J. Food Process. Preserv. 2010, 34, 167–179. [Google Scholar] [CrossRef]
- Uluata, S.; Özdemir, N. Antioxidant activities and oxidative stabilities of some unconventional oilseeds. J. Am. Oil Chem. Soc. 2011, 89, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Martysiak-Żurowska, D.; Wenta, W. A comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Sci. Pol. Technol. Aliment. 2012, 11, 83–89. [Google Scholar] [PubMed]
- Okoh, S.O.; Asekun, O.T.; Familoni, O.B.; Afolayan, A.J. Antioxidant and free radical scavenging Ccapacity of seed and shell essential oils extracted from Abrus precatorius (L). Antioxidants 2014, 3, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachman, J.; Sulc, M.; Faitová, K.; Pivec, V. Major factors influencing antioxidant contents and antioxidant activity in grapes and wines. Int. J. Wine Res. 2009, 1, 101–121. [Google Scholar] [CrossRef] [Green Version]
- Šulc, M.; Lachman, J.; Hamouz, K.; Dvořák, P. Impact of Phenolic Content on Antioxidant Activity in Yellow and Purple-fleshed Potatoes Grown in the Czech Republic. Biol. Agric. Hortic. 2008, 26, 45–54. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, Y.; Beta, T. Comparison of antioxidant activities of different colored wheat grains and analysis of phenolic compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef]
- Kaur, P.; Waghmare, R.; Kumar, V.; Rasane, P.; Kaur, S.; Gat, Y. Recent advances in utilization of flaxseed as potential source for value addition. OCL-Oilseeds Fats Crop. Lipids 2018, 25, A304. [Google Scholar] [CrossRef] [Green Version]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, C.S. The effects of dietary fibre addition on the quality of common cereal products. J. Cereal Sci. 2013, 58, 216–227. [Google Scholar] [CrossRef]
- Grasso, S.; Omoarukhe, E.; Wen, X.; Papoutsis, K.; Methven, L. The use of upcycled defatted sunflower seed flour as a functional ingredient in biscuits. Foods 2019, 8, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Oil Cake | Yield (%) | |
|---|---|---|
| A250 | B250 | |
| Flax | 51.95 | 45.15 |
| Hemp | 61.99 | 35.20 |
| Milk thistle | 50.01 | 49.06 |
| Poppy | 41.41 | 56.50 |
| Pumpkin | 38.04 | 59.79 |
| Rapeseed | 49.57 | 48.00 |
| Safflower | 68.45 | 30.64 |
| Sunflower | 56.53 | 41.51 |
| Oilseed Cake | Crude Protein (%) | Crude Fat (%) | Carbohydrates (%) | Ash (%) | Moisture (%) |
|---|---|---|---|---|---|
| Flax | |||||
| WF | 28.46 ± 0.44 hi | 16.16 ± 0.11 b | 41.74 ± 0.37 j | 4.73 ± 0.03 o | 8.92 ± 0.10 b |
| A250 | 24.81 ± 0.60 j | 13.03 ± 0.26 d | 47.97 ± 0.29 gh | 4.80 ± 0.01 o | 9.38 ± 0.10 a |
| B250 | 33.58 ± 0.47 f | 19.56 ± 0.10 a | 33.65 ± 0.44 m | 5.00 ± 0.04 n | 8.21 ± 0.15 cde |
| Hemp | |||||
| WF | 28.25 ± 0.42 hi | 8.61 ± 0.24 i | 50.23 ± 0.66 f | 5.34 ± 0.02 m | 7.56 ± 0.33 fg |
| A250 | 18.83 ± 2.42 l | 6.30 ± 0.14 m | 62.19 ± 2.31 d | 4.22 ± 0.08 p | 8.46 ± 0.09 c |
| B250 | 45.69 ± 0.44 c | 10.24 ± 0.11 g | 27.84 ± 0.41 o | 7.65 ± 0.04 g | 8.58 ± 0.12 bc |
| Milk thistle | |||||
| WF | 22.56 ± 0.36 k | 4.84 ± 0.23 o | 63.73 ± 0.23 d | 5.78 ± 0.03 jk | 3.09 ± 0.66 l |
| A250 | 10.75 ± 1.20 n | 2.27 ± 0.44 p | 78.17 ± 1.36 a | 3.61 ± 0.03 q | 5.20 ± 0.05 k |
| B250 | 31.78 ± 1.61 g | 5.46 ± 0.21 n | 49.49 ± 1.74 fg | 8.11 ± 0.04 f | 5.16 ± 0.50 k |
| Poppy | |||||
| WF | 34.75 ± 0.44 ef | 10.49 ± 0.13 g | 36.03 ± 0.43 kl | 10.90 ± 0.01 b | 7.84 ± 0.15 ef |
| A250 | 34.66 ± 0.36 ef | 9.75 ± 0.05 h | 37.32 ± 0.23 k | 10.94 ± 0.09 b | 7.33 ± 0.09 gh |
| B250 | 35.42 ± 0.49 e | 11.25 ± 0.23 f | 34.62 ± 0.67 lm | 11.19 ± 0.02 a | 7.52 ± 0.03 fg |
| Pumpkin | |||||
| WF | 59.12 ± 0.47 a | 12.36 ± 0.26 e | 14.39 ± 0.67 p | 9.14 ± 0.06 d | 4.99 ± 0.14 k |
| A250 | 60.38 ± 0.31 a | 8.95 ± 0.06 i | 14.67 ± 0.39 p | 9.42 ± 0.11 c | 6.58 ± 0.18 i |
| B250 | 57.47 ± 0.46 b | 13.65 ± 0.05 c | 14.06 ± 0.65 p | 8.94 ± 0.03 e | 5.88 ± 0.31 j |
| Rapeseed | |||||
| WF | 29.84 ± 1.50 h | 12.52 ± 0.19 e | 43.84 ± 1.67 i | 5.73 ± 0.12 k | 8.08 ± 0.14 de |
| A250 | 24.96 ± 0.54 j | 8.82 ± 0.42 i | 52.30 ± 0.76 e | 5.49 ± 0.08 l | 8.43 ± 0.09 cd |
| B250 | 33.52 ± 0.18 f | 15.89 ± 0.13 b | 37.42 ± 0.35 k | 5.90 ± 0.02 ij | 7.27 ± 0.28 gh |
| Safflower | |||||
| WF | 14.21 ± 1.20 m | 8.00 ± 0.16 j | 69.30 ± 0.92 c | 2.51 ± 0.31 r | 5.98 ± 0.03 j |
| A250 | 8.38 ± 1.97 o | 7.17 ± 0.15 k | 75.19 ± 2.10 b | 2.07 ± 0.03 s | 7.20 ± 0.03 gh |
| B250 | 34.29 ± 1.06 ef | 6.75 ± 0.09 l | 46.72 ± 0.86 h | 5.11 ± 0.04 n | 13 ± 0.12 h |
| Sunflower | |||||
| WF | 34.41 ± 0.51 ef | 12.47 ± 0.07 e | 40.52 ± 0.77 j | 5.99 ± 0.01 i | 6.60 ± 0.34 i |
| A250 | 27.87 ± 1.60 i | 11.12 ± 0.64 f | 48.38 ± 1.44 gh | 5.25 ± 0.08 m | 7.37 ± 0.05 gh |
| B250 | 41.66 ± 0.22 d | 12.20 ± 0.01 e | 31.49 ± 0.27 n | 7.42 ± 0.04 h | 7.24 ± 0.06 gh |
| Oil Cake | WS (%) | WHC (g/g of Flour) | WHC after Boiling (g/g of Flour) | FHC (g/g of Flour) |
|---|---|---|---|---|
| Flax | ||||
| WF | 21.84 ± 2.49 d | 4.15 ± 0.24 b | 8.12 ± 0.15 a | 0.97 ± 0.01 c |
| A250 | 22.26 ± 0.92 cd | 4.90 ± 0.03 a | 8.32 ± 0.06 a | 1.09 ± 0.04 a |
| B250 | 26.68 ± 1.26 b | 3.17 ± 0.29 cd | 7.42 ± 0.37 b | 0.91 ± 0.01 d |
| Hemp | ||||
| WF | 15.36 ± 0.52 jkl | 1.67 ± 0.04 kl | 1.91 ± 0.15 hij | 0.77 ± 0.03 jk |
| A250 | 13.08 ± 0.23 lm | 1.61 ± 0.06 lmn | 1.70 ± 0.01 jkl | 0.82 ± 0.01 hi |
| B250 | 18.98 ± 1.66 efgh | 1.83 ± 0.08 k | 1.98 ± 0.05 hi | 0.83 ± 0.01 hi |
| Milk thistle | ||||
| WF | 15.95 ± 0.68 ijk | 2.73 ± 0.09 f | 2.99 ± 0.16 d | 0.83 ± 0.01 hi |
| A250 | 12.89 ± 0.31 m | 2.19 ± 0.09 j | 2.58 ± 0.18 ef | 0.85 ± 0.00 gh |
| B250 | 20.60 ± 1.58 def | 3.33 ± 0.09 c | 3.18 ± 0.20 cd | 0.90 ± 0.02 de |
| Poppy | ||||
| WF | 21.13 ± 0.71 de | 2.67 ± 0.09 fg | 2.35 ± 0.02 fg | 0.91 ± 0.01 d |
| A250 | 19.34 ± 0.41 efgh | 3.07 ± 0.08 de | 2.55 ± 0.09 ef | 0.99 ± 0.03 bc |
| B250 | 20.87 ± 0.47 def | 2.55 ± 0.17 ghi | 2.16 ± 0.14 gh | 1.01 ± 0.01 b |
| Pumpkin | ||||
| WF | 19.91 ± 1.37 defg | 1.45 ± 0.06 mn | 1.65 ± 0.06 kl | 0.65 ± 0.01 m |
| A250 | 18.11 ± 4.96 ghi | 1.60 ± 0.13 lmn | 1.84 ± 0.06 ijk | 0.65 ± 0.01 m |
| B250 | 19.10 ± 0.13 efgh | 1.46 ± 0.06 mn | 1.55 ± 0.06 l | 0.71 ± 0.03 l |
| Rapeseed | ||||
| WF | 25.08 ± 1.59 b | 2.42 ± 0.19 hi | 3.10 ± 0.15 cd | 0.90 ± 0.01 de |
| A250 | 20.62 ± 0.64 def | 2.37 ± 0.05 ij | 3.33 ± 0.34 c | 0.86 ± 0.07 efgh |
| B250 | 33.12 ± 0.35 a | 2.42 ± 0.02 hi | 2.40 ± 0.28 fg | 0.89 ± 0.02 def |
| Safflower | ||||
| WF | 13.56 ± 0.54 klm | 1.56 ± 0.04 lmn | 1.95 ± 0.07 hij | 0.74 ± 0.01 kl |
| A250 | 12.31 ± 1.78 m | 1.44 ± 0.03 n | 1.88 ± 0.08 ijk | 0.79 ± 0.01 ij |
| B250 | 17.30 ± 0.58 hij | 1.63 ± 0.04 lm | 1.89 ± 0.06 ijk | 0.85 ± 0.02 fgh |
| Sunflower | ||||
| WF | 22.31 ± 1.67 cd | 2.66 ± 0.07 fg | 2.67 ± 0.07 e | 0.86 ± 0.00 efgh |
| A250 | 18.54 ± 0.28 fgh | 2.96 ± 0.05 e | 2.99 ± 0.22 d | 1.03 ± 0.03 b |
| B250 | 24.63 ± 0.96 bc | 2.58 ± 0.04 fgh | 2.49 ± 0.04 ef | 0.88 ± 0.03 defg |
| Oilseed Cake | Total Phenolics Content (mg GAE/g) | Antioxidant Activity ABTS•+ (mg AAE/g) | Antioxidant Activity DPPH• (mg AAE/g) |
|---|---|---|---|
| Flax | |||
| WF | 6.13 ± 0.59 hi | 13.50 ± 1.15 f | 2.35 ± 0.03 f |
| A250 | 7.42 ± 0.41 fg | 17.45 ± 0.34 e | 2.55 ± 0.03 f |
| B250 | 3.59 ± 0.28 j | 8.16 ± 0.25 gij | 1.84 ± 0.01 g |
| Hemp | |||
| WF | 2.72 ± 0.13 jk | 7.70 ± 0.51 ijk | 1.66 ± 0.04 g |
| A250 | 2.99 ± 0.33 jk | 9.36 ± 0.53 gi | 1.96 ± 0.03 g |
| B250 | 2.61 ± 0.37 jkl | 8.34 ± 0.43 gij | 1.25 ± 0.06 h |
| Milk thistle | |||
| WF | 30.44 ± 0.42 b | 86.42 ± 6.43 b | 11.36 ± 0.56 b |
| A250 | 40.89 ± 1.60 a | 101.95 ± 4.14 a | 11.37 ± 0.43 b |
| B250 | 17.68 ± 1.20 c | 55.71 ± 1.42 c | 9.63 ± 0.65 c |
| Poppy | |||
| WF | 2.84 ± 0.36 jk | 3.02 ± 0.07 l | 0.89 ± 0.01 i |
| A250 | 2.39 ± 0.12 klm | 3.66 ± 0.21 l | 0.90 ± 0.00 I |
| B250 | 3.02 ± 0.34 jk | 3.61 ± 0.24 l | 0.87 ± 0.03 i |
| Pumpkin | |||
| WF | 1.45 ± 0.11 m | 4.34 ± 0.19 kl | 0.92 ± 0.02 hi |
| A250 | 1.41 ± 0.12 m | 5.20 ± 0.13 jkl | 0.90 ± 0.05 i |
| B250 | 1.52 ± 0.18 lm | 6.19 ± 0.31 ijkl | 0.90 ± 0.04 i |
| Rapeseed | |||
| WF | 8.47 ± 0.56 f | 17.42 ± 0.47 e | 9.33 ± 0.08 c |
| A250 | 6.97 ± 0.23 gh | 11.56 ± 1.22 fg | 7.74 ± 0.13 d |
| B250 | 10.51 ± 1.03 e | 20.51 ± 1.66 e | 11.05 ± 0.09 b |
| Safflower | |||
| WF | 6.79 ± 0.47 gh | 19.95 ± 0.15 e | 3.07 ± 0.02 e |
| A250 | 5.49 ± 0.29 i | 17.59 ± 0.59 e | 2.90 ± 0.13 e |
| B250 | 11.99 ± 1.15 d | 33.82 ± 1.13 d | 3.07 ± 0.04 e |
| Sunflower | |||
| WF | 17.21 ± 0.89 c | 17.67 ± 6.51 e | 15.37 ± 0.11 a |
| A250 | 12.93 ± 0.40 d | 18.85 ± 2.47 e | 15.29 ± 0.17 a |
| B250 | 17.65 ± 1.19 c | 31.03 ± 1.26 d | 15.49 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárta, J.; Bártová, V.; Jarošová, M.; Švajner, J.; Smetana, P.; Kadlec, J.; Filip, V.; Kyselka, J.; Berčíková, M.; Zdráhal, Z.; et al. Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences. Foods 2021, 10, 2766. https://doi.org/10.3390/foods10112766
Bárta J, Bártová V, Jarošová M, Švajner J, Smetana P, Kadlec J, Filip V, Kyselka J, Berčíková M, Zdráhal Z, et al. Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences. Foods. 2021; 10(11):2766. https://doi.org/10.3390/foods10112766
Chicago/Turabian StyleBárta, Jan, Veronika Bártová, Markéta Jarošová, Josef Švajner, Pavel Smetana, Jaromír Kadlec, Vladimír Filip, Jan Kyselka, Markéta Berčíková, Zbyněk Zdráhal, and et al. 2021. "Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences" Foods 10, no. 11: 2766. https://doi.org/10.3390/foods10112766







