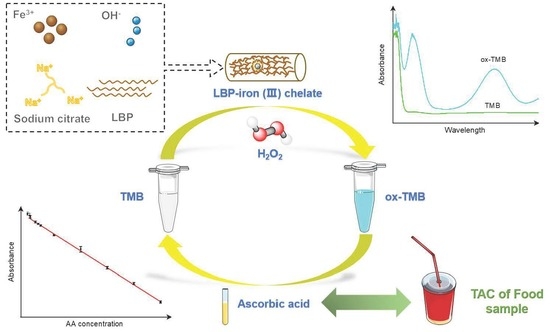
Lycium Barbarum Polysaccharide-Iron (III) Chelate as Peroxidase Mimics for Total Antioxidant Capacity Assay of Fruit and Vegetable Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Purification and Decoloration of Crude LBP
2.3. Synthesis of LBPIC
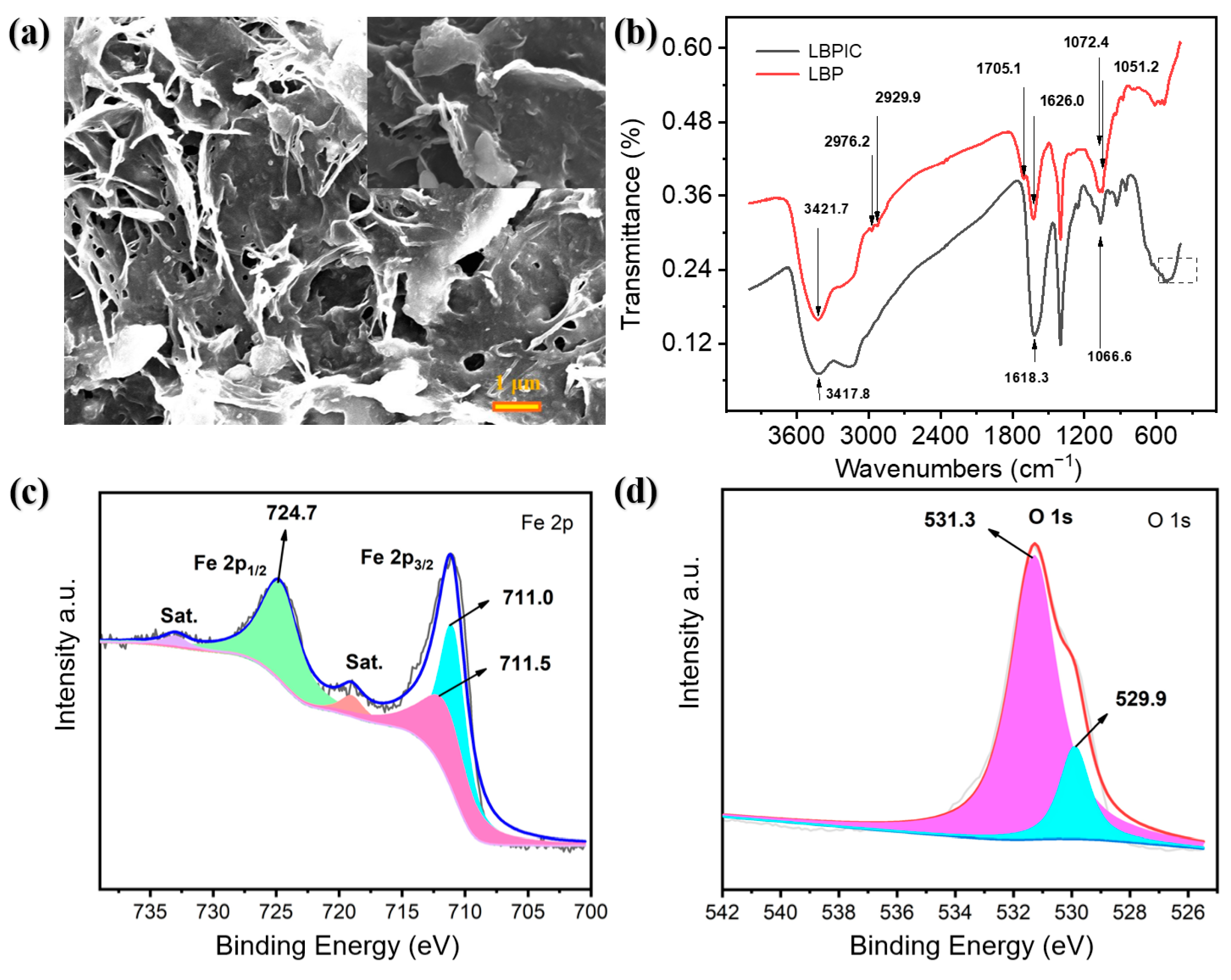
2.4. Characterization of LBPIC
2.5. Peroxidase-Like Activity of LBPIC
2.6. Enzyme Kinetics Studies of LBPIC
2.7. Detection of AA
2.8. Selectivity Test of the AA Detection Assay
2.9. TAC Detection in Food Samples
3. Results and Discussion
3.1. Characterization of LBPIC
3.2. Peroxidase-Like Activity of LBPIC
3.3. Steady-State Kinetics
3.4. Colorimetric Detection of AA
3.5. TAC Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LBP | Lycium barbarum polysaccharide |
| LBPIC | Lycium barbarum polysaccharide-iron (III) chelate |
| TAC | total antioxidant capacity |
| POD | peroxidase |
| TMB | 3,3’,5,5’-tetramethyl-benzidine |
| H2O2 | hydrogen peroxide |
| AA | ascorbic acid |
| LOD | limit of detection |
| HRP | horseradish peroxidase |
| ROS | reactive oxygen species |
| OS | oxidative stress |
| EPR | electron paramagnetic resonance |
| ATR-IR | attenuated total reflectance infrared spectroscopy |
| DPPH | 2,2′-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) |
| ORAC | oxygen radical absorbance capacity |
| FC | Folin–Ciocalteu |
| MOFs | metal-organic frameworks |
| NH2-rGO | aminated reduced graphene oxide |
| CA | citric acid |
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Bio. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Antioxidants bound to an insoluble food matrix: Their analysis, regeneration behavior, and physiological importance. Compr. Rev. Food Sci. Food Saf. 2017, 16, 382–399. [Google Scholar] [CrossRef] [Green Version]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Merits and limitations. J. Sci. Food Agric. 2020, 100, 5064–5078. [Google Scholar] [CrossRef]
- Polak, J.; Bartoszek, M. The study of antioxidant capacity of varieties of nalewka, a traditional Polish fruit liqueur, using EPR, NMR and UV-vis spectroscopy. J. Food Compos. Anal. 2015, 40, 114–119. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Xu, E.B.; Long, J.; Pan, X.W.; Xu, X.M.; Jin, Z.Y.; Jiao, A.Q. Comparison between ATR-IR, Raman, concatenated ATR-IR and Raman spectroscopy for the determination of total antioxidant capacity and total phenolic content of Chinese rice wine. Food Chem. 2016, 194, 671–679. [Google Scholar] [CrossRef]
- Andrei, V.; Bunea, A.-I.; Tudorache, A.; Gáspár, S.; Vasilescu, A. Simple DPPH.-based electrochemical assay for the evaluation of the antioxidant capacity: A thorough comparison with spectrophotometric assays and evaluation with real-world samples. Electroanalysis 2014, 26, 2677–2685. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Iranifam, M.; Al Lawati, H.A.J. Monitoring the antioxidant capacity in honey and fruit juices using a microfluidic device with a NaHCO3-H2O2-Co2+ chemiluminescence reaction. Food Chem. 2019, 297, 124930. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Berglund, G.I.; Carlsson, G.H.; Smith, A.T.; Szoke, H.; Henriksen, A.; Hajdu, J. The catalytic pathway of horseradish peroxidase at high resolution. Nature 2002, 417, 463–468. [Google Scholar] [CrossRef]
- Chen, Z.W.; Yin, J.J.; Zhou, Y.T.; Zhang, Y.; Song, L.; Song, M.J.; Hu, S.L.; Gu, N. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Huang, L.; Zhang, Z.; Dong, S. One-pot synthesis of Fe3O4 nanoparticle loaded 3D porous graphene nanocomposites with enhanced nanozyme activity for glucose detection. ACS Appl. Mater. Interfaces 2017, 9, 7465–7471. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Fan, K.; Yan, X. Iron oxide nanozyme: A multifunctional enzyme mimetic for biomedical applications. Theranostics 2017, 7, 3207–3227. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liu, B.; Yuan, Q.; Liu, J. Magnetic iron oxide nanoparticle seeded growth of nucleotide coordinated polymers. ACS Appl. Mater. Interfaces 2016, 8, 15615–15622. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiao, L.; Luo, X.; Xu, W.; Wei, X.; Wang, H.; Yan, H.; Gu, W.; Xu, B.Z.; Du, D.; et al. Oxidase-like Fe-N-C single-atom nanozymes for the detection of acetylcholinesterase activity. Small 2019, 15, 1903108. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kang, Y.; Jiao, L.; Wu, Y.; Yan, H.; Li, J.; Gu, W.; Song, W.; Zhu, C. Tuning atomically dispersed Fe sites in metal-organic frameworks boosts peroxidase-like activity for sensitive biosensing. Nano-Micro Lett. 2020, 12, 184. [Google Scholar] [CrossRef]
- London, E. The molecular formula and proposed structure of the iron-dextran complex, imferon. J. Pharm. Sci. 2004, 93, 1838–1846. [Google Scholar] [CrossRef]
- Boury, B.; Plumejeau, S. Metal oxides and polysaccharides: An efficient hybrid association for materials chemistry. Green Chem. 2015, 17, 72–88. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, structural characterization, and biological functions of Lycium barbarum polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Jiao, L.; Yan, H.; Xu, W.; Wu, Y.; Wang, H.; Gu, W.; Zhu, C. Hierarchically porous S/N co-doped carbon nanozymes with enhanced peroxidase-like activity for total antioxidant capacity biosensing. Anal. Chem. 2020, 92, 13518–13524. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, W.; Liu, X.; Ren, X.; Li, M.; Sun, J.; Wang, Y.; Yue, T.; Wang, J. A sustainable and nondestructive method to high-throughput decolor Lycium barbarum L. polysaccharides by graphene-based nano-decoloration. Food Chem. 2021, 338, 127749. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Chen, C.; Fu, X. Fructus mori L. polysaccharide-iron chelates formed by self-embedding with iron (III) as the core exhibit good antioxidant activity. Food Funct. 2019, 10, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.P.; Zhao, S.; Wang, Q.; Wei, H. N-Doped carbon as peroxidase-like nanozymes for total antioxidant capacity assay. Anal. Chem. 2019, 91, 15267–15274. [Google Scholar] [CrossRef]
- Liu, T.; Liu, T.; Liu, H.; Fan, H.; Chen, B.; Wang, D.; Zhang, Y.; Sun, F. Preparation and characterization of a novel polysaccharide-iron (III) complex in Auricularia auricula potentially used as an iron supplement. BioMed Res. Int. 2019, 2019, 6416941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, X.; Gu, S.; Pan, L.; Sun, H.; Gong, E.; Zhu, Z.; Wen, T.; Daba, G.M.; Elkhateeb, W.A. Structure analysis and antioxidant activity of polysaccharide-iron (III) from Cordyceps militaris mycelia. Int. J. Biol. Macromol. 2021, 178, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.Z.; Li, Y.P.; Zhang, G.L.; Gao, Y.Q.; Ye, H.; Gao, J.; Wang, P. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohydr. Polym. 2018, 181, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xu, L.; Meng, Y.; Liu, Y.; Li, J.; Zu, Y.; Zhu, M. Preparation and characterization of a novel Astragalus membranaceus polysaccharide-iron (III) complex. Int. J. Biol. Macromol. 2016, 93, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.H.; Huang, Y.P.; He, R.X.; Zeng, X.A. Synthesis and characterization of a new soluble soybean polysaccharide-iron (III) complex using ion exchange column. Int. J. Biol. Macromol. 2018, 108, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.P.; Chen, Z.X.; Zhang, Y.; Wang, P.P.; Wang, J.H.; Dai, L.Q. Molecular weight and proposed structure of the Angelica sinensis polysaccharide-iron complex. Chin. J. Chem. 2008, 26, 1068–1074. [Google Scholar] [CrossRef]
- Ganie, S.A.; Naik, R.A.; Ali, A.; Mir, T.A.; Mazumdar, N. Preparation, characterization, release and antianemic studies of guar gum functionalized iron complexes. Int. J. Biol. Macromol. 2021, 183, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Wang, Y.; Xing, L. Synthesis and characterization of a new Inonotus obliquus polysaccharide-iron (III) complex. Int. J. Biol. Macromol. 2015, 75, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; He, X.J.; Hong, Y.K.; Ma, T.; Xu, Y.P.; Li, H.H. Chemical characterization of Lycium barbarum polysaccharides and its inhibition against liver oxidative injury of high-fat mice. Int. J. Biol. Macromol. 2010, 46, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.F.; Zhou, C.L.; Li, Y.P.; Zhang, M.H.; Tao, P.X.; Liu, Q.L.; Cui, W.Q. Flower-like FeOOH hybridized with carbon quantum dots for efficient photo-Fenton degradation of organic pollutants. Appl. Surf. Sci. 2021, 540, 148362. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Ding, M.M.; Chen, W.; Xu, H.; Shen, Z.; Lin, T.; Hu, K.; Lu, C.H.; Xie, Z.L. Novel alpha-Fe2O3/MXene nanocomposite as heterogeneous activator of peroxymonosulfate for the degradation of salicylic acid. J. Hazard. Mater. 2020, 382, 121064. [Google Scholar] [CrossRef]
- Wang, K.; Du, H.; He, S.; Liu, L.; Yang, K.; Sun, J.; Liu, Y.; Du, Z.; Xie, L.; Ai, W.; et al. Kinetically controlled, scalable synthesis of gamma-FeOOH nanosheet arrays on nickel foam toward efficient oxygen evolution: The key role of in-situ-generated gamma-NiOOH. Adv. Mater. 2021, 33, 2005587. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Zhang, Y.; Gao, K.; Dai, L.; Shen, M.; Wang, W.; Wang, J.; Ni, Y. Cellulose-based electrospun nanofiber membrane with core-sheath structure and robust photocatalytic activity for simultaneous and efficient oil emulsions separation, dye degradation and Cr(VI) reduction. Carbohydr. Polym. 2021, 258, 117676. [Google Scholar] [CrossRef]
- Li, W.F.; Ma, H.H.; Yuan, S.; Zhang, X.F. Production of Pyracantha polysaccharide-iron (III) complex and its biologic activity. Molecules 2021, 26, 1949. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Li, C.; Chen, J. Antioxidant and digestion properties of polysaccharides from Rosa roxburghii Tratt fruit and polysacchride-iron (III) complex. J. Food Process. Preserv. 2021, 45, e15617. [Google Scholar] [CrossRef]
- Luo, L.; Huang, L.; Liu, X.; Zhang, W.; Yao, X.; Dou, L.; Zhang, X.; Nian, Y.; Sun, J.; Wang, J. Mixed-valence Ce-BPyDC metal-organic framework with dual enzyme-like activities for colorimetric biosensing. Inorg. Chem. 2019, 58, 11382–11388. [Google Scholar] [CrossRef]
- Wang, L.J.; Xu, X.C.; Niu, X.H.; Pan, J.M. Colorimetric detection and membrane removal of arsenate by a multifunctional L-arginine modified FeOOH. Sep. Purif. Technol. 2021, 258, 118021. [Google Scholar] [CrossRef]
- Frey, A.; Meckelein, B.; Externest, D.; Schmidt, M.A. A stable and highly sensitive 3,3′,5,5′-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J. Immunol. Methods 2000, 233, 47–56. [Google Scholar] [CrossRef]
- Song, N.; Zhong, M.; Xu, J.; Wang, C.; Lu, X. Single-atom iron confined within polypyrrole-derived carbon nanotubes with exceptional peroxidase-like activity for total antioxidant capacity. Sens. Actuators B Chem. 2021, 351, 130969. [Google Scholar] [CrossRef]
- Markowski, J.; Zbrzezniak, M.; Mieszczakowska-Frac, M.; Rutkowski, K.; Popinska, W. Effect of cultivar and fruit storage on basic composition of clear and cloudy pear juices. LWT Food Sci. Technol. 2012, 49, 263–266. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Feng, J.; Liang, Y.; Sun, H.; Yang, X.; Su, Z.; Luo, L.; Wang, J.; Zhang, W. Lycium Barbarum Polysaccharide-Iron (III) Chelate as Peroxidase Mimics for Total Antioxidant Capacity Assay of Fruit and Vegetable Food. Foods 2021, 10, 2800. https://doi.org/10.3390/foods10112800
Shi S, Feng J, Liang Y, Sun H, Yang X, Su Z, Luo L, Wang J, Zhang W. Lycium Barbarum Polysaccharide-Iron (III) Chelate as Peroxidase Mimics for Total Antioxidant Capacity Assay of Fruit and Vegetable Food. Foods. 2021; 10(11):2800. https://doi.org/10.3390/foods10112800
Chicago/Turabian StyleShi, Shuo, Jianxing Feng, Yanmin Liang, Hao Sun, Xuewei Yang, Zehui Su, Linpin Luo, Jianlong Wang, and Wentao Zhang. 2021. "Lycium Barbarum Polysaccharide-Iron (III) Chelate as Peroxidase Mimics for Total Antioxidant Capacity Assay of Fruit and Vegetable Food" Foods 10, no. 11: 2800. https://doi.org/10.3390/foods10112800