Influence of Fresh Palm Fruit Sterilization in the Production of Carotenoid-Rich Virgin Palm Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Cold-Press Extraction Process
2.3. Design of Experiment for the Sterilization Oil Palm Fruits
2.4. Analysis of VPO and CPO
2.5. Statistical Analyses
3. Results and Discussion
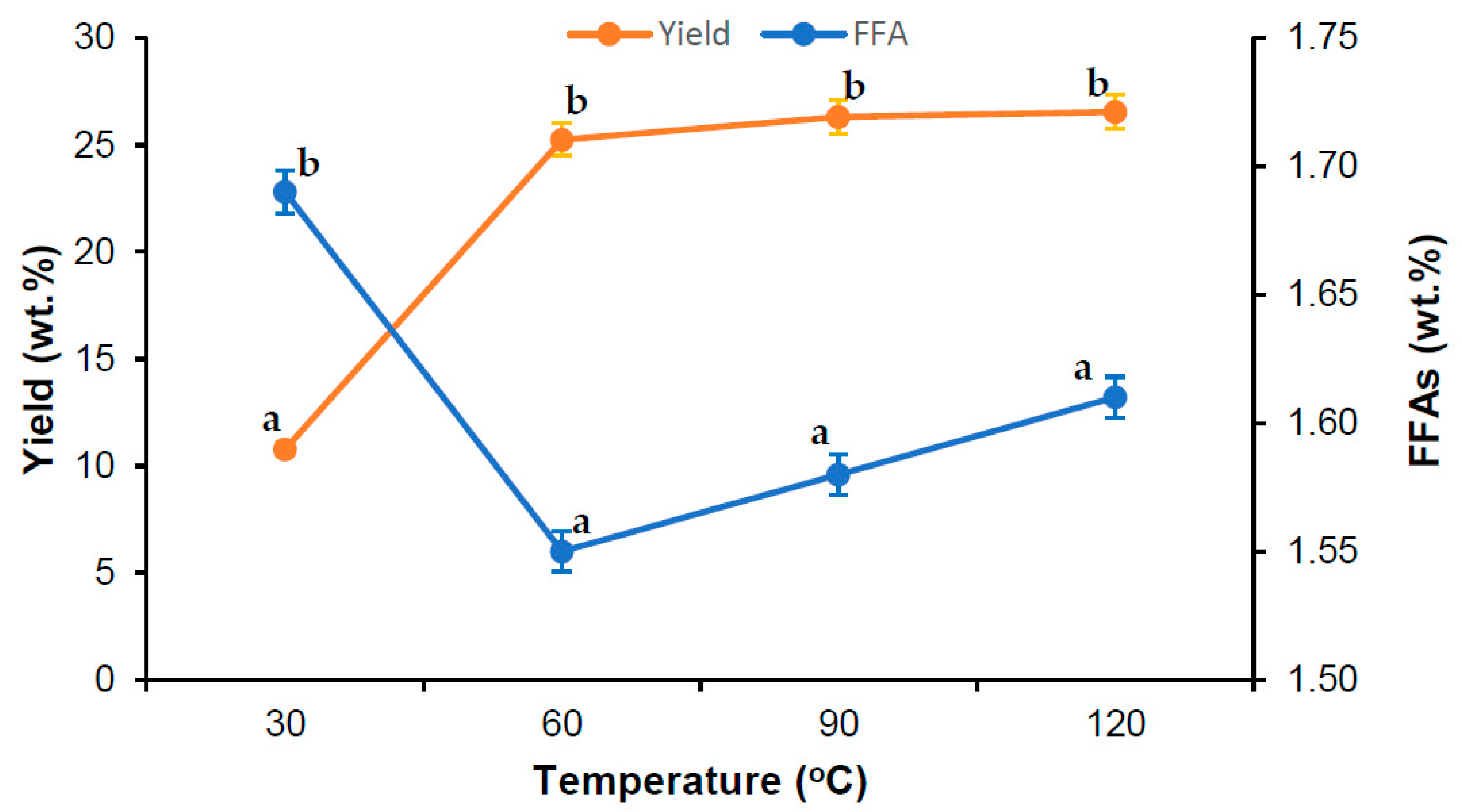
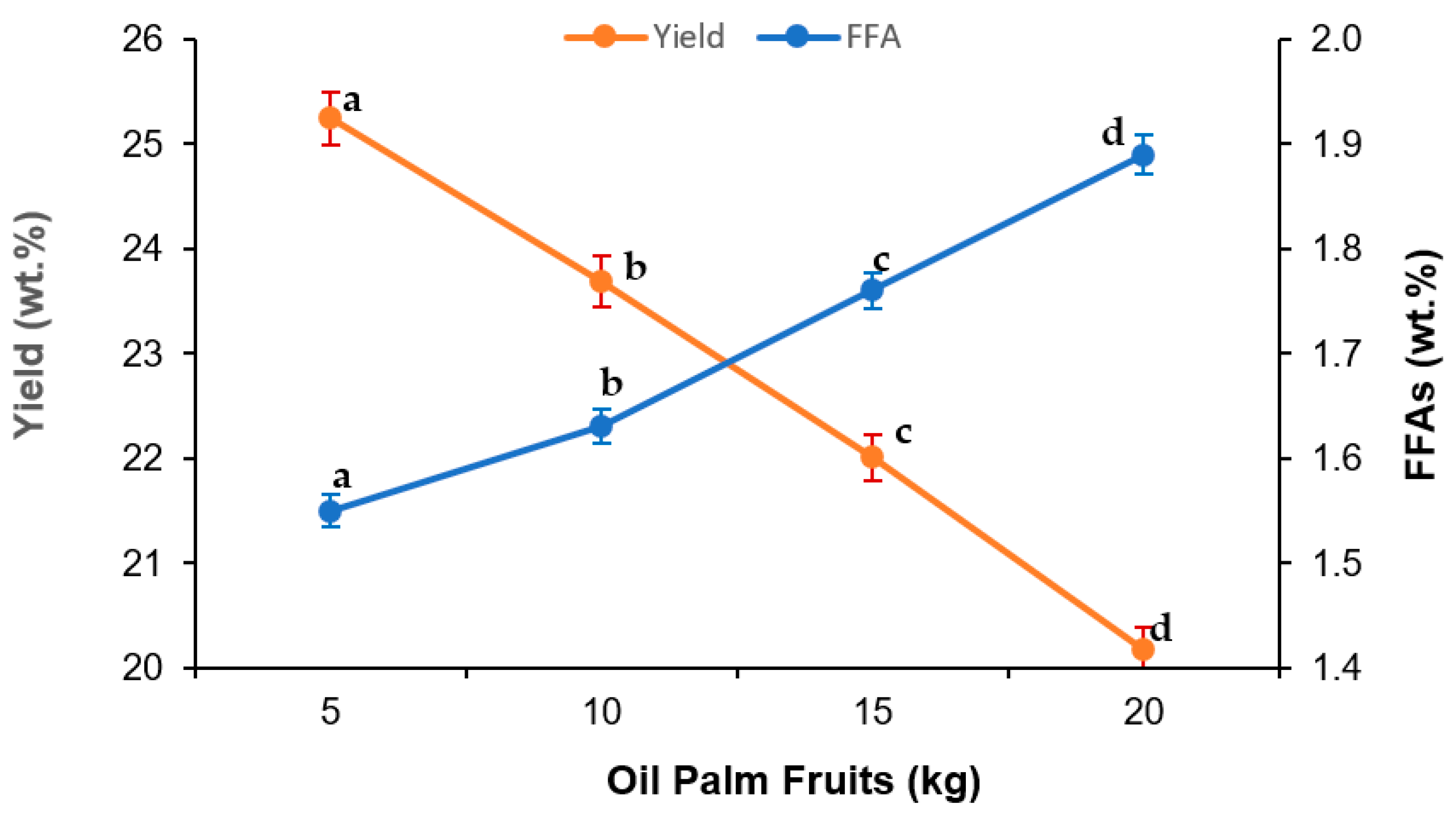
3.1. Effect of Sterilization Parameters in VPO Extraction
3.2. Regression Model
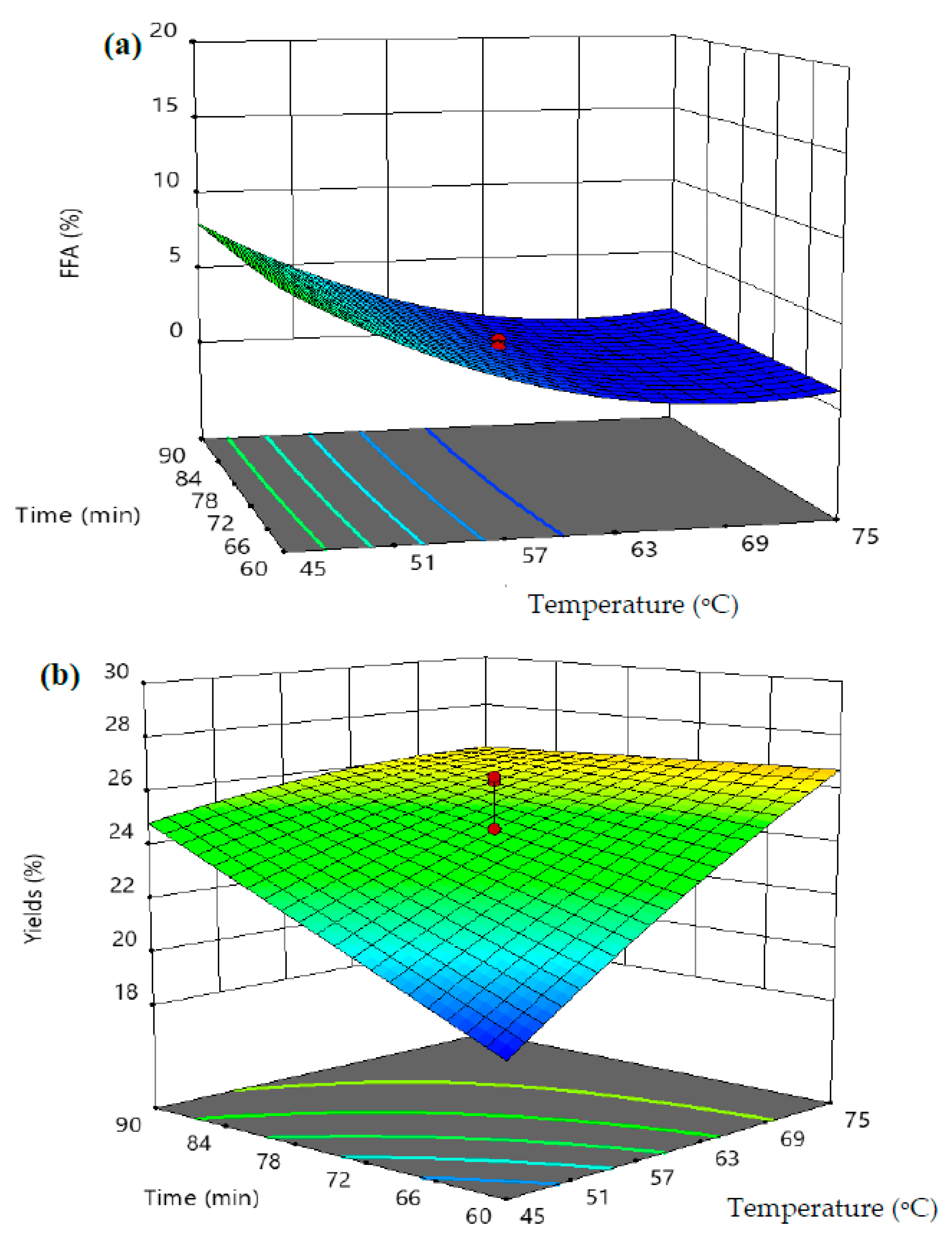
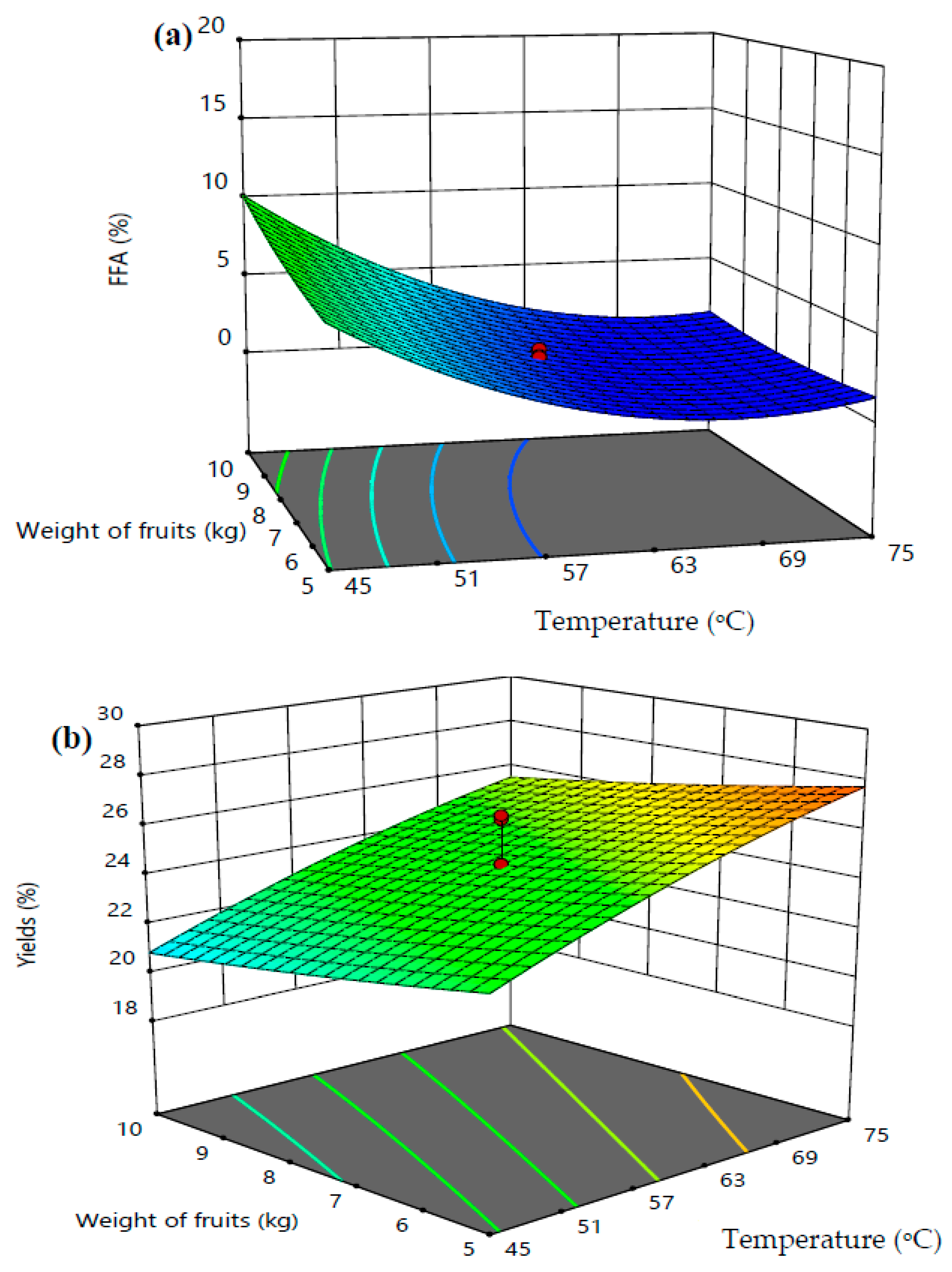
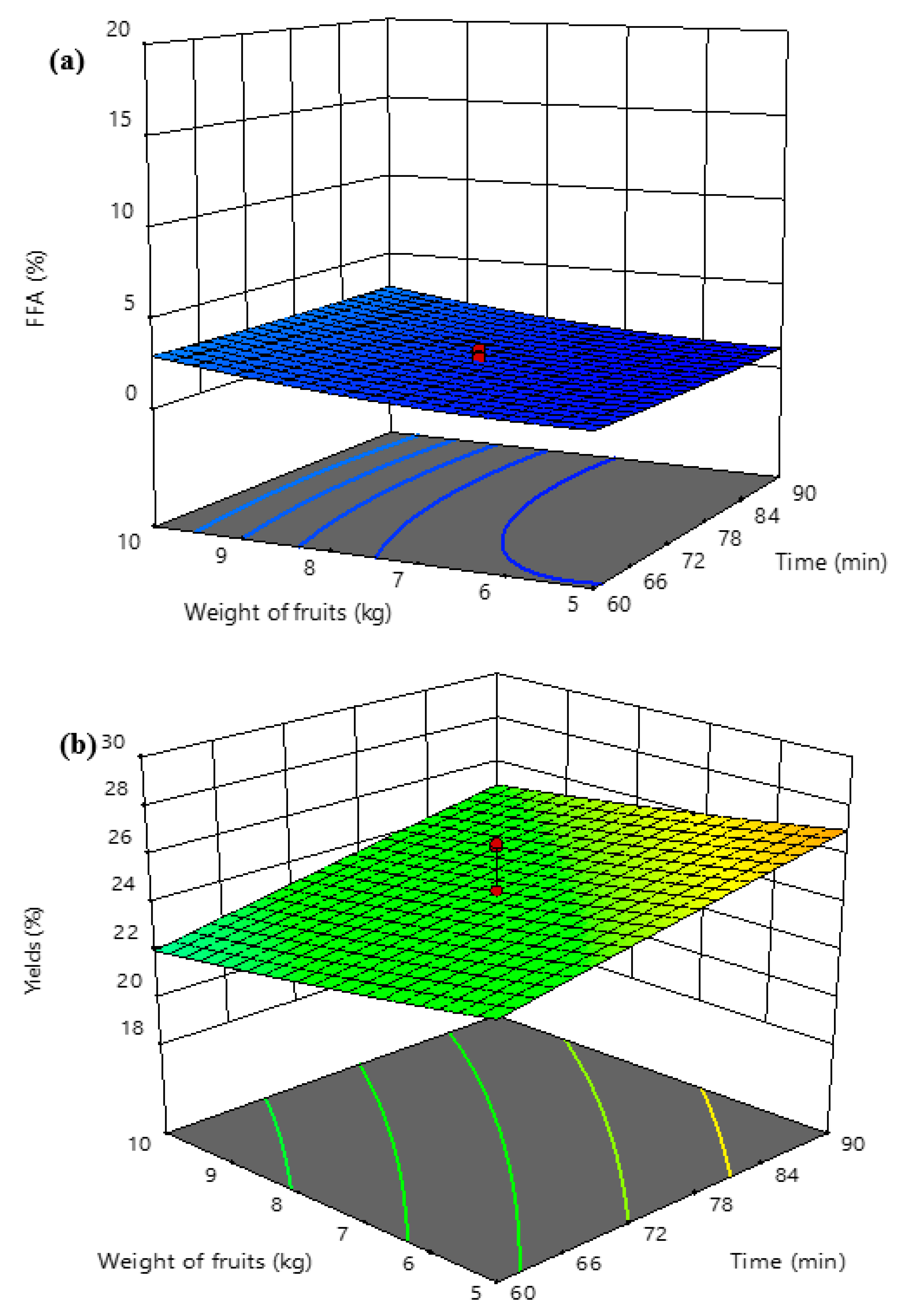
3.3. Response Surface Analyses
3.4. Physicochemical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusoff, M.N.A.M.; Zulkifli, N.W.M.; Sukiman, N.L.; Chyuan, O.H.; Hassan, M.H.; Hasnul, M.H.; Zulkifli, M.S.A.; Abbas, M.M.; Zakaria, M.Z. Sustainability of Palm Biodiesel in Transportation: A Review on Biofuel Standard, Policy and International Collabration Between Malaysia and Colombia. BioEnergy Res. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Foong, S.Z.Y.; Lam, Y.L.; Andiappan, V.; Foo, D.C.Y.; Ng, D.K.S. A Systematic Approach for the Synthesis and Optimization of Palm Oil Milling Processes. Ind. Eng. Chem. Res. 2018, 57, 2945–2955. [Google Scholar] [CrossRef]
- Mohd Omar, A.K.; Tengku Norsalwani, T.L.; Asmah, M.S.; Badrulhisham, Z.Y.; Easa, A.M.; Omar, F.M.; Hossain, M.S.; Zuknik, M.H.; Nik Norulaini, N.A. Implementation of the supercritical carbon dioxide technology in oil palm fresh fruits bunch sterilization: A review. J. CO2 Util. 2018, 25, 205–215. [Google Scholar] [CrossRef]
- Mohd Omar, A.K.; Tengku Norsalwani, T.L.; Abdul Khalil, H.P.S.; Nagao, H.; Zuknik, M.H.; Sohrab Hossain, M.; Nik Norulaini, N.A. Waterless sterilization of oil palm fruitlets using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 126, 65–71. [Google Scholar] [CrossRef]
- Arris, F.A.; Thai, V.T.S.; Manan, W.N.; Sajab, M.S. A Revisit to the Formation and Mitigation of 3-Chloropropane-1,2-Diol in Palm Oil Production. Foods 2020, 9, 1769. [Google Scholar] [CrossRef]
- Abd Rashid, S.N.A.; Ab Malik, S.; Embi, K.; Mohd Ropi, N.A.; Yaakob, H.; Cheng, K.K.; Sarmidi, M.R.; Leong, H.Y. Carotenoids and antioxidant activity in virgin palm oil (VPO) produced from palm mesocarp with low heat aqueous-enzyme extraction techniques. Mater. Today Proc. 2021, 42, 148–152. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Musa Özcan, M.; Al-Juhaimi, F.Y.; Mohamed Ahmed, I.A.; Osman, M.A.; Gassem, M.A. Effect of different microwave power setting on quality of chia seed oil obtained in a cold press. Food Chem. 2019, 278, 190–196. [Google Scholar] [CrossRef]
- Östbring, K.; Malmqvist, E.; Nilsson, K.; Rosenlind, I.; Rayner, M. The Effects of Oil Extraction Methods on Recovery Yield and Emulsifying Properties of Proteins from Rapeseed Meal and Press Cake. Foods 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytaç, E. Comparison of extraction methods of virgin coconut oil: Cold press, soxhlet and supercritical fluid extraction. Sep. Sci. Technol. in press.
- Gabriel, A.A.; Nepomuceno, I.N. Thermal and ultraviolet-c inactivation of Salmonella enterica in cold-pressed virgin coconut oil. LWT 2020, 123, 109092. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Nunes, M.A.; da Silva, B.V.; Pimentel, F.B.; Costa, A.S.G.; Alvarez-Ortí, M.; Pardo, J.E.; Oliveira, M.B.P.P. Almond cold-pressed oil by-product as ingredient for cookies with potential health benefits: Chemical and sensory evaluation. Food Sci. Hum. Wellness 2019, 8, 292–298. [Google Scholar] [CrossRef]
- Hunthayung, K.; Klinkesorn, U.; Hongsprabhas, P.; Chanput, W. Controlled release and macrophage polarizing activity of cold-pressed rice bran oil in a niosome system. Food Funct. 2019, 10, 3272–3281. [Google Scholar] [CrossRef]
- Gonzalez-Diaz, A.; Pataquiva-Mateus, A.; García-Núñez, J.A. Recovery of palm phytonutrients as a potential market for the by-products generated by palm oil mills and refineries—A review. Food Biosci. 2021, 41, 100916. [Google Scholar] [CrossRef]
- Phan, V.M.; Junyusen, T.; Liplap, P.; Junyusen, P. Effects of ultrasonication and thermal cooking pretreatments on the extractability and quality of cold press extracted rice bran oil. J. Food Process. Eng. 2019, 42, e12975. [Google Scholar] [CrossRef]
- Rukunudin, I.H.; White, P.J.; Bern, C.J.; Bailey, T.B. A modified method for determining free fatty acids from small soybean oil sample sizes. J. Am. Oil Chem. Soc. 1998, 75, 563–568. [Google Scholar] [CrossRef]
- AOCS. Color: Lovibond Method Using Color Glasses Calibrated in Accordance with the Lovibond Tintometer Color Scale. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS Press: Champaign, IL, USA, 1993. [Google Scholar]
- AOCS. AOCS Official Method Cd 8b-90: Peroxide Value, Acetic Acid, Isooctane Method; AOCS Press: Champaign, IL, USA, 2017. [Google Scholar]
- Malaysian Palm oil Board (MPOB). Palm Oil Research Institute of Malaysia Test Method in 2018; Malaysian Palm oil Board: Kula Lumpur, Malaysia, 2018.
- PORAM. Handbook, Palm oil Refiners Association of Malaysia (PORAM) Standard Specifications for Processed Palm Oil; PORAM: Selangor, Malaysia, 2012. [Google Scholar]
- Abbas, S.A.; Ali, S.; Halim, S.I.M.; Fakhrul-Razi, A.; Yunus, R.; Choong, T.S.Y. Effect of thermal softening on the textural properties of palm oil fruitlets. J. Food Eng. 2006, 76, 626–631. [Google Scholar] [CrossRef]
- Norsalwani, T.L.T.; Hossain, M.S.; Omar, F.M.; Easa, A.M.; Sofian, A.M.; Kadir, M.O.A. Scale up study on the supercritical carbon dioxide sterilization of oil palm fresh fruits bunch. J. Oil Palm Res. 2020, 32, 64–74. [Google Scholar]
- Monié, A.; David, A.; Clemens, K.; Malet-Martino, M.; Balayssac, S.; Perez, E.; Franceschi, S.; Crepin, M.; Delample, M. Enzymatic hydrolysis of rapeseed oil with a non-GMO lipase: A strategy to substitute mono- and diglycerides of fatty acids and improve the softness of sponge cakes. LWT 2021, 137, 110405. [Google Scholar] [CrossRef]
- Davoudpour, Y.; Hossain, S.; Khalil, H.P.S.A.; Haafiz, M.K.M.; Ishak, Z.A.M.; Hassan, A.; Sarker, Z.I. Optimization of high pressure homogenization parameters for the isolation of cellulosic nanofibers using response surface methodology. Ind. Crop. Prod. 2015, 74, 381–387. [Google Scholar] [CrossRef]
- Hossain, M.S.; Nik Ab Rahman, N.N.; Balakrishnan, V.; Alkarkhi, A.F.M.; Ahmad Rajion, Z.; Ab Kadir, M.O. Optimizing supercritical carbon dioxide in the inactivation of bacteria in clinical solid waste by using response surface methodology. Waste Manag. 2015, 38, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Lietz, G.; Henry, C.J.K.; Mulokozi, G.; Mugyabuso, J.K.; Ballart, A.; Ndossi, G.D.; Lorri, W.; Tomkins, A. Comparison of the effects of supplemental red palm oil and sunflower oil on maternal vitamin A status. Am. J. Clin. Nutr. 2001, 74, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variable | Symbol | Factor Level | ||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | +1 | +1.682 | ||
| Temperature (°C) | X1 | 35 | 45 | 60 | 75 | 85 |
| Sterilization time (min) | X2 | 50 | 60 | 75 | 90 | 100 |
| Amount of fruits (kg) | X3 | 3.3 | 5 | 7.5 | 10 | 11.7 |
| Run | X1 | X2 | X3 | FFAs (%) | Yield (%) | ||
|---|---|---|---|---|---|---|---|
| Actual | Predicted | Actual | Predicted | ||||
| 1 | −1 | −1 | −1 | 7.25 | 7.30 | 18.81 | 19.99 |
| 2 | 1 | −1 | −1 | 1.58 | 1.60 | 27.33 | 28.04 |
| 3 | −1 | 1 | −1 | 6.74 | 6.81 | 26.32 | 25.96 |
| 4 | 1 | 1 | −1 | 1.56 | 1.45 | 25.79 | 27.42 |
| 5 | −1 | −1 | 1 | 10.31 | 10.32 | 19.35 | 17.69 |
| 6 | 1 | −1 | 1 | 1.62 | 1.44 | 25.34 | 25.67 |
| 7 | −1 | 1 | 1 | 10.23 | 10.11 | 24.69 | 23.95 |
| 8 | 1 | 1 | 1 | 1.72 | 1.57 | 26.55 | 25.34 |
| 9 | −1.682 | 0 | 0 | 15.98 | 15.93 | 18.6 | 19.52 |
| 10 | 1.682 | 0 | 0 | 3.75 | 3.95 | 28.32 | 27.45 |
| 11 | 0 | −1.682 | 0 | 2.02 | 2.03 | 22.45 | 22.10 |
| 12 | 0 | 1.682 | 0 | 1.60 | 1.73 | 26.45 | 26.84 |
| 13 | 0 | 0 | −1.682 | 1.56 | 1.49 | 28.6 | 26.71 |
| 14 | 0 | 0 | 1.682 | 3.91 | 4.13 | 21.09 | 23.03 |
| 15 | 0 | 0 | 0 | 1.58 | 1.64 | 26.35 | 24.53 |
| 16 | 0 | 0 | 0 | 1.65 | 1.64 | 24.56 | 24.53 |
| 17 | 0 | 0 | 0 | 2.18 | 2.32 | 21.65 | 24.53 |
| 18 | 0 | 0 | 0 | 1.76 | 1.64 | 26.51 | 24.53 |
| 19 | 0 | 0 | 0 | 1.68 | 1.64 | 24.55 | 24.53 |
| 20 | 0 | 0 | 0 | 1.01 | 1.64 | 23.56 | 24.53 |
| Term | Coefficient | Standard Error | T-Value | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| FFAs | Yield | FFAs | Yield | FFAs | Yield | FFAs | Yield | |
| Constant | 1.639 | 24.529 | 0.123 | 0.766 | 13.29 | 32.04 | 0.000 | 0.000 |
| X1 | −3.559 | 2.356 | 0.082 | 0.508 | −43.51 | 4.64 | 0.000 | 0.001 |
| X2 | −0.089 | 1.409 | 0.082 | 0.508 | −1.09 | 2.77 | 0.303 | 0.020 |
| X3 | 0.783 | −1.094 | 0.082 | 0.508 | 9.57 | −2.15 | 0.000 | 0.057 |
| X12 | 2.935 | −0.370 | 0.079 | 0.494 | 36.85 | −0.75 | 0.000 | 0.471 |
| X22 | 0.087 | −0.020 | 0.079 | 0.494 | 1.09 | −0.04 | 0.301 | 0.968 |
| X32 | 0.414 | 0.119 | 0.079 | 0.494 | 5.20 | 0.24 | 0.000 | 0.814 |
| X1X2 | 0.085 | −1.648 | 0.107 | 0.664 | 0.79 | −2.48 | 0.447 | 0.032 |
| X1X3 | −0.794 | −0.017 | 0.107 | 0.664 | −7.43 | −0.03 | 0.000 | 0.980 |
| X2X3 | 0.068 | 0.073 | 0.107 | 0.664 | 0.64 | 0.11 | 0.537 | 0.914 |
| Source | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| a FFAs | b Yield | FFAs | Yield | FFAs | Yield | FFAs | Yield | ||
| Model | 9 | 311.71 | 143.44 | 34.63 | 15.94 | 379.32 | 4.53 | 0.0001 | 0.0136 |
| Residual | 10 | 0.9131 | 35.22 | 0.0913 | 3.52 | ||||
| Lack of Fit | 5 | 0.2049 | 18.75 | 0.0410 | 3.75 | 0.2894 | 1.14 | 0.9001 | 0.4452 |
| Pure Error | 5 | 0.7081 | 16.47 | 0.1416 | 3.29 | ||||
| Total | 19 | 312.62 | 178.66 | ||||||
| Parameters | Optimum Value | VPO Yield (wt.%) | FFAs (wt.%) | ||
|---|---|---|---|---|---|
| Predicted | Actual | Predicted | Actual | ||
| Temperature (°C) | 62 | 28.61 | 27.94 | 1.27 | 1.32 |
| Time (min) | 90 | ||||
| Weight of Fruits (kg) | 8 | ||||
| Properties | Unit | VPO | CPO |
|---|---|---|---|
| Color | Red/Yellow | 30R/20Y | - |
| Moisture & Impurities | wt.% | 0.12 a ± 0.01 | 0.10 a ± 0.01 |
| Free fatty acids | wt.% | 1.32 a ± 0.51 | 3.56 b ± 0.14 |
| Peroxide value | meq/kg | 0.42 a ± 0.08 | 3.39 b ± 0.11 |
| Phosphorus | mg/kg | 1.92 a ± 0.19 | 2.0 a ± 0.20 |
| IV values | mg/kg | 57.17 a ± 4.58 | 52.31 a ± 2 |
| Cloud point | °C | 9.61 ± 0.84 | NA |
| Rancimat at 120 °C | 5.63 ± 0.56 | NA | |
| Carotenoids | μg/g | 708 a ± 24 | 343 b ± 12 |
| Fatty acids | |||
| Dodecanoic acid (C12:0) | % | 0.04 a ± 0.01 | 0.341 b ± 0.01 |
| Myristic (C14:0) | % | 1.19 a ± 0.11 | 1.08 a ± 0.02 |
| Palmitic (C16:0) | % | 40.82 a ± 2.14 | 43.48 a ± 1.52 |
| Palmitoleic (C16:1) | % | 0.21 a ± 0.01 | 0.118 a ± 0.11 |
| Stearic (C18:0) | % | 3.84 a ± 0.11 | 4.436 b ± 0.16 |
| Oleic (C18:1) | % | 40.95 a ± 2.43 | 40.22 b ± 1.05 |
| Linoleic (C18:2) | % | 12.01 a ± 1.42 | 9.39 b ± 0.40 |
| Linolenic (C18:3) | % | 0.37 a ± 0.02 | 0.27 a ± 0.01 |
| Arachidic (C20:0) | % | 0.62 a ± 0.02 | 0.47 a ± 0.12 |
| Saturated | % | 46.51 a ± 1.21 | 49.82 b ± 0.56 |
| Monounsaturated | % | 41.16 a ± 1.65 | 40.34 a ± 1.32 |
| Polyunsaturated | % | 12.38 a ± 1.02 | 9.66 b ± 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, N.S.M.; Hossain, M.S.; Balakrishnan, V.; Zuknik, M.H.; Mustaner, M.; Easa, A.M.; Al-Gheethi, A.; Yahaya, A.N.A. Influence of Fresh Palm Fruit Sterilization in the Production of Carotenoid-Rich Virgin Palm Oil. Foods 2021, 10, 2838. https://doi.org/10.3390/foods10112838
Hassan NSM, Hossain MS, Balakrishnan V, Zuknik MH, Mustaner M, Easa AM, Al-Gheethi A, Yahaya ANA. Influence of Fresh Palm Fruit Sterilization in the Production of Carotenoid-Rich Virgin Palm Oil. Foods. 2021; 10(11):2838. https://doi.org/10.3390/foods10112838
Chicago/Turabian StyleHassan, Nik Suhaimi Mat, Md. Sohrab Hossain, Venugopal Balakrishnan, Mark Harris Zuknik, Muliadi Mustaner, Azhar Mat Easa, Adel Al-Gheethi, and Ahmad Naim Ahmad Yahaya. 2021. "Influence of Fresh Palm Fruit Sterilization in the Production of Carotenoid-Rich Virgin Palm Oil" Foods 10, no. 11: 2838. https://doi.org/10.3390/foods10112838
APA StyleHassan, N. S. M., Hossain, M. S., Balakrishnan, V., Zuknik, M. H., Mustaner, M., Easa, A. M., Al-Gheethi, A., & Yahaya, A. N. A. (2021). Influence of Fresh Palm Fruit Sterilization in the Production of Carotenoid-Rich Virgin Palm Oil. Foods, 10(11), 2838. https://doi.org/10.3390/foods10112838










