Microbiological Quality and Resistance to an Artificial Gut Environment of Two Probiotic Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Products
2.2. Quantification of Living Microbes
2.3. Culture-Dependent Identification of Probiotic Microbes by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
2.4. Culture-Independent Identification of Probiotic Microbes by Genomic DNA Extraction and Metagenomic Analysis
2.5. Resistance of Probiotic Microbes to Artificial Gastric Juices
2.6. Resistance of Probiotic Microbes in Simulated Intestinal Fluid
2.7. Behavior of Microbiosys Intact Capsules in Simulated Gastric and Intestinal Conditions
2.8. Statistical Analysis
3. Results
3.1. Quantification and Identification of the Microbes Contained in the Commercial Formulations
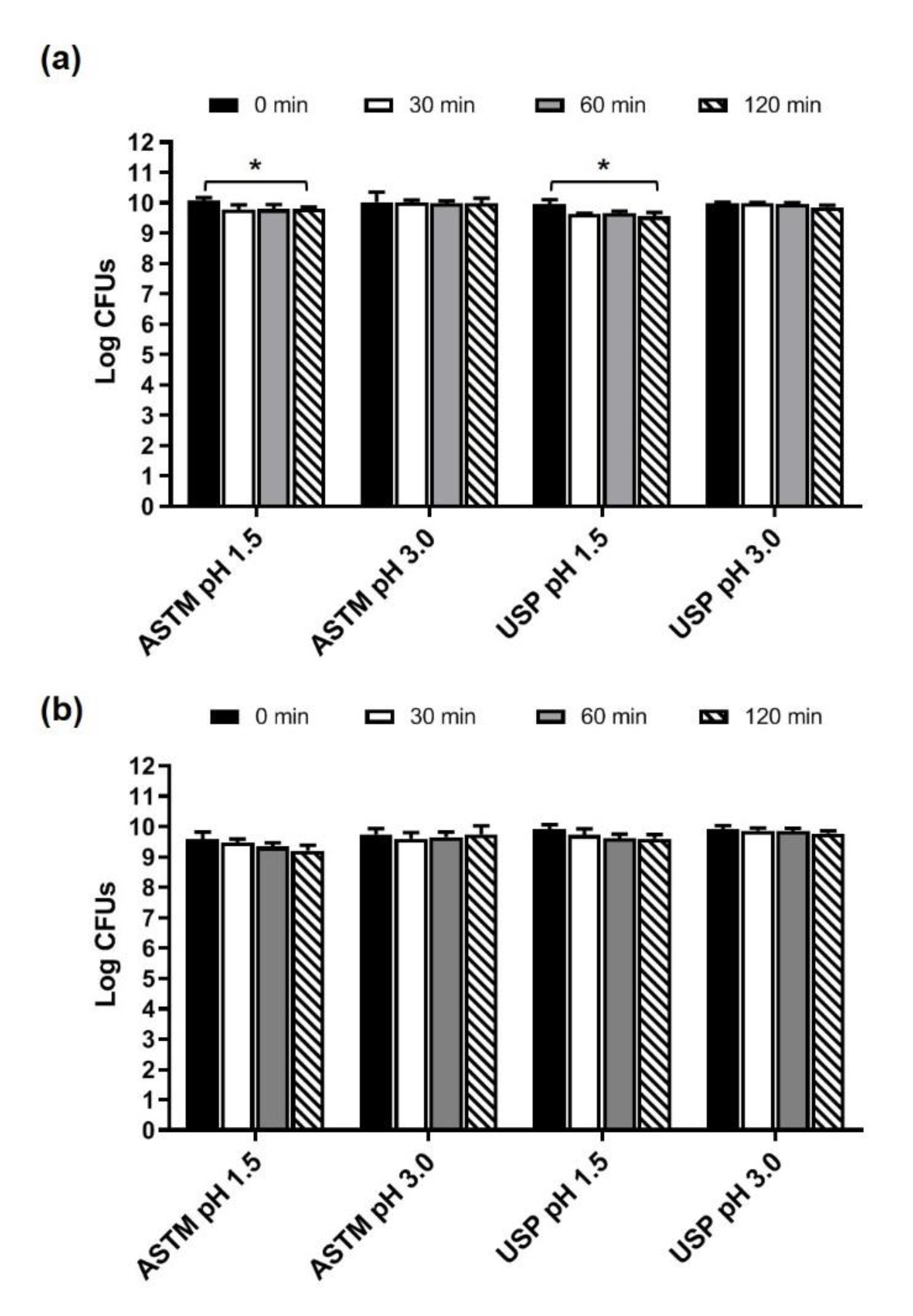
3.2. Survival of Microbes Contained in Microbiosys and in Enterogermina Viaggi in Simulated Gastric Juices
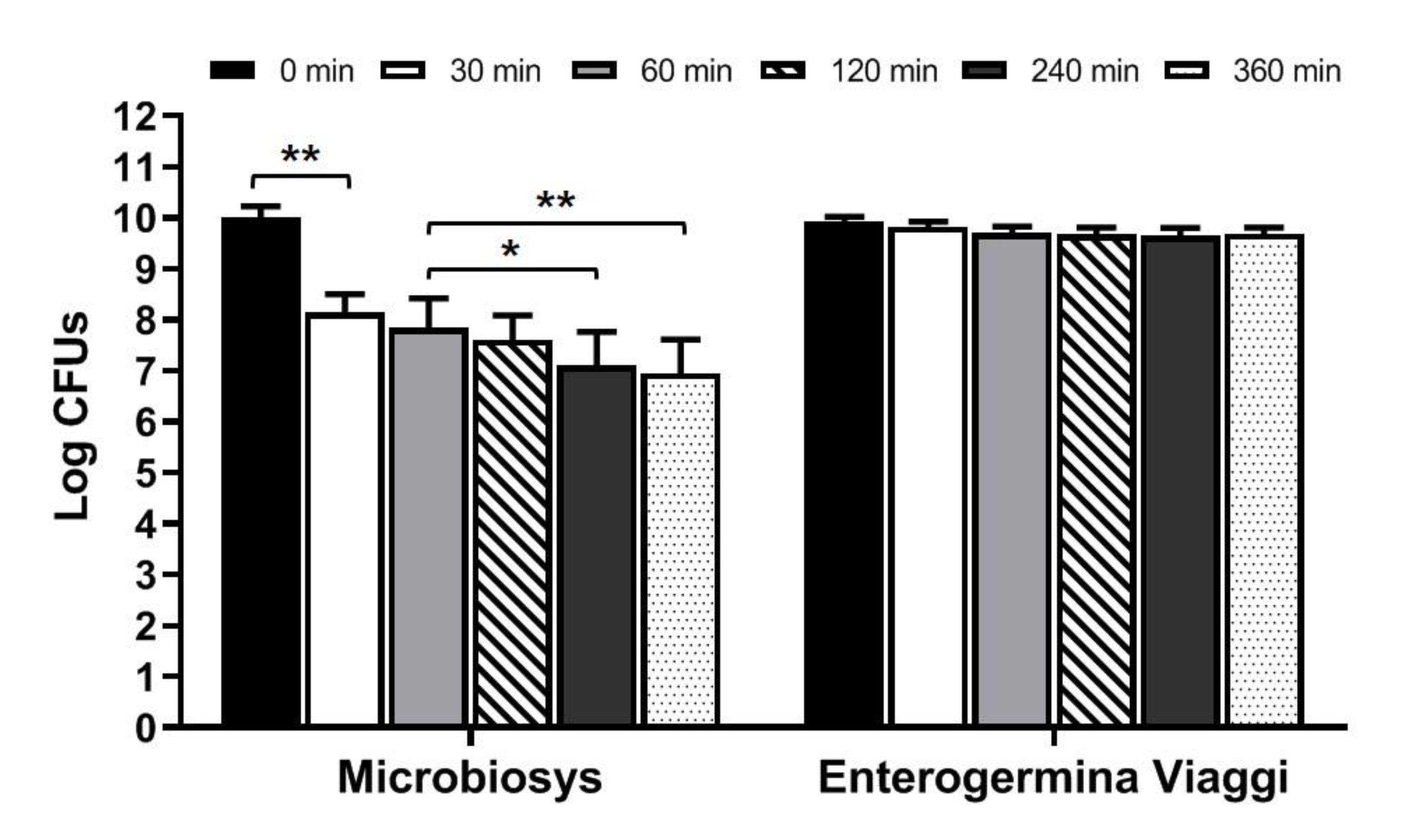
3.3. Behavior of Microbes Contained in Microbiosys and in Enterogermina Viaggi in Simulated Intestinal Fluid
3.4. Behavior of Microbiosys Intact Capsules in Simulated Gastrointestinal Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J. Expert consensus document. The international scientific association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, E.J.; Citron, D.M.; Claros, M.C.; Tyrrell, K.L. Bacterial counts from five over-the-counter probiotics: Are you getting what you paid for? Anaerobe 2014, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fredua-Agyeman, M.; Parab, S.; Gaisford, S. Evaluation of commercial probiotic products. Br. J. Pharm. 2016, 1, 84–89. [Google Scholar] [CrossRef]
- Ansari, J.M.; Colasacco, C.; Emmanouil, E.; Kohlhepp, S.; Harriott, O. Strain-level diversity of commercial probiotic isolates of Bacillus, Lactobacillus, and Saccharomyces species illustrated by molecular identification and phenotypic profiling. PLoS ONE 2019, 14, e0213841. [Google Scholar] [CrossRef]
- Lugli, G.A.; Mangifesta, M.; Mancabelli, L.; Milani, C.; Turroni, F.; Viappiani, A.; van Sinderen, D.; Ventura, M. Compositional assessment of bacterial communities in probiotic supplements by means of metagenomic techniques. Int. J. Food Microbiol. 2019, 294, 1–9. [Google Scholar] [CrossRef]
- Zimmer, C.; Dorea, C. Enumeration of Escherichia coli in probiotic products. Microorganisms 2019, 7, 437. [Google Scholar] [CrossRef] [Green Version]
- Dioso, C.M.; Vital, P.; Arellano, K.; Park, H.; Todorov, S.D.; Ji, Y.; Holzapfel, W. Do Your Kids Get What You Paid for? Evaluation of Commercially Available Probiotic Products Intended for Children in the Republic of the Philippines and the Republic of Korea. Foods 2020, 9, 1229. [Google Scholar] [CrossRef]
- Mazzantini, D.; Calvigioni, M.; Celandroni, F.; Lupetti, A.; Ghelardi, E. Spotlight on the Compositional Quality of Probiotic Formulations Marketed Worldwide. Front. Microbiol. 2021, 12, 693973. [Google Scholar] [CrossRef]
- Kolaček, S.; Hojsak, I.; Berni Canani, R.; Guarino, A.; Indrio, F.; Orel, R.; Pot, B.; Shamir, R.; Szajewska, H.; Vandenplas, Y.; et al. ESPGHAN Working Group for probiotics and prebiotics. Commercial probiotic products: A call for improved quality control. a position paper by the ESPGHAN Working Group for probiotics and prebiotics. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 117–124. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 27 September 2021).
- Council for Responsible Nutrition and International Probiotics Association. Best Practices Guidelines for Probiotics. 2017. Available online: https://www.crnusa.org/sites/default/files/pdfs/CRN-IPA-Best-Practices-Guidelines-for-Probiotics.pdf (accessed on 17 September 2021).
- Ouwehand, A.C. A review of dose-responses of probiotics in human studies. Benef. Microbes 2017, 8, 143–151. [Google Scholar] [CrossRef]
- Ministero della Salute. Direzione Generale per L’igiene e la Sicurezza degli Alimenti e la Nutrizione—Ufficio 4. Guidelines on Probiotics and Prebiotics. 2018. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1016_allegato.pdf (accessed on 17 September 2021).
- Vitetta, L.; Coulson, S.; Thomsen, M.; Nguyen, T.; Hall, S. Probiotics, D-Lactic acidosis, oxidative stress and strain specificity. Gut Microbes 2017, 8, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Fusco, V.; Fanelli, F.; Chieffi, D. Authenticity of probiotic foods and dietary supplements: A pivotal issue to address. Crit. Rev. Food Sci. Nutr. 2021, 5, 1–18. [Google Scholar] [CrossRef]
- FAO/WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. 2001. Available online: http://www.fao.org/3/a0512e/a0512e.pdf (accessed on 27 September 2021).
- Jackson, S.A.; Schoeni, J.L.; Vegge, C.; Pane, M.; Stahl, B.; Bradley, M.; Goldman, V.S.; Burguière, P.; Atwater, J.B.; Sanders, M.E. Improving End-User Trust in the Quality of Commercial Probiotic Products. Front. Microbiol. 2019, 10, 739. [Google Scholar] [CrossRef] [Green Version]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Tuomola, E.; Crittenden, R.; Playne, M.; Isolauri, E.; Salminen, S. Quality assurance criteria for probiotic bacteria. Am. J. Clin. Nutr. 2001, 73, 393S–398S. [Google Scholar] [CrossRef]
- Šipailienė, A.; Petraitytė, S. Encapsulation of Probiotics: Proper Selection of the Probiotic Strain and the Influence of Encapsulation Technology and Materials on the Viability of Encapsulated Microorganisms. Probiotics Antimicrob. Proteins 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Tavanti, A.; Hensgens, L.A.; Ghelardi, E.; Campa, M.; Senesi, S. Genotyping of Candida orthopsilosis clinical isolates by amplification fragment length polymorphism reveals genetic diversity among independent isolates and strain maintenance within patients. J. Clin. Microbiol. 2007, 45, 1455–1462. [Google Scholar] [CrossRef] [Green Version]
- American Society of Testing Materials. D5517-03: Standard Test Method for Determining Extractability of Metals from Art Materials. 2003. Available online: www.astm.org (accessed on 17 September 2021).
- USP. Pharmacopeia and National Formulary; United States Pharmacopeia Convention Inc.: Rockville, MD, USA, 2003. [Google Scholar]
- Musikasang, H.; Tani, A.; H-kittikun, A.; Maneerat, S. Probiotic potential of lactic acid bacteria isolated from chicken gastrointestinal digestive tract. World J. Microbiol. Biotechnol. 2009, 25, 1337–1345. [Google Scholar] [CrossRef]
- Tokatli, M.; Gülgör, G.; Elmacı, S.B.; İşleyen, N.A.; Özçelik, F. In Vitro properties of potential probiotic indigenous lactic acid bacteria originating from traditional pickles. Biomed Res. Int. 2015, 2015, 315819. [Google Scholar] [CrossRef] [Green Version]
- Vecchione, A.; Celandroni, F.; Mazzantini, D.; Senesi, S.; Lupetti, A.; Ghelardi, E. Compositional Quality and Potential Gastrointestinal Behavior of Probiotic Products Commercialized in Italy. Front. Med. 2018, 5, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taipale, T.J.; Pienihäkkinen, K.; Isolauri, E.; Jokela, J.T.; Söderling, E.M. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatr. Res. 2016, 79, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jebava, I.; Chuat, V.; Lortal, S.; Valence, F. Peptidoglycan hydrolases as species-specific markers to differentiate Lactobacillus helveticus from Lactobacillus gallinarum and other closely related homofermentative lactobacilli. Curr. Microbiol. 2014, 68, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulero-Cerezo, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Saccharomyces Cerevisiae Var. Boulardii: Valuable Probiotic Starter for Craft Beer Production. Appl. Sci. 2019, 9, 3250. [Google Scholar] [CrossRef] [Green Version]
- Borneman, A.R.; Pretorius, I.S. Genomic insights into the Saccharomyces sensu stricto complex. Genetics 2015, 199, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C. Enumeration of probiotic strains: Review of culture-dependent and alternative techniques to quantify viable bacteria. J. Microbiol. Methods 2014, 103, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, S.J.Z.; Tang, P.; Kiefer, A.; Galles, K.; Wong, C.; Morovic, W. Droplet Digital PCR Is an Improved Alternative Method for High-Quality Enumeration of Viable Probiotic Strains. Front. Microbiol. 2020, 10, 3025. [Google Scholar] [CrossRef]
- Michelutti, L.; Bulfoni, M.; Nencioni, E. A novel pharmaceutical approach for the analytical validation of probiotic bacterial count by flow cytometry. J. Microbiol. Methods 2020, 170, 105834. [Google Scholar] [CrossRef]
- International Standards Organisation [ISO]. Milk Products—Enumeration of Presumptive Lactobacillus Acidophilus on a Selective Medium—Colony-Count Technique at 37 Degrees C. ISO 20128:2006 (IDF 192:2006); International Standards Organisation: Geneva, Switzerland, 2006. [Google Scholar]
- International Standards Organisation [ISO]. Milk Products—Enumeration of Presumptive Bifidobacteria—Colony Count Technique at 37 Degrees C. ISO 29981:2010 (IDF 220:2010); International Standards Organisation: Geneva, Switzerland, 2010. [Google Scholar]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Grumet, L.; Tromp, Y.; Stiegelbauer, V. The Development of High-Quality Multispecies Probiotic Formulations: From Bench to Market. Nutrients 2020, 12, 2453. [Google Scholar] [CrossRef]
- Angelakis, E.; Million, M.; Henry, M.; Raoult, D. Rapid and accurate bacterial identification in probiotics and yoghurts by MALDI-TOF mass spectrometry. J. Food Sci. 2011, 76, M568–M572. [Google Scholar] [CrossRef]
- Patro, J.N.; Ramachandran, P.; Barnaba, T.; Mammel, M.K.; Lewis, J.L.; Elkins, C.A. Culture- independent metagenomic surveillance of commercially available probiotics with high-throughput next-generation sequencing. mSphere 2016, 1, e00057-16. [Google Scholar] [CrossRef] [Green Version]
- Celandroni, F.; Vecchione, A.; Cara, A.; Mazzantini, D.; Lupetti, A.; Ghelardi, E. Identification of Bacillus species: Implication on the quality of probiotic formulations. PLoS ONE 2019, 14, e0217021. [Google Scholar] [CrossRef]
- Mora, D.; Filardi, R.; Arioli, S.; Boeren, S.; Aalvink, S.; de Vos, W.M. Development of omics-based protocols for the microbiological characterization of multi-strain formulations marketed as probiotics: The case of VSL#3. Microb. Biotechnol. 2019, 12, 1371–1386. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.; Raza, A.; Ye, L.; Yu, Z. Viability and Composition Validation of Commercial Probiotic Products by Selective Culturing Combined with Next-Generation Sequencing. Microorganisms 2019, 7, 188. [Google Scholar] [CrossRef] [Green Version]
- Kesavelu, D., Sr.; Rohit, A.; Karunasagar, I.; Karunasagar, I. Composition and Laboratory Correlation of Commercial Probiotics in India. Cureus 2020, 12, e11334. [Google Scholar] [CrossRef]
- Celandroni, F.; Salvetti, S.; Gueye, S.A.; Mazzantini, D.; Lupetti, A.; Senesi, S.; Ghelardi, E. Identification and Pathogenic Potential of Clinical Bacillus and Paenibacillus Isolates. PLoS ONE 2016, 11, e0152831. [Google Scholar] [CrossRef]
- Mohar Lorbeg, P.; Golob, M.; Kramer, M.; Treven, P.; Bogovič Matijašić, B. Evaluation of Dietary Supplements Containing Viable Bacteria by Cultivation/MALDI-TOF Mass Spectrometry and PCR Identification. Front. Microbiol. 2021, 12, 700138. [Google Scholar] [CrossRef]
- Gao, B.; Chi, L.; Zhu, Y.; Shi, X.; Tu, P.; Li, B.; Yin, J.; Gao, N.; Shen, W.; Schnabl, B. An Introduction to Next Generation Sequencing Bioinformatic Analysis in Gut Microbiome Studies. Biomolecules 2021, 11, 530. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [Green Version]
- Fietto, J.L.; Araújo, R.S.; Valadão, F.N.; Fietto, L.G.; Brandão, R.L.; Neves, M.J.; Gomes, F.C.; Nicoli, J.R.; Castro, I.M. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can. J. Microbiol. 2004, 50, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.N.; Afrin, S.; Humayun, S.; Ahmed, M.M.; Saha, B.K. Identification and growth characterization of a novel strain of Saccharomyces boulardii isolated from soya paste. Front. Nutr. 2020, 7, 27. [Google Scholar] [CrossRef]
- De Vecchi, E.; Nicola, L.; Zanini, S.; Drago, L. In Vitro screening of probiotic characteristics of some Italian products. J. Chemother. 2008, 20, 341–347. [Google Scholar] [CrossRef]
- Grimoud, J.; Durand, H.; Courtin, C.; Monsan, P.; Ouarné, F.; Theodorou, V.; Roques, C. In Vitro screening of probiotic lactic acid bacteria and prebiotic glucooligosaccharides to select effective synbiotics. Anaerobe 2010, 16, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Crittenden, R.G.; Morris, L.F.; Harvey, M.L.; Tran, L.T.; Mitchell, H.L.; Playne, M.J. Selection of a Bifidobacterium strain to complement resistant starch in a synbiotic yoghurt. J. Appl. Microbiol. 2001, 90, 268–278. [Google Scholar] [CrossRef]
| Formulation | Form | Claimed CFUs | Total CFUs (Mean ± S.D.) |
|---|---|---|---|
| Microbiosys | Capsule | 5 × 109 | 2.86 ± 2.28 × 1010 |
| Enterogermina Viaggi | Sachet | 6 × 109 | 1.22 ± 0.82 × 1010 |
| Product | Claimed Species | MALDI-TOF | Metagenomic |
|---|---|---|---|
| Microbiosys | Lactobacillus rhamnosus Rosell®-11 1 Lactobacillus rhamnosus GG 1 | L. rhamnosus | L. rhamnosus |
| Lactobacillus helveticus Rosell®-52 | L. helveticus | L.gallinarum | |
| Bifidobacterium animalis subsp. lactis LAFTI® B94 | N.I. 2 | B. animalis | |
| Bifidobacterium bifidum HA-132 | N.I. 2 | B. bifidum | |
| Enterogermina Viaggi | Saccharomyces boulardii | S. cerevisiae | S. cariocanus |
| Juice | Inoculum | 30 Min | 60 Min | 120 Min |
|---|---|---|---|---|
| ASTM pH 1.5 | 10.330 ± 0.304 1 | 9.190 ± 0.117 1 88.97 2 | 8.890 ± 0.288 1 86.07 2 | 9.293 ± 0.223 1 88.27 2 |
| ASTM pH 3.0 | 10.330 ± 0.304 1 | 8.540 ± 0.368 1 82.68 2 | 8.974 ± 0.267 1 86.87 2 | 9.295 ± 0.095 1 89.98 2 |
| USP pH 1.5 | 10.330 ± 0.304 1 | 8.287 ± 0.360 1 80.67 2 | 8.463 ± 0.391 1 83.84 2 | 9.102 ± 0.257 1 89.78 2 |
| USP pH 3.0 | 10.330 ± 0.304 1 | 8.204 ± 0.698 1 79.42 2 | 9.192 ± 0.122 1 88.98 2 | 9.423 ± 0.08 1 91.22 2 |
| Log CFUs 1 | Release Rate (%) | |
|---|---|---|
| Inoculum | 10.330 ± 0.304 | N.A. 2 |
| 30 min | 7.367 ± 0.156 | 71.32 |
| 60 min | 8.110 ± 0.671 | 78.51 |
| 120 min | 8.280 ± 0.144 | 80.16 |
| 240 min | 8.645 ± 0.145 | 83.70 |
| 360 min | 8.137 ± 0.121 | 78.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzantini, D.; Celandroni, F.; Calvigioni, M.; Panattoni, A.; Labella, R.; Ghelardi, E. Microbiological Quality and Resistance to an Artificial Gut Environment of Two Probiotic Formulations. Foods 2021, 10, 2781. https://doi.org/10.3390/foods10112781
Mazzantini D, Celandroni F, Calvigioni M, Panattoni A, Labella R, Ghelardi E. Microbiological Quality and Resistance to an Artificial Gut Environment of Two Probiotic Formulations. Foods. 2021; 10(11):2781. https://doi.org/10.3390/foods10112781
Chicago/Turabian StyleMazzantini, Diletta, Francesco Celandroni, Marco Calvigioni, Adelaide Panattoni, Roberto Labella, and Emilia Ghelardi. 2021. "Microbiological Quality and Resistance to an Artificial Gut Environment of Two Probiotic Formulations" Foods 10, no. 11: 2781. https://doi.org/10.3390/foods10112781






