Pharmacokinetics and Excretion Study of Lycium barbarum Polysaccharides in Rats by FITC-Fluorescence Labeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Instrumentation
2.4. Establishment of Quantitative Analysis Method for LBP-FITC
2.4.1. Fabrication of LBP-FITC
2.4.2. Preparation of LBP-FITC Standard Solution
2.5. Sample Collection
2.5.1. Urine and Fecal Samples
2.5.2. Plasma Sample
2.6. Sample Determination
2.6.1. Determination Conditions
2.6.2. Plasma Samples Determination
2.6.3. Urine Samples Determination
2.6.4. Fecal Samples Determination
2.7. Fabrication of Standard Curve
2.7.1. Plasma Samples
2.7.2. Urine Samples
2.7.3. Fecal Samples
2.8. Method Validation
2.8.1. Precision
2.8.2. Stability
2.8.3. Recovery Rate and Matrix Effect
2.9. Plasma Pharmacokinetics
2.9.1. Administration Method
2.9.2. Sampling Method
2.10. Excretion Study
2.10.1. Administration Method
2.10.2. Sample Collection and Processing
2.11. Sample Determination
2.12. Data Processing
2.12.1. Pharmacokinetic Data Processing
2.12.2. Data Processing of Excretion Experiment
3. Results and Discussion
3.1. Establishment and Validation of Quantitative Analysis Method
3.1.1. Standard Curve Establishment
3.1.2. Precision
3.1.3. Stability
3.1.4. Recovery and Matrix Effect
3.2. Pharmacokinetics Study
3.2.1. Plasma Concentration-Time Data
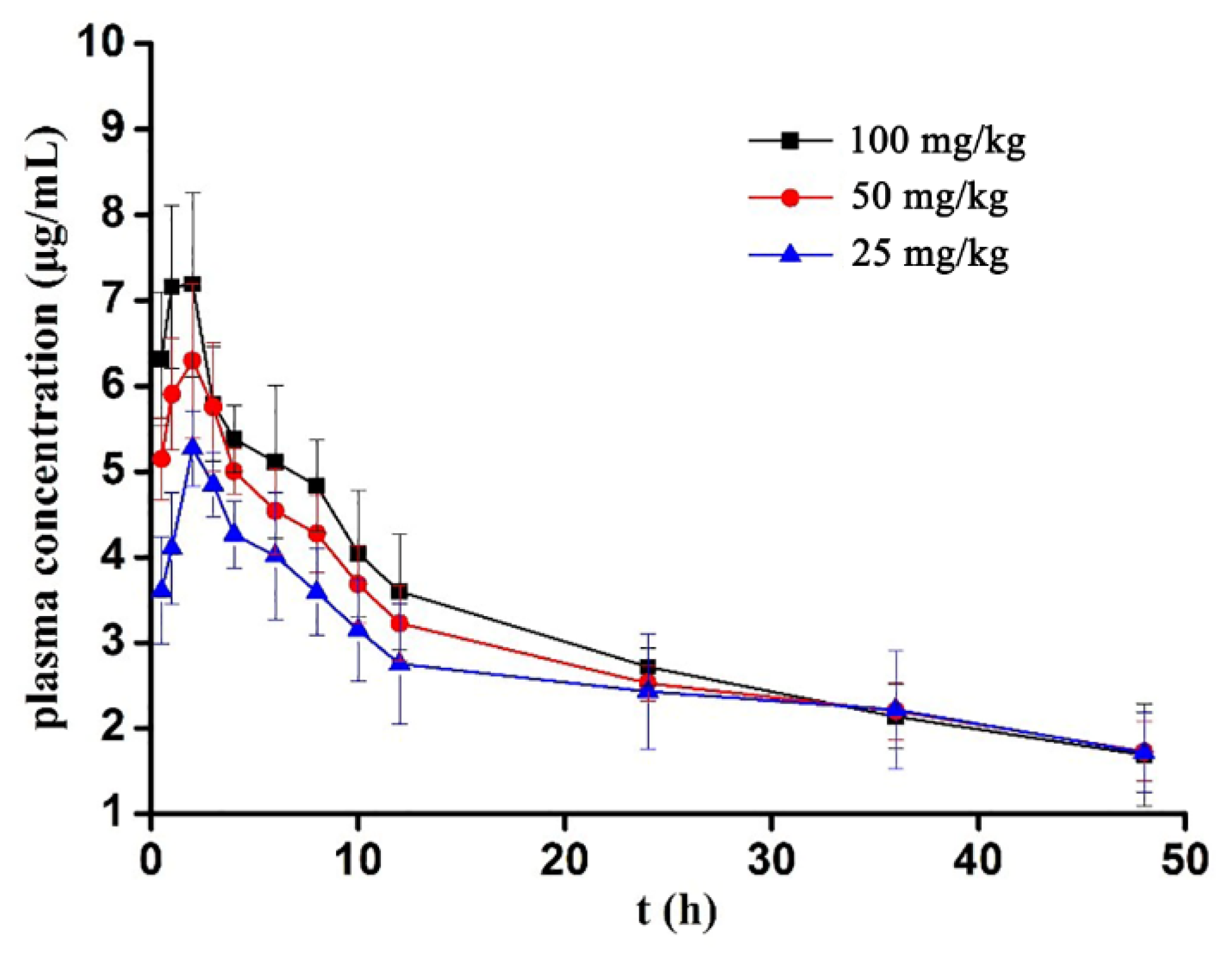
3.2.2. Mean Plasma Concentration–Time Curve
3.2.3. Pharmacokinetic Parameters
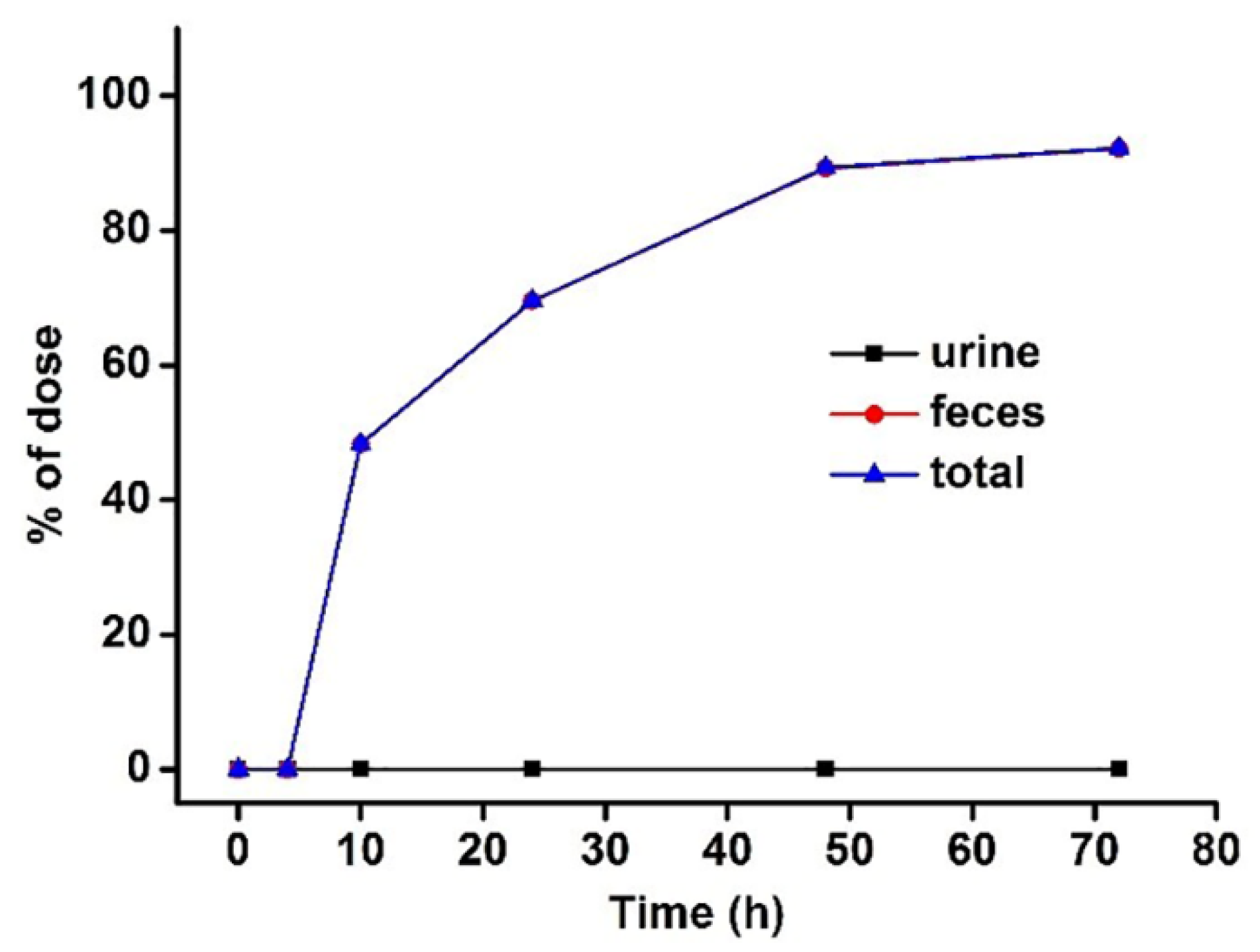
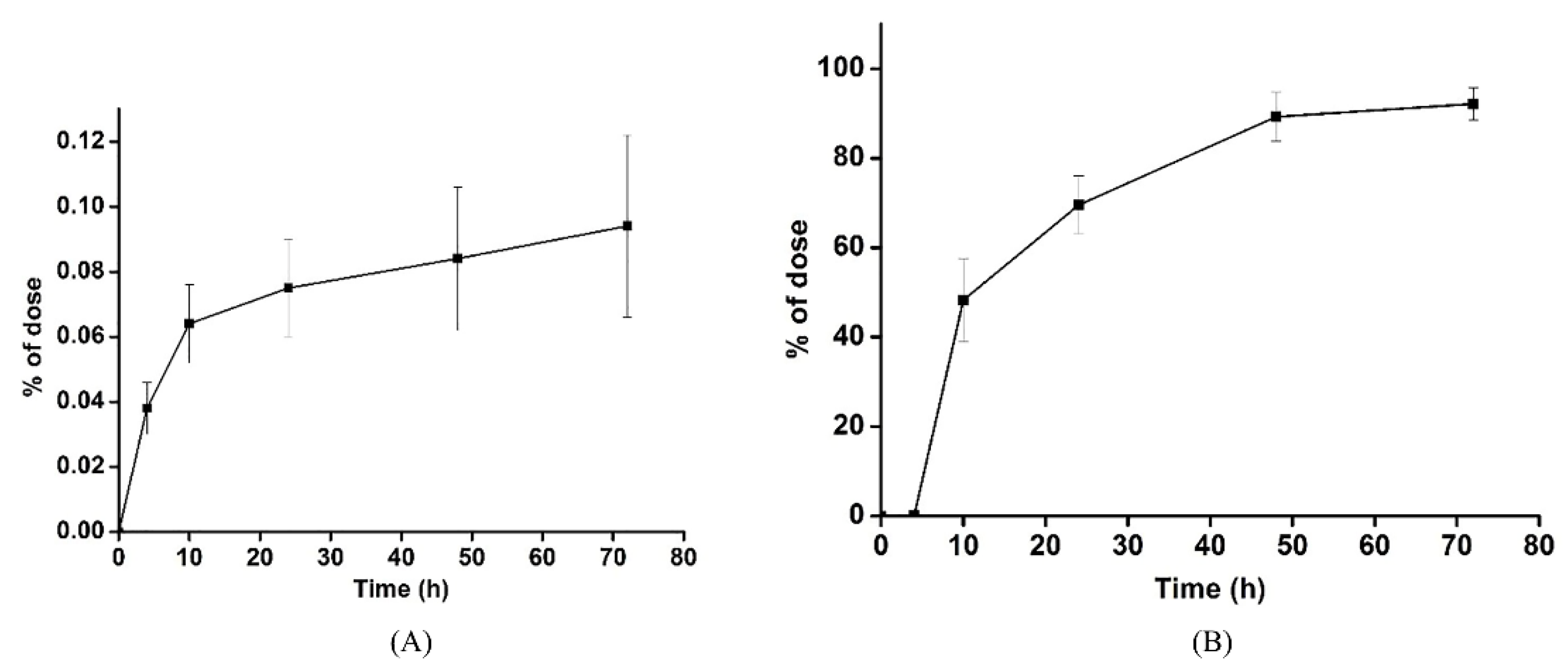
3.3. Excretion Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.-L.; Huang, M.-Y.; Yang, Y.; Huang, M.-Q.; Shi, J.-J.; Zou, L.; Lu, J.-J. Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chem. 2020, 327, 127029. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Washio, Y.; Hara, M.; Toyoda, H.; Koshiishi, I.; Toida, T.; Imanari, T. Simultaneous Determination of Dermatan Sulfate and Oversulfated Dermatan Sulfate in Plasma by High-Performance Liquid Chromatography with Postcolumn Fluorescence Derivatization. Anal. Biochem. 1996, 240, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lv, Z.; Jiang, T.; Wang, Y. Bioanalysis and pharmacokinetics of chitosan ester in rabbit serum by HPLC with postcolumn fluorescence derivatization. J. Chromatogr. B 2007, 845, 138–142. [Google Scholar] [CrossRef]
- Lin, X.; Xu, D.-S.; Feng, Y.; Shen, L. Determination of Ophiopogon japonicus polysaccharide in plasma by HPLC with modified postcolumn fluorescence derivatization. Anal. Biochem. 2005, 342, 179–185. [Google Scholar] [CrossRef]
- Li, P.-L.; Li, C.-X.; Xue, Y.-T.; Li, H.-H.; Liu, H.-B.; He, X.-X.; Yu, G.-L.; Guan, H.-S. An HPLC Method for Microanalysis and Pharmacokinetics of Marine Sulfated Polysaccharide PSS-Loaded Poly Lactic-co-Glycolic Acid (PLGA) Nanoparticles in Rat Plasma. Mar. Drugs 2013, 11, 1113–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, L.; Jin, Y.; Fu, H.; Ma, J.; Wang, X.; Jin, Y.; Wen, C. Pharmacokinetics and UPLC-MS/MS of Delsoline in Mouse Whole Blood. J. Anal. Methods Chem. 2018, 2018, 9412708. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, D.-S.; Feng, Y.; Li, S.-M.; Lu, Z.-L.; Shen, L. Release-Controlling Absorption Enhancement of Enterally Administered Ophiopogon Japonicus Polysaccharide by Sodium Caprate in Rats. J. Pharm. Sci. 2006, 95, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Z.; Sun, G.; Shen, L.; Xu, D.; Feng, Y. A sensitive and specific HPGPC-FD method for the study of pharmacokinetics and tissue distribution of Radix Ophiopogonis polysaccharide in rats. Biomed. Chromatogr. 2009, 24, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.J.; Lockhart, B.E.; Barker, L.A.; Adams, E.L.; Ensley, H.E.; Williams, D.L. Pharmacokinetics of fungal (1-3)-beta-D-glucans following intravenous administration in rats. Int. Immunopharmacol. 2004, 4, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.; Yagi, Y.; Matsunaga, K.; Ishihara, Y.; Yasuhara, T. Development of an enzyme-linked immunosorbent assay to detect an immunomodulatory alpha-D-glucan-protein complex, MPG-1, in basidiomycete Tricholoma matsutake and related processed foods. J. Agric. Food Chem. 2007, 55, 8508–8515. [Google Scholar] [CrossRef]
- Mousa, S.A.; Zhang, F.; Aljada, A.; Chaturvedi, S.; Takieddin, M.; Zhang, H.; Chi, L.; Castelli, M.C.; Friedman, K.; Goldberg, M.M.; et al. Pharmacokinetics and Pharmacodynamics of Oral Heparin Solid Dosage Form in Healthy Human Subjects. J. Clin. Pharmacol. 2007, 47, 1508–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, M.C.; Wong, D.F.; Friedman, K.; Riley, M.G.I. Pharmacokinetics of Oral Cyanocobalamin Formulated With Sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC): An Open-Label, Randomized, Single-Dose, Parallel-Group Study in Healthy Male Subjects. Clin. Ther. 2011, 33, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, J.; Zhou, Q.; Meng, D.; He, Y.; Chen, F.; Wang, S.; Ji, W. Effects of naringenin on the pharmacokinetics of tofacitinib in rats. Pharm. Biol. 2020, 58, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Zhang, E.; Hu, B.; Liang, H.; Song, S.; Ji, A. Study on Absorption Mechanism and Tissue Distribution of Fucoidan. Molecules 2020, 25, 1087. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, Y.; Guo, L.P.; Huang, L.Q.; Zhang, X.B.; Yang, J. Study on quality regionalization of Lycii Fructus. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2019, 44, 1156–1163. [Google Scholar]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef]
- Shi, G.-J.; Zheng, J.; Wu, J.; Qiao, H.-Q.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.-X.; Yu, J.-Q. Beneficial effects of Lycium barbarum polysaccharide on spermatogenesis by improving antioxidant activity and inhibiting apoptosis in streptozotocin-induced diabetic male mice. Food Funct. 2017, 8, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wei, Y.; Liao, B.; Wei, G.; Qin, H.; Pang, X.; Wang, J. Lycium barbarum polysaccharide prevents cisplatin-induced MLTC-1 cell apoptosis and autophagy via regulating endoplasmic reticulum stress pathway. Drug Des. Dev. Ther. 2018, 12, 3211–3219. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.-X.; Liu, L.; Sha, X.-Y.; Wu, Y.-N.; Chen, M.-T. Lycium barbarum polysaccharides protects retinal ganglion cells against oxidative stress injury. Neural Regen. Res. 2020, 15, 1526–1531. [Google Scholar] [CrossRef]
- Hao, W.; Wang, S.-F.; Zhao, J.; Li, S.-P. Effects of extraction methods on immunology activity and chemical profiles of Lycium barbarum polysaccharides. J. Pharm. Biomed. Anal. 2020, 185, 113219. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhang, Z.; Zhao, J.; Ma, Q.; Liu, H.; Nie, C.; Ma, Z.; An, W.; Li, J. Dietary whole Goji berry (Lycium barbarum) intake improves colonic barrier function by altering gut microbiota composition in mice. Int. J. Food Sci. Technol. 2021, 56, 103–114. [Google Scholar] [CrossRef]
- Azhdarinia, A.; Ghosh, P.; Ghosh, S.; Wilganowski, N.; Sevick-Muraca, E.M. Dual-Labeling Strategies for Nuclear and Fluorescence Molecular Imaging: A Review and Analysis. Mol. Imaging Biol. 2012, 14, 261–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L. Uptake of FITC-Chitosan Nanoparticles by A549 Cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Dey, T.; Kundu, S.C. Fabrication of sericin nanoparticles for controlled gene delivery. RSC Adv. 2014, 4, 2137–2142. [Google Scholar] [CrossRef]
- Remuñán-López, C.; Lorenzo-Lamosa, M.; Vila-Jato, J.; Alonso, M.J. Development of new chitosan–cellulose multicore microparticles for controlled drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 49–56. [Google Scholar] [CrossRef]
- Raveendran, S.; Poulose, A.C.; Yoshida, Y.; Maekawa, T.; Kumar, S. Bacterial exopolysaccharide based nanoparticles for sustained drug delivery, cancer chemotherapy and bioimaging. Carbohydr. Polym. 2013, 91, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.H.; Choi, J.H.; Been, S.Y.; Kim, N.; Choi, J.M.; Kim, W.; Kim, D.; Jung, J.J.; Song, J.E.; Khang, G. Development of fluorescein isothiocyanate conjugated gellan gum for application of bioimaging for biomedical application. Int. J. Biol. Macromol. 2020, 164, 2804–2812. [Google Scholar] [CrossRef]
- Zheng, Z.; Pan, X.; Xu, J.; Wu, Z.; Zhang, Y.; Wang, K. Advances in tracking of polysaccharides in vivo: Labeling strategies, potential factors and applications based on pharmacokinetic characteristics. Int. J. Biol. Macromol. 2020, 163, 1403–1420. [Google Scholar] [CrossRef]
- Tang, H.-L.; Chen, C.; Wang, S.-K.; Sun, G.-J. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int. J. Biol. Macromol. 2015, 77, 235–242. [Google Scholar] [CrossRef]
- Tichá, M.; Kocourek, J. Fluorescein-labeled O-glycosyloxyalkenyl-aminoalkenyl-acrylamide copolymers in lectin-saccharide binding studies. Carbohydr. Res. 1991, 213, 339–343. [Google Scholar] [CrossRef]
- Li, G.; You, J.; Suo, Y.; Song, C.; Sun, Z.; Xia, L.; Shi, J. Studies on Separation and Fluorescent Labeling of Lycium barbarum Polysaccharides. Lishizhen Med. Mater. Med. Res. 2014, 25, 2312–2315. [Google Scholar]
- Chen, C.; Tang, H.; Ma, Y.; Wang, S.; Yang, L.; Peng, J.; Sun, G. Establishment of A Method for Detecting Fluorescence Labeled Lycium barbarum Polysaccharide in Rat Plasma. Food Sci. 2015, 36, 90–95. [Google Scholar]
- Guo, C.; Jiang, Y.; Li, L.; Hong, L.; Wang, Y.; Shen, Q.; Lou, Y.; Hu, H.; Zhou, H.; Yu, L.; et al. Application of a liquid chromatography–tandem mass spectrometry method to the pharmacokinetics, tissue distribution and excretion studies of Dactylicapnos scandens in rats. J. Pharm. Biomed. Anal. 2013, 74, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Cappiello, A.; Famiglini, G.; Palma, P.; Pierini, E.; Termopoli, V.; Trufelli, H. Overcoming Matrix Effects in Liquid Chromatography−Mass Spectrometry. Anal. Chem. 2008, 80, 9343–9348. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Z.-J.; Wang, S.; Shen, L.; Feng, Y.; Ruan, K.-F.; Xu, D.-S. Comparison of tissue distribution of a PEGylated Radix Ophiopogonis polysaccharide in mice with normal and ischemic myocardium. Eur. J. Pharm. Biopharm. 2011, 79, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, T.; Luo, L.; Cui, Z.; Wang, N.; Shu, Y.; Wang, K.-P. Pharmacokinetics, biodistribution and receptor mediated endocytosis of a natural Angelica sinensis polysaccharide. Artif. Cells Nanomed. Biotechnol. 2018, 46, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Y.; Wang, H.-X.; He, J.-R. Research progresses of pharmacokinetics of polysaccharides. Yao Xue Xue Bao Acta Pharm. Sin. 2014, 49, 443–449. [Google Scholar]
- Kaneo, Y.; Uemura, T.; Tanaka, T.; Kanoh, S. Polysaccharides as Drug Carriers: Biodisposition of Fluorescein-Labeled Dextrans in Mice. Biol. Pharm. Bull. 1997, 20, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Mehvar, R.; Robinson, M.A.; Reynolds, J.M. Dose Dependency of the Kinetics of Dextrans in Rats: Effects of Molecular Weight. J. Pharm. Sci. 1995, 84, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, Z.; Yang, X.; Pan, X.; Yin, L.; Huang, X.; Li, Q.; Shu, Y.; Zhang, Q.; Wang, K. A sensitive and rapid radiolabelling method for the in vivo pharmacokinetic study of lentinan. Food Funct. 2018, 9, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yao, R.; Zhu, Z.; Pang, D.; Cao, X.; Feng, B.; Paulsen, B.S.; Li, L.; Yin, Z.; Chen, X.; et al. A pectic polysaccharide from water decoction of Xinjiang Lycium barbarum fruit protects against intestinal endoplasmic reticulum stress. Int. J. Biol. Macromol. 2019, 130, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bai, H.; Cai, W.; Li, J.; Zhou, Q.; Wang, Y.; Han, J.; Zhu, X.; Dong, M.; Hu, D. Lycium barbarum polysaccharides reduce intestinal ischemia/reperfusion injuries in rats. Chem. Interact. 2013, 204, 166–172. [Google Scholar] [CrossRef] [PubMed]


| Biological Sample | Theoretical Concentration (μg/mL) | Inter-Day, RSD (%) | Intra-Day, RSD (%) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Plasma | 0.2 | 7.28 | 6.9 | 5.37 | 4.88 | 4.92 | 7.75 |
| 2.0 | 3.45 | 4.01 | 4.92 | 2.67 | 5.39 | 4.83 | |
| 20.0 | 1.86 | 2.24 | 3.28 | 2.48 | 1.97 | 2.62 | |
| Urine | 0.25 | 4.26 | 5.9 | 5.37 | 4.88 | 4.92 | 7.16 |
| 50.0 | 1.45 | 2.01 | 1.92 | 2.67 | 1.39 | 1.83 | |
| 500.0 | 0.86 | 1.04 | 0.58 | 0.68 | 0.47 | 0.78 | |
| Feces | 5.0 | 3.68 | 3.92 | 6.21 | 4.86 | 3.9 | 6.12 |
| 50.0 | 1.66 | 1.91 | 2.04 | 1.69 | 1.12 | 2.86 | |
| 300.0 | 0.89 | 1.53 | 1.24 | 1.08 | 1.37 | 1.74 | |
| Biological Sample | Theoretical Concentration (μg/mL) | 24 h at Room Temperature | Repeated Freeze-Thaw Test for 3 Times | Frozen at −20 °C for 15 Days | |||
|---|---|---|---|---|---|---|---|
| Measured Concentration (μg/mL) | RSD (%) | Measured Concentration (μg/mL) | RSD (%) | Measured Concentration (μg/mL) | RSD (%) | ||
| Plasma | 0.2 | 0.196 ± 0.004 | 4.90 | 0.194 ± 0.005 | 4.98 | 0.191 ± 0.003 | 3.80 |
| 2.0 | 1.89 ± 0.02 | 2.07 | 1.93 ± 0.04 | 4.03 | 1.96 ± 0.03 | 3.24 | |
| 20.0 | 19.17 ± 0.34 | 3.74 | 19.03 ± 0.24 | 2.72 | 19.49 ± 0.17 | 1.87 | |
| Urine | 0.25 | 0.246 ± 0.004 | 4.61 | 0.244 ± 0.005 | 4.98 | 0.247 ± 0.003 | 4.78 |
| 50 | 48.89 ± 0.82 | 1.07 | 49.93 ± 0.74 | 1.83 | 49.96 ± 0.83 | 1.34 | |
| 500 | 498.17 ± 3.34 | 0.74 | 496.03 ± 2.24 | 0.72 | 498.49 ± 3.17 | 0.89 | |
| Feces | 5.0 | 4.92 ± 0.14 | 3.64 | 4.86 ± 0.12 | 4.02 | 4.96 ± 0.13 | 3.82 |
| 50.0 | 48.89 ± 0.22 | 2.11 | 46.93 ± 0.34 | 5.36 | 48.96 ± 0.23 | 4.64 | |
| 300.0 | 287.63 ± 0.34 | 3.28 | 279.08 ± 0.24 | 2.72 | 292.44 ± 0.31 | 2.81 | |
| Biological Sample | Theoretical Concentration (μg/mL) | Inter-Group | Intra-Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||||
| Rate (%) (M ± SD) | RSD (%) | Rate (%) (M ± SD) | RSD (%) | Rate (%) (M ± SD) | RSD (%) | Rate (%) (M ± SD) | RSD (%) | Rate (%) (M ± SD) | RSD (%) | Rate (%) (M ± SD) | RSD (%) | ||
| Plasma | 0.2 | 99.0 ± 7.9 | 7.28 | 96.0 ± 6.1 | 6.90 | 99.5 ± 7.8 | 5.37 | 104.0 ± 5.6 | 4.88 | 97.5 ± 7.2 | 4.92 | 100.2 ± 4.6 | 4.58 |
| 2.0 | 93.2 ± 3.8 | 3.45 | 87.1 ± 3.5 | 4.01 | 84.6 ± 2.9 | 4.92 | 95.2 ± 3.4 | 2.67 | 94.3 ± 5.2 | 5.39 | 91.6 ± 4.4 | 3.82 | |
| 20.0 | 98.4 ± 1.2 | 1.86 | 96.9 ± 1.8 | 2.24 | 98.9 ± 1.6 | 3.28 | 94.6 ± 0.9 | 2.48 | 97.2 ± 1.9 | 1.97 | 98.3 ± 2.8 | 2.04 | |
| Urine | 0.25 | 99.2 ± 4.8 | 4.28 | 100.0 ± 5.1 | 5.90 | 98.4 ± 5.6 | 5.37 | 100.8 ± 4.8 | 4.88 | 98.0 ± 6.4 | 4.92 | 98.4 ± 1.4 | 1.42 |
| 50 | 97.7 ± 1.7 | 1.45 | 95.5 ± 1.6 | 2.01 | 97.4 ± 1.9 | 1.92 | 99.8 ± 1.7 | 2.67 | 95.8 ± 1.6 | 1.39 | 97.6 ± 1.5 | 1.64 | |
| 500 | 99.5 ± 0.5 | 0.86 | 99.1 ± 0.7 | 1.04 | 99.4 ± 0.4 | 0.58 | 98.8 ± 0.6 | 0.68 | 99.7 ± 0.7 | 0.47 | 99.3 ± 0.4 | 0.38 | |
| Feces | 5.0 | 99.6 ± 4.78 | 3.68 | 97.2 ± 4.20 | 3.92 | 94.6 ± 2.72 | 6.21 | 99.2 ± 6.40 | 4.86 | 100.6 ± 3.18 | 3.90 | 98.2 ± 2.72 | 2.78 |
| 50.0 | 96.5 ± 1.64 | 1.66 | 96.3 ± 1.56 | 1.91 | 96.2 ± 1.86 | 2.04 | 95.6 ± 1.34 | 1.69 | 95.4 ± 1.06 | 1.12 | 98.3 ± 0.50 | 0.52 | |
| 300.0 | 99.6 ± 1.05 | 0.89 | 99.3 ± 1.21 | 1.53 | 99.4 ± 1.50 | 1.24 | 98.9 ± 1.02 | 1.08 | 99.8 ± 1.24 | 1.37 | 99.4 ± 0.98 | 1.00 | |
| Concentration | 100 mg/kg | 50 mg/kg | 25 mg/kg | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time(h) | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 5.176 | 5.722 | 6.454 | 6.425 | 6.69 | 7.426 | 4.933 | 5.082 | 5.358 | 5.527 | 4.333 | 5.632 | 3.514 | 4.050 | 4.180 | 4.132 | 3.121 | 2.645 |
| 1 | 5.305 | 7.451 | 7.084 | 7.679 | 7.463 | 7.975 | 4.724 | 6.331 | 6.174 | 6.564 | 5.666 | 5.963 | 4.391 | 4.641 | 4.578 | 4.427 | 3.519 | 3.054 |
| 2 | 5.160 | 7.543 | 7.624 | 7.706 | 6.878 | 8.228 | 4.623 | 6.494 | 6.634 | 7.214 | 6.078 | 6.747 | 4.741 | 5.976 | 5.403 | 5.194 | 5.404 | 4.898 |
| 3 | 6.085 | 6.343 | 5.407 | 5.023 | 5.219 | 6.677 | 5.902 | 6.056 | 5.401 | 4.98 | 5.146 | 7.021 | 4.496 | 5.184 | 5.315 | 4.413 | 5.038 | 4.635 |
| 4 | 5.677 | 5.158 | 5.321 | 5.186 | 4.93 | 6.001 | 4.897 | 5.235 | 5.111 | 4.666 | 4.789 | 5.336 | 4.193 | 4.237 | 4.872 | 3.938 | 4.524 | 3.816 |
| 6 | 5.555 | 4.09 | 5.231 | 4.844 | 4.394 | 6.578 | 4.206 | 4.004 | 4.859 | 4.758 | 4.143 | 5.282 | 3.988 | 3.320 | 4.855 | 4.672 | 4.273 | 2.974 |
| 8 | 4.045 | 4.713 | 4.88 | 4.784 | 4.875 | 5.714 | 3.981 | 3.651 | 4.546 | 4.256 | 4.254 | 4.967 | 3.728 | 3.019 | 4.026 | 3.947 | 3.949 | 2.874 |
| 10 | 3.582 | 3.11 | 3.726 | 4.987 | 4.027 | 4.832 | 3.515 | 2.897 | 3.669 | 4.034 | 3.812 | 4.185 | 3.413 | 2.478 | 3.640 | 3.773 | 3.213 | 2.376 |
| 12 | 2.903 | 3.923 | 2.76 | 4.557 | 3.777 | 3.658 | 2.808 | 2.682 | 3.528 | 3.879 | 3.221 | 3.241 | 3.051 | 2.566 | 3.449 | 3.296 | 2.642 | 1.511 |
| 24 | 2.592 | 2.631 | 2.756 | 2.612 | 2.551 | 3.146 | 2.349 | 2.476 | 2.743 | 2.581 | 2.756 | 2.248 | 3.026 | 2.126 | 2.996 | 2.551 | 2.638 | 1.239 |
| 36 | 1.884 | 1.858 | 2.507 | 1.748 | 2.161 | 2.668 | 1.964 | 1.869 | 2.721 | 2.511 | 2.108 | 2.046 | 3.018 | 1.992 | 2.671 | 2.498 | 2.088 | 1.034 |
| 48 | 1.592 | 1.161 | 2.065 | 1.499 | 1.118 | 2.689 | 1.362 | 1.558 | 2.014 | 2.123 | 1.964 | 1.337 | 2.094 | 1.423 | 1.941 | 2.039 | 1.901 | 0.892 |
| Parameter | Unit | 100 mg/kg | 50 mg/kg | 25 mg/kg | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Cmax a | mg/L | 7.44 | 0.72 | 6.56 | 0.51 | 5.27 | 0.44 |
| Tmax b | h | 2 | 0.63 | 2.33 | 0.52 | 2 | 0 |
| AUC(0–t) c | mg/L.h | 151.09 | 15.10 | 141.25 | 12.02 | 128.21 | 27.64 |
| AUC(0–∞) d | mg/L.h | 230.49 | 73.26 | 236.18 | 35.08 | 242.57 | 64.09 |
| MRT(0–t) e | h | 18.38 | 1.01 | 19.15 | 0.84 | 20.07 | 1.49 |
| t1/2 f | h | 31.39 | 14.64 | 38.09 | 9.43 | 45.76 | 4.57 |
| CLz/F g | L/h/kg | 0.47 | 0.12 | 0.22 | 0.03 | 0.11 | 0.04 |
| Vz/F h | L/kg | 18.96 | 3.66 | 11.57 | 2.16 | 7.29 | 2.38 |
| Time | Urine | Feces | ||
|---|---|---|---|---|
| Amount (μg) | % of Dose | Amount (μg) | % of Dose | |
| 0–4 | 3.87 ± 0.04 | 0.038 ± 0.008 | 0.05 ± 0.02 | 0.052 ± 0.018 |
| 0–10 | 6.44 ± 0.06 | 0.064 ± 0.012 | 4.81 ± 1.21 | 48.28 ± 9.349 |
| 0–24 | 7.49 ± 0.09 | 0.075 ± 0.015 | 6.94 ± 1.18 | 69.53 ± 6.483 |
| 0–48 | 8.36 ± 0.13 | 0.084 ± 0.022 | 8.88 ± 1.12 | 89.24 ± 5.487 |
| 0–72 | 9.38 ± 0.23 | 0.094 ± 0.036 | 9.23 ± 0.50 | 92.18 ± 3.609 |
| Time (h) | Δt (h) | Urinary | Feces | ||
|---|---|---|---|---|---|
| Excretion Amount (mg) | Excretion Rate (mg/h) | Excretion Amount (mg) | Excretion Rate (mg/h) | ||
| 0 | 0 | ||||
| 4 | 4 | 3.87 ± 0.06 | 0.968 ± 0.211 | 0.05 ± 0.02 | 0.013 ± 0.005 |
| 10 | 6 | 2.57 ± 1.24 | 0.428 ± 0.107 | 4.76 ± 1.12 | 0.793 ± 0.187 |
| 24 | 14 | 1.05 ± 0.89 | 0.075 ± 0.064 | 2.13 ± 1.08 | 0.152 ± 0.077 |
| 48 | 24 | 0.87 ± 1.03 | 0.036 ± 0.043 | 1.94 ± 0.51 | 0.081 ± 0.021 |
| 72 | 24 | 1.02 ± 0.92 | 0.043 ± 0.038 | 0.35 ± 0.13 | 0.015 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Yang, C.; Zhou, B.; Tang, H.; Yang, L.; Liao, W.; Sun, G. Pharmacokinetics and Excretion Study of Lycium barbarum Polysaccharides in Rats by FITC-Fluorescence Labeling. Foods 2021, 10, 2851. https://doi.org/10.3390/foods10112851
Xia H, Yang C, Zhou B, Tang H, Yang L, Liao W, Sun G. Pharmacokinetics and Excretion Study of Lycium barbarum Polysaccharides in Rats by FITC-Fluorescence Labeling. Foods. 2021; 10(11):2851. https://doi.org/10.3390/foods10112851
Chicago/Turabian StyleXia, Hui, Chao Yang, Beijia Zhou, Huali Tang, Ligang Yang, Wang Liao, and Guiju Sun. 2021. "Pharmacokinetics and Excretion Study of Lycium barbarum Polysaccharides in Rats by FITC-Fluorescence Labeling" Foods 10, no. 11: 2851. https://doi.org/10.3390/foods10112851






