Nutritional Properties of Larval Epidermis and Meat of the Edible Insect Clanis bilineata tsingtauica (Lepidoptera: Sphingidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Materials
2.2. Determination of Protein Content
2.3. Determination of Amino Acid Content
2.4. Determination of Mineral Content
2.5. Determination of Phytic Acid Content
2.6. Statistical Analyses
3. Results
3.1. Classification and Determination of Total Protein
3.2. Essential and Nonessential Amino Acid Content
3.3. Determination of Mineral Content
3.4. Phytic Acid (PA) Content and Mineral Bioavailability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.K.; Ellur, R.K.; Singh, A.K.; Nagarajan, M.; Singh, B.D.; Singh, N.K. Effect of qGN4.1 QTL for grain number per panicle in genetic backgrounds of twelve different mega varieties of rice. Rice 2018, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Rochow, V.B. Can insects help to ease the problem of world food shortage? Search 1975, 6, 261–262. [Google Scholar]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an alternative protein source for monogastric animal: A review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Huis, V.A.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013; Volume 171, pp. 1–201. [Google Scholar]
- Kim, S.Y.; Kwak, K.-W.; Park, E.-S.; Yoon, H.J.; Kim, Y.-S.; Park, K.; Kim, E.; Kim, S.-D. Evaluation of subchronic oral dose toxicity of freeze-dried skimmed powder of Zophobas atratus Larvae (frpfdZAL) in Rats. Foods 2020, 9, 995. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A.; Schwabe, M.; Brinkrolf, K.; Plarre, R.; Wielsch, N.; Vogel, H. Larvae of the clothing moth Tineola bisselliella maintain gut bacteria that secrete enzyme cocktails to facilitate the digestion of keratin. Microorganisms 2020, 8, 1415. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Nakane, W.; Nakamura, H.; Nakazato, T.; Kaminaga, N.; Nakano, M.; Sakamoto, T.; Nishiko, M.; Bono, H.; Ogiwara, I.; Kitano, Y.; et al. Construction of TUATinsecta database that integrated plant and insect database for screening phytophagous insect metabolic products with medicinal potential. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Elhassan, M.; Wendin, K.; Olsson, V.; Langton, M. Quality aspects of insects as food—Nutritional, sensory, and related concepts. Foods 2019, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Huis, A.V. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Lyke, M.M.; Di Fiore, A.; Fierer, N.; Madden, A.A.; Lambert, J.E. Metagenomic analyses reveal previously unrecognized variation in the diets of sympatric Old World monkey species. PLoS ONE 2019, 14, e0218245. [Google Scholar] [CrossRef]
- McGrew, W.C. The ‘other faunivory’ revisited: Insectivory in human and non-human primates and the evolution of human diet. J. Hum. Evol. 2014, 71, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.M.; Raubenheimer, D.; Bryer, M.A.; Takahashi, M.; Gilbert, C. Nutritional contributions of insects to primate diets: Implications for primate evolution. J. Hum. Evol. 2014, 71, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Claudia, C.; Miranda, M.; John, B. Potential of extracted Locusta Migratoria protein fractions as value-added ingredients. Insects 2018, 9, 20. [Google Scholar]
- Wendin, K.; Mårtensson, L.; Djerf, H.; Langton, M. Product quality during the storage of foods with insects as an ingredient: Impact of particle size, antioxidant, oil content and salt content. Foods 2020, 9, 791. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef] [Green Version]
- Gasco, L.; Biasato, I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals fed insect-based diets: State-of-the-art on digestibility, performance and product quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Jung, C. Nutritional value of bee-collected pollens of hardy kiwi, Actinidia arguta (Actinidiaceae) and oak, Quercus sp. (Fagaceae). J. Asia-Pac. Entomol. 2017, 20, 245–251. [Google Scholar] [CrossRef]
- Chakravorty, J.; Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of Chondacris rosea and brachytrupes orientalis: Two common insects used as food by tribes of arunachal pradesh, india. J. Asia-Pac. Entomol. 2014, 17, 407–415. [Google Scholar] [CrossRef]
- Terova, G.; Gini, E.; Gasco, L.; Moroni, F.; Antonini, M.; Rimoldi, S. Effects of full replacement of dietary fishmeal with insect meal from Tenebrio molitor on rainbow trout gut and skin microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Fontaneto, D.; Tommaseo-Ponzetta, M.; Galli, C.; Risé, P.; Glew, R.H.; Paoletti, M.G. Differences in fatty acid composition between aquatic and terrestrial insects used as food in human nutrition. Ecol. Food Nutr. 2011, 50, 351–367. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.-M.; Zhao, M.; He, Z.; Sun, L.; Wang, C.-Y.; Ding, W.-F. Edible insects in China: Utilization and prospects. Insect Sci. 2018, 25, 184–198. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Ma, G.C.; Jin, Q.A.; Lü, B.Q.; Peng, Z.Q.; Jin, T.; Wen, H.B. Effect of temperature on developmental duration and feeding amount of Clanis bilineata tsingtauica. Chin. J. Trop. Crop. 2014, 35, 2442–2444. [Google Scholar]
- Guo, M.M.; Li, X.F.; Deng, P.; Li, D.W.; Li, J.L.; Fan, J.W.; Chen, F. Diapause termination and post-diapause of overwintering Clanis bilineata tsingtauica larvae. Chin. J. Appl. Entomol. 2021, 58, 966–972. [Google Scholar]
- Tian, H.; Zhang, Y.M. Advances of integrated utilization on resource insect Clanis bilineata tsingtauica. Guizhou Agric. Sci. 2009, 37, 111–113. [Google Scholar]
- Guo, M.M.; Liao, H.J.; Deng, P.; Li, D.W.; Li, J.L.; Zhang, J.Q.; Fan, J.W.; Chen, F. Effects of soybean varieties (lines) and planting density on survival and development of Clanis bilineata tsingtauica Mell larvae. J. Environ. Entomol. 2020, 42, 1401–1408. [Google Scholar]
- Tian, H.; Zhang, Y.M. The nutritional components analysis and evaluation of Clanis bilineata tsingtauica Mell. Acta Nutr. Sin. 2012, 34, 289–291. [Google Scholar]
- Doumani, N.; Severin, I.; Dahbi, L.; Bou-Maroun, E.; Tueni, M.; Sok, N.; Chagnon, M.-C.; Maalouly, J.; Cayot, P. Lemon juice, sesame paste, and autoclaving influence iron bioavailability of hummus: Assessment by an in vitro digestion/Caco-2 cell model. Foods 2020, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Jobarteh, M.L.; McArdle, H.J.; Holtrop, G.; Sise, E.A.; Prentice, A.M.; Moore, S.E. mRNA levels of placental iron and zinc transporter genes are upregulated in Gambian women with low iron and zinc status. J. Nutr. 2017, 147, 1401–1409. [Google Scholar] [CrossRef] [Green Version]
- Rebholz, C.M.; Lichtenstein, A.H.; Zheng, Z.; Appel, L.J.; Coresh, J. Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am. J. Clin. Nutr. 2018, 108, 243–255. [Google Scholar] [CrossRef]
- Clements, R.S.; Darnell, B. Myo-inositol content of common foods: Development of a high-myo-inositol diet. Am. J. Clin. Nutr. 1980, 33, 1954–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steadman, K.J.; Burgoon, M.S.; Schuster, R.L.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Fagopyritols, D-chiro-inositol, and other soluble carbohydrates in buckwheat seed milling fractions. J. Agric. Food Chem. 2000, 48, 2843–2847. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.O.; Bracarense, A.P.F.; Bracarense, A.P. Phytic acid: From antinutritional to multiple protection factor of organic systems. J. Food Sci. 2016, 81, R1357–R1362. [Google Scholar] [CrossRef] [Green Version]
- Joseph, L. D-chiro-inositol—Its functional role in insulin action and its deficit in insulin resistance. Int. J. Exp. Diabetes Res. 2002, 3, 47–60. [Google Scholar]
- Gupta, S.; Habeych, E.; Scheers, N.; Merinat, S.; Rey, B.; Galaffu, N.; Sandberg, A.-S. The development of a novel ferric phytate compound for iron fortification of bouillons (part I). Sci. Rep. 2020, 10, 5340. [Google Scholar] [CrossRef]
- Sandberg, A.-S.; Andersson, H. The effect of dietary phytase on the digestion of phytate in the stomach and small intestine of humans. J. Nutr. 1988, 118, 469–473. [Google Scholar] [CrossRef]
- Hurrell, R.F. Phytic acid degradation as a means of improving iron absorption. Int. J. Vitam. Nutr. Res. 2004, 74, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Brune, M.; Rossander-Hultén, L.; Hallberg, L.; Gleerup, A.; Sandberg, A.-S. Iron absorption from bread in humans: Inhibiting effects of cereal fiber, phytate and inositol phosphates with different numbers of phosphate groups. J. Nutr. 1992, 122, 442–449. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.; Gahukar, R.; Ghosh, S.; Jung, C. Chemical composition, nutrient quality and acceptability of edible insects are affected by species, developmental stage, gender, diet, and processing method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef]
- Chakravorty, J.; Ghosh, S.; Megu, K.; Jung, C.; Meyer-Rochow, V.B. Nutritional and anti-nutritional composition of Oecophylla smaragdina (Hymenoptera: Formicidae) and Odontotermes sp. (Isoptera: Termitidae): Two preferred edible insects of Arunachal Pradesh, India. J. Asia-Pac. Entomol. 2016, 19, 711–720. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Zhou, J.; Wang, Y.; Peng, B.; Zhu, J.; Yang, L.; Wang, Y. Elevated tropospheric ozone increased grain protein and amino acid content of a hybrid rice without manipulation by planting density. J. Sci. Food Agric. 2014, 95, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Hettiarachchy, N.; Rath, N. Extraction, denaturation and hydrophobic properties of rice flour proteins. J. Food Sci. 2001, 66, 229–232. [Google Scholar] [CrossRef]
- Toshio, S.; Kunisuke, T.; Zenzaburo, K. Improved extraction of rice prolamin. Agric. Biol. Chem. 1986, 50, 2409–2411. [Google Scholar]
- Jing, L.; Chen, C.; Hu, S.; Dong, S.; Pan, Y.; Wang, Y.; Lai, S.; Wang, Y.; Yang, L. Effects of elevated atmosphere CO2 and temperature on the morphology, structure and thermal properties of starch granules and their relationship to cooked rice quality. Food Hydrocoll. 2021, 112, 106360. [Google Scholar] [CrossRef]
- Qiao, T.S.; Tang, H.C.; Liu, J.X.; Li, L. Analysis of nutritional components and evaluation of protein quality of Oxya chinensis. Chin. Bull. Entomol. 1992, 29, 113–117. [Google Scholar]
- Huis, A.V. Prospects of insects as food and feed. Org. Agric. 2021, 11, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Khampakool, A.; Soisungwan, S.; You, S.G.; Park, S.H. Infrared assisted freeze-drying (IRAFD) to produce shelf-stable insect food from Protaetia brevitarsis (white-spotted flower chafer) larva. Food Sci. Anim. Resour. 2020, 40, 813–830. [Google Scholar] [CrossRef]
- Mi, Y.C.; Gwon, E.Y.; Hwang, J.S.; Goo, T.W.; Yun, E.Y. Analysis of general composition and harmful material of Protaetia brevitarsis. J. Life Sci. 2013, 23, 664–668. [Google Scholar]
- Melis, R.; Braca, A.; Mulas, G.; Sanna, R.; Spada, S.; Serra, G.; Leonarda, F.M.; Roggio, T.; Uzzau, S.; Anedda, R. Effect of freezing and drying processes on the molecular traits of edible yellow mealworm. Innov. Food Sci. Emerg. Technol. 2018, 48, 138–149. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Lee, H.S.; Park, J.Y.; Lee, J.W. Associating intake proportion of carbohydrate, fat, and protein with all-cause mortality in Korean adults. Nutrients 2020, 12, 3208. [Google Scholar] [CrossRef]
- Carreiro, A.L.; Dhillon, J.; Gordon, S.; Higgins, K.A.; Jacobs, A.G.; McArthur, B.M.; Redan, B.W.; Rivera, R.L.; Schmidt, L.R.; Mattes, R.D. The macronutrients, appetite, and energy intake. Annu. Rev. Nutr. 2016, 36, 73–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.K.; Yong, H.I.; Chang, H.J.; Han, S.G.; Kim, Y.B.; Paik, H.D.; Choi, Y.S. Technical functional properties of water- and salt-soluble proteins extracted from edible insects. Food Sci. Anim. Resour. 2019, 39, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Pali-Scholl, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodriguez-Monroy, F.A.; Einhorm, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jager, H. Edible insects: Cross-recognition of IgE from crustacean and house dust mite allergic patients, and reduction of allergenicity by food processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Li, K.; Dai, R.H. Analysis and evaluation of nutrition of Opisthoplata orientalis Buron. J. Environ. Entomol. 2013, 35, 466–472. [Google Scholar]
- Boulos, S.; Tnnler, A.; Nystrm, L. Nitrogen-to-protein conversion factors for edible insects on the Swiss market: T. molitor, A. domesticus, and L. migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef]
- Liu, J.; Huang, L.; Li, T.X.; Liu, Y.X.; Yan, Z.H.; Tang, G.; Zheng, Y.L.; Liu, D.C.; Wu, B.H. Genome-wide association study for grain micronutrient concentrations in wheat advanced lines derived from wild emmer. Front. Plant Sci. 2021, 12, 651283. [Google Scholar] [CrossRef]
- Calayugan, M.; Formantes, A.K.; Amparado, A.; Descalsota-Empleo, G.I.; Nha, C.T.; Inabangan-Asilo, M.A.; Swe, Z.M.; Hernandez, J.E.; Borromeo, T.H.; Lalusin, A.G.; et al. Genetic analysis of agronomic traits and grain iron and zinc concentrations in a doubled haploid population of rice (Oryza sativa L.). Sci. Rep. 2020, 10, 2283. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Sandstead, H.H. Is zinc deficiency a public problem? Nutrition 1995, 11, 87–92. [Google Scholar]
- Baqui, A.H.; Black, R.E.; Arifeen, S.E.; Yunus, M.; Chakraborty, J.; Ahmed, S.; Vaughan, J.P. Effect of zinc supplementation started during diarrhoea on morbidity and mortality in Bangladeshi children: Community randomised trial. BMJ 2002, 325, 1059–1062. [Google Scholar] [CrossRef] [Green Version]
- Caproni, L.; Raggi, L.; Talsma, E.F.; Wenzl, P.; Negri, V. European landrace diversity for common bean biofortification: A genome-wide association study. Sci. Rep. 2020, 10, 19775. [Google Scholar] [CrossRef]
- Luzak, A.; Heier, M.; Thorand, B.; Laxy, M.; Nowak, D.; Peters, A.; Schulz, H. Physical activity levels, duration pattern and adherence to WHO recommendations in german adults. PLoS ONE 2017, 12, e0172503. [Google Scholar] [CrossRef] [Green Version]
- Lowe, N.M.; Khan, M.J.; Broadley, M.R.; Zia, M.H.; Mcardle, H.J.; Joy, E.J.; Ohly, H.; Shahzad, B.; Ullah, U.; Kabana, G.; et al. Examining the effectiveness of consuming flour made from agronomically biofortified wheat (Zincol-2016/NR-421) for improving Zn status in women in a low-resource setting in Pakistan: Study protocol for a randomised, double-blind, controlled cross-over trial (BiZiFED). BMJ Open 2018, 8, e021364. [Google Scholar]
- Petry, N.; Boy, E.; Wirth, J.P.; Hurrell, R.F. Review: The potential of the common bean (Phaseolus vulgaris) as a vehicle for iron biofortification. Nutrients 2015, 7, 1144–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.F.; Black, R.E. Zinc and the risk for infectious disease. Annu. Rev. Nutr. 2004, 24, 255–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, C.R.; Grant, F.K.; Dawn, S.; Smith, J.L.; Anne, J.; Northrop-Clewes, C.A.; Calawell, K.L.; Pfeiffer, C.M.; Ziegler, T.R. Zinc and iron deficiency and their interrelations in low-income African American and Hispanic children in Atlanta. Am. J. Clin. Nutr. 2010, 91, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.F.; Gao, X.; Lan, M.X.; Yuan, Y.; Guo, Z.J.; Tang, P.; Li, M.Y.; Liao, X.B.; Zhu, J.Y.; Li, Z.Y.; et al. De novo transcriptome analysis and identification of genes associated with immunity, detoxification and energy metabolism from the fat body of the tephritid gall fly, Procecidochares utilis. PLoS ONE 2019, 14, e0226039. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]

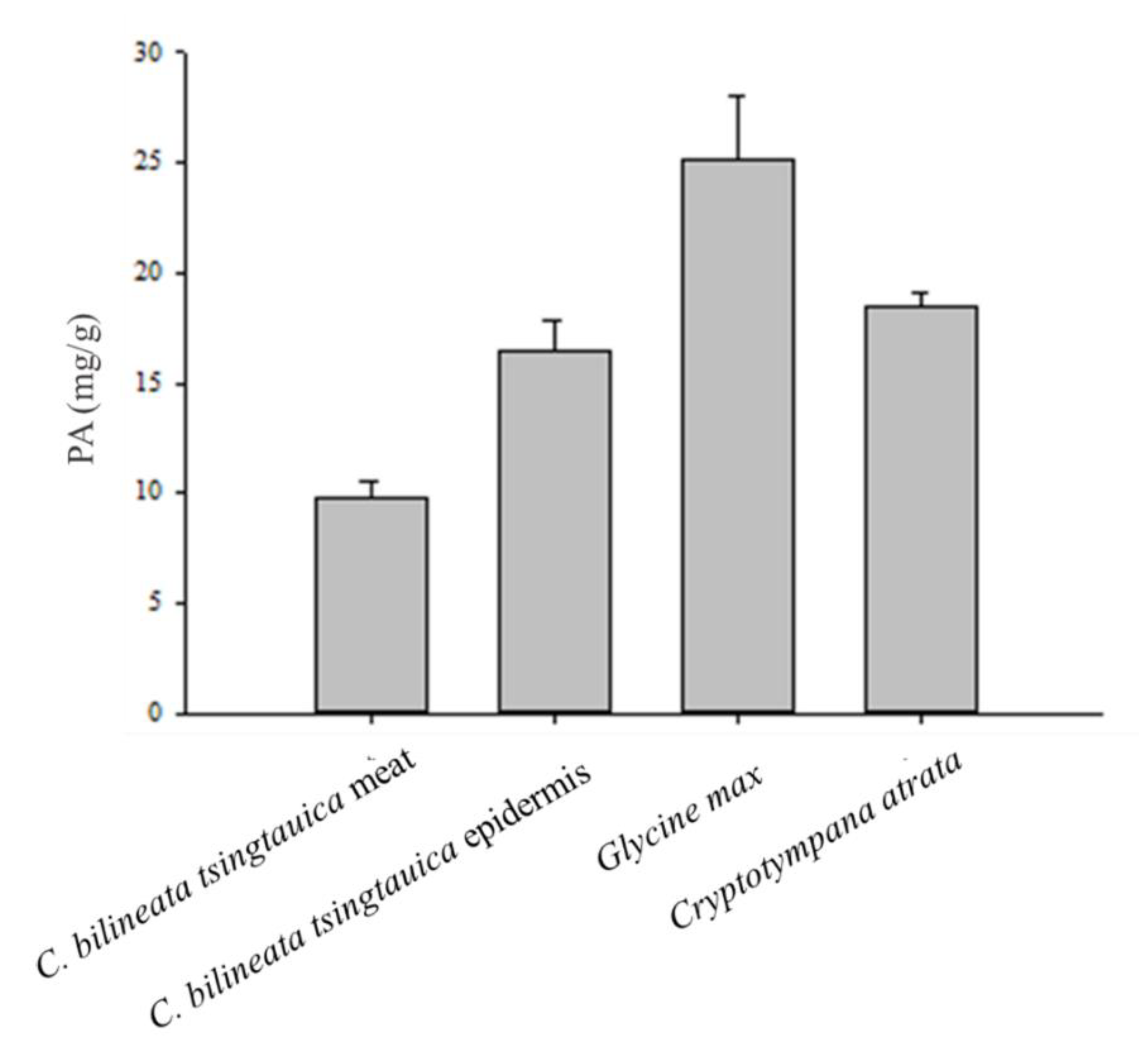
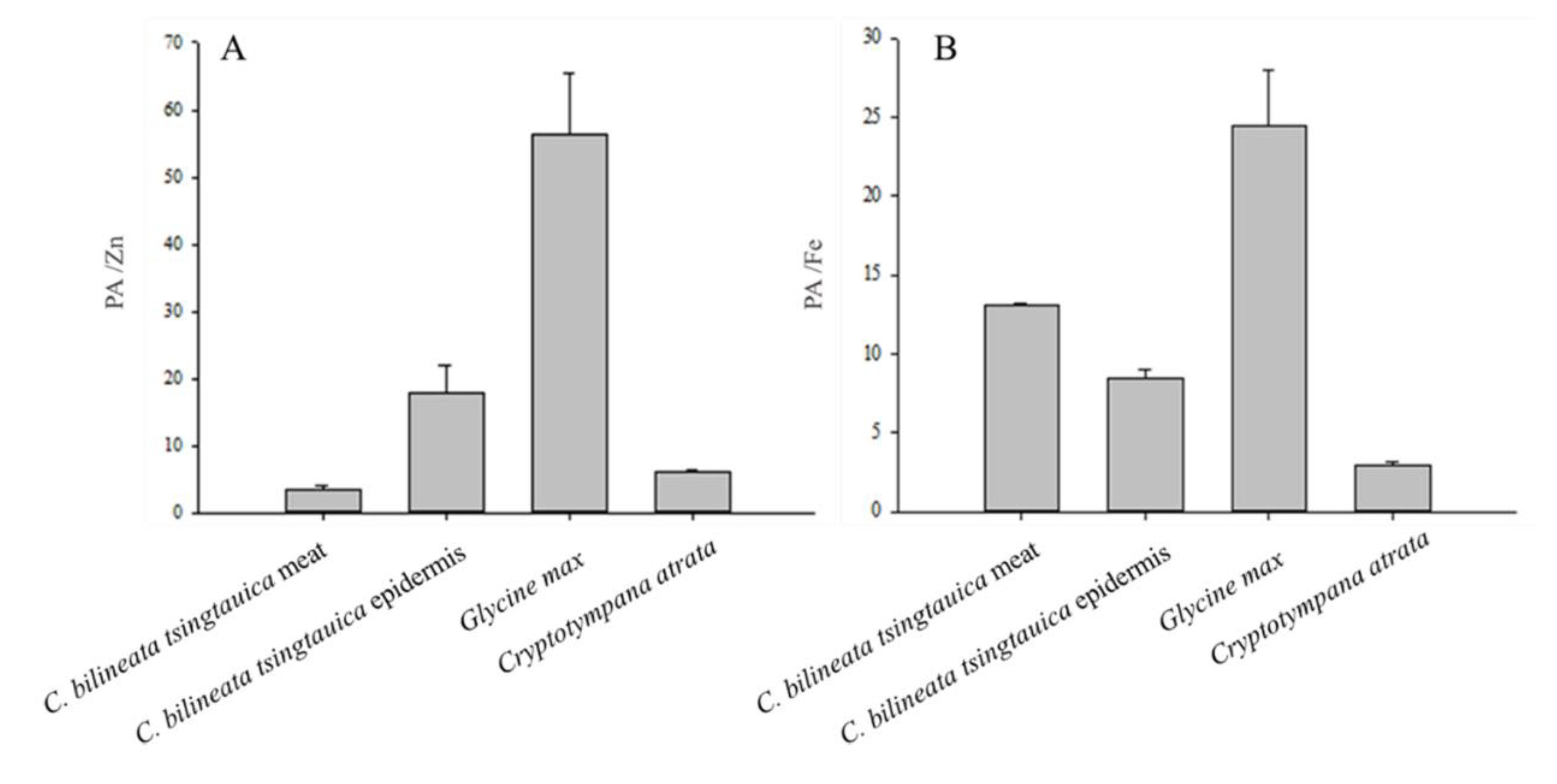
| Clanis bilineata tsingtauica Meat | Clanis bilineata tsingtauica Epidermis | Glycine max (CK1) | Cryptotympana atrata (CK2) | |
|---|---|---|---|---|
| Albumin | 19.50 ± 2.40 | 22.25 ± 8.56 | 33.55 ± 2.05 | 12.75 ± 4.03 |
| Globulin | 5.27 ± 1.20 | 3.70 ± 0.57 | 4.56 ± 0.30 | 3.53 ± 0.39 |
| Glutelin | 34.35 ± 4.43 | 34.23 ± 3.49 | 8.17 ± 0.52 | 39.07 ± 3.20 |
| Prolamin | 5.13 ± 0.98 | 11.67 ± 1.32 | 1.28 ± 0.35 | 11.02 ± 2.01 |
| Total protein | 64.25 ± 0.21 | 71.85 ± 3.18 | 47.55 ± 1.63 | 66.35 ± 4.74 |
| Clanis bilineata tsingtauica Meat | Clanis bilineata tsingtauica Epidermis | Glycine max (CK1) | Cryptotympana atrata (CK2) | |
|---|---|---|---|---|
| Thr * | 23.35 ± 4.34 | 44.22 ± 2.81 | 42.37 ± 5.59 | 29.95 ± 1.51 |
| Val * | 22.85 ± 1.00 | 33.17 ± 4.12 | 37.85 ± 4.91 | 25.05 ± 1.78 |
| Met * | 9.49 ± 1.22 | 18.81 ± 1.21 | 10.40 ± 1.09 | 6.73 ± 0.74 |
| Ile * | 21.02 ± 1.71 | 40.79 ± 2.08 | 36.74 ± 4.41 | 29.73 ± 2.76 |
| Leu * | 40.81 ± 2.55 | 50.89 ± 6.54 | 41.23 ± 6.50 | 39.67 ± 2.67 |
| Phe * | 24.81 ± 3.10 | 40.64 ± 3.21 | 107.26 ± 6.93 | 34.65 ± 1.76 |
| Lys * | 26.91 ± 1.82 | 59.05 ± 2.38 | 48.73 ± 14.81 | 42.03 ± 1.51 |
| Trp * | - | - | - | - |
| Asp | 45.49 ± 7.47 | 66.59 ± 2.47 | 69.51 ± 7.28 | 64.38 ± 2.86 |
| Ser | 24.53 ± 4.26 | 35.12 ± 3.02 | 40.15 ± 1.53 | 31.56 ± 2.89 |
| Glu | 78.40 ± 2.18 | 99.21 ± 2.04 | 93.21 ± 4.42 | 97.59 ± 1.44 |
| Gly | 28.43 ± 1.84 | 42.37 ± 3.56 | 48.97 ± 2.99 | 30.74 ± 2.65 |
| Ala | 35.52 ± 3.28 | 47.76 ± 2.98 | 52.52 ± 4.02 | 28.34 ± 2.58 |
| Tyr | 18.38 ± 0.78 | 39.11 ± 3.04 | 58.96 ± 2.26 | 24.50 ± 2.69 |
| Arg | 30.92 ± 3.08 | 54.82 ± 4.94 | 40.30 ± 1.54 | 48.68 ± 0.52 |
| Pro | 3.60 ± 0.48 | 3.69 ± 0.11 | 6.28 ± 0.39 | 3.79 ± 0.45 |
| His | 21.13 ± 1.25 | 33.68 ± 2.24 | 39.60 ± 4.16 | 21.29 ± 3.14 |
| Cys | - | - | - | - |
| EAA | 169.23 ± 1.97 | 287.75 ± 15.49 | 324.56 ± 3.43 | 207.72 ± 7.87 |
| NEAA | 286.39 ± 20.95 | 422.33 ± 1.22 | 449.48 ± 16.70 | 350.84 ± 7.29 |
| TAA | 455.62 ± 22.92 | 710.07 ± 14.27 | 774.04 ± 20.13 | 558.55 ± 15.16 |
| EAA/NEAA (%) | 59.23 ± 3.64 | 68.14 ± 3.86 | 72.24 ± 1.92 | 59.20 ± 1.01 |
| EAA/TAA (%) | 37.18 ± 1.44 | 40.51 ± 1.37 | 41.94 ± 0.65 | 37.19 ± 0.40 |
| Content (mg/g DW) | AAS | CS | ||||||
|---|---|---|---|---|---|---|---|---|
| FAO * | Eggs * | Meat | Epidermis | Meat | Epidermis | Meat | Epidermis | |
| Ile | 40 | 52.4 | 21.02 | 40.99 | 0.53 | 1.02 | 0.40 | 0.78 |
| Leu | 70 | 84.1 | 40.81 | 50.89 | 0.58 | 0.73 | 0.49 | 0.61 |
| Lys | 55 | 64.9 | 26.91 | 59.05 | 0.49 | 1.07 | 0.41 | 0.91 |
| Met | 35 | 62.7 | 9.49 | 18.81 | 0.27 | 0.54 | 0.15 | 0.30 |
| Phe + Tyr | 60 | 95.5 | 43.19 | 79.75 | 0.72 | 1.33 | 0.45 | 0.84 |
| Thr | 40 | 53.9 | 23.35 | 44.22 | 0.58 | 1.11 | 0.43 | 0.82 |
| Trp | 10 | 16.2 | - | - | - | - | - | - |
| Val | 50 | 57.6 | 22.85 | 33.17 | 0.46 | 0.66 | 0.40 | 0.58 |
| TAA | 360 | 487.3 | 217.94 | 362.60 | - | - | - | - |
| Clanis bilineata tsingtauica Meat | Clanis bilineata tsingtauica Epidermis | Glycine max (CK1) | Cryptotympana atrata (CK2) | |
|---|---|---|---|---|
| Ca (mg/g) * | 0.57 ± 0.12 | 0.73 ± 0.01 | 0.98 ± 0.03 | 1.59 ± 0.15 |
| K (mg/g) * | 12.53 ± 2.08 | 9.52 ± 0.13 | 13.56 ± 1.02 | 5.45 ± 0.16 |
| Mg (mg/g) * | 13.92 ± 2.21 | 11.24 ± 0.48 | 21.75 ± 0.49 | 12.09 ± 1.45 |
| P (mg/g) * | 7.91 ± 0.44 | 3.06 ± 0.15 | 5.10 ± 0.50 | 5.30 ± 0.24 |
| Cu (μg/g) | 6.79 ± 0.62 | 11.48 ± 0.29 | 14.00 ± 0.91 | 33.15 ± 0.16 |
| Fe (μg/g) | 63.05 ± 7.94 | 163.82 ± 4.08 | 87.88 ± 4.19 | 532.80 ± 30.92 |
| Mn (μg/g) | 10.57 ± 2.39 | 11.04 ± 0.02 | 23.37 ± 1.44 | 391.98 ± 13.51 |
| Zn (μg/g) | 299.31 ± 39.69 | 94.80 ± 20.57 | 44.71 ± 3.11 | 296.06 ± 33.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Lu, M.-X.; Jing, L.-Q.; Qian, L.; Zhao, M.; Du, Y.-Z.; Liao, H.-J. Nutritional Properties of Larval Epidermis and Meat of the Edible Insect Clanis bilineata tsingtauica (Lepidoptera: Sphingidae). Foods 2021, 10, 2895. https://doi.org/10.3390/foods10122895
Su Y, Lu M-X, Jing L-Q, Qian L, Zhao M, Du Y-Z, Liao H-J. Nutritional Properties of Larval Epidermis and Meat of the Edible Insect Clanis bilineata tsingtauica (Lepidoptera: Sphingidae). Foods. 2021; 10(12):2895. https://doi.org/10.3390/foods10122895
Chicago/Turabian StyleSu, Ying, Ming-Xing Lu, Li-Quan Jing, Lei Qian, Ming Zhao, Yu-Zhou Du, and Huai-Jian Liao. 2021. "Nutritional Properties of Larval Epidermis and Meat of the Edible Insect Clanis bilineata tsingtauica (Lepidoptera: Sphingidae)" Foods 10, no. 12: 2895. https://doi.org/10.3390/foods10122895
APA StyleSu, Y., Lu, M.-X., Jing, L.-Q., Qian, L., Zhao, M., Du, Y.-Z., & Liao, H.-J. (2021). Nutritional Properties of Larval Epidermis and Meat of the Edible Insect Clanis bilineata tsingtauica (Lepidoptera: Sphingidae). Foods, 10(12), 2895. https://doi.org/10.3390/foods10122895






