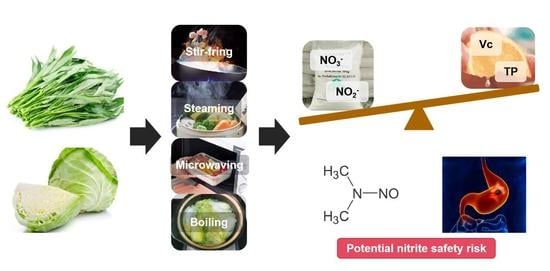
Assessment of Potential Nitrite Safety Risk of Leafy Vegetables after Domestic Cooking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cooking Process
2.3. Storage Process
2.4. Parametric Measurement
2.4.1. Determination of Moisture Content
2.4.2. Determination of Nitrate and Nitrite
2.4.3. Determination of Antioxidant Capacity
2.4.4. Determination of Ascorbic Acid and Total Phenol
2.4.5. Determination of Membrane Permeability
2.5. Potential Nitrite Safety Risk (PNSR) Assessment
2.6. Calculation Method and Statistical Analysis
3. Results and Discussion
3.1. Cooking Process
3.1.1. Changes in Nitrate and Nitrite
3.1.2. Changes in Antioxidant Capacity
3.1.3. Assessment of PNSR of Cooked Vegetables
3.2. Storage Process
3.2.1. Changes in Nitrate and Nitrite
3.2.2. Changes in Antioxidant Capacity and Key Antioxidants Content
3.2.3. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyriacou, M.C.; Soteriou, G.A.; Colla, G.; Rouphael, Y. The Occurrence of Nitrate and Nitrite in Mediterranean Fresh Salad Vegetables and Its Modulation by Preharvest Practices and Postharvest Conditions. Food Chem. 2019, 285, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Urrestarazu, L.; Lobillo-Eguíba, J.; Fernández-Cañero, R.; Fernández-Cabanás, V.M. Food Safety Concerns in Urban Aquaponic Production: Nitrate Contents in Leafy Vegetables. Urban For. Urban Green. 2019, 44, 126431. [Google Scholar] [CrossRef]
- Habermeyer, M.; Roth, A.; Guth, S.; Diel, P.; Engel, K.-H.; Epe, B.; Fürst, P.; Heinz, V.; Humpf, H.-U.; Joost, H.-G.; et al. Nitrate and Nitrite in the Diet: How to Assess Their Benefit and Risk for Human Health. Mol. Nutr. Food Res. 2015, 59, 106–128. [Google Scholar] [CrossRef]
- Martín León, V.; Luzardo, O.P. Evaluation of Nitrate Contents in Regulated and Non-Regulated Leafy Vegetables of High Consumption in the Canary Islands, Spain: Risk Assessment. Food Chem. Toxicol. 2020, 146, 111812. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in Fruits and Vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Zhang, X.; Liu, J.; Hao, Y.; Yang, X.; Wang, Y. Comparative Evaluation of the Antioxidant Effects of the Natural Vitamin C Analog 2-O-β-D-Glucopyranosyl-L-Ascorbic Acid Isolated from Goji Berry Fruit. Arch. Pharm. Res. 2011, 34, 801–810. [Google Scholar] [CrossRef]
- Sun, J.; He, X.-M.; Zhao, M.-M.; Li, L.; Li, C.-B.; Dong, Y. Antioxidant and Nitrite-Scavenging Capacities of Phenolic Compounds from Sugarcane (Saccharum Officinarum L.) Tops. Molecules 2014, 19, 13147–13160. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-G.; Li, Y.-Y.; Lin, H.-L. Excellent Nutritional Value in Fruits of Three Guava Cultivars in Taiwan. Acta Hortic. 2017, 1166, 209–213. [Google Scholar] [CrossRef]
- Niu, P.; Wang, F.; Yuan, K.; Li, X.; Yang, X.; Guo, Y. Alkaline-Extracted Thinned Young Apple Polyphenols as an Effective Scavenger against Nitrite in Pickles: A Comparative Study with Ethanol-Extracted Polyphenols. Food Control 2021, 130, 108387. [Google Scholar] [CrossRef]
- Ding, Z.; Johanningsmeier, S.D.; Price, R.; Reynolds, R.; Truong, V.-D.; Payton, S.C.; Breidt, F. Evaluation of Nitrate and Nitrite Contents in Pickled Fruit and Vegetable Products. Food Control 2018, 90, 304–311. [Google Scholar] [CrossRef]
- Leszczyńska, T.; Filipiak-Florkiewicz, A.; Cieślik, E.; Sikora, E.; Pisulewski, P.M. Effects of Some Processing Methods on Nitrate and Nitrite Changes in Cruciferous Vegetables. J. Food Compos. Anal. 2009, 22, 315–321. [Google Scholar] [CrossRef]
- Mehmood, A.; Zeb, A. Effects of Different Cooking Techniques on Bioactive Contents of Leafy Vegetables. Int. J. Gastron. Food Sci. 2020, 22, 100246. [Google Scholar] [CrossRef]
- Huarte-Mendicoa, J.C.; Astiasarán, I.; Bello, J. Nitrate and Nitrite Levels in Fresh and Frozen Broccoli. Effect of Freezing and Cooking. Food Chem. 1997, 58, 39–42. [Google Scholar] [CrossRef]
- Prasad, S.; Chetty, A.A. Nitrate-N Determination in Leafy Vegetables: Study of the Effects of Cooking and Freezing. Food Chem. 2008, 106, 772–780. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y. The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Wong, K.W.; Benzie, I.F.F. The Effect of Cooking on Brassica Vegetables. Food Chem. 2008, 110, 706–710. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, Y.; Zou, G.; Zhang, Q.; Lu, R.; Yan, H.; Cao, L.; Liu, T.; Ruan, R. Enhancement of Nutrients Removal and Biomass Accumulation of Chlorella Vulgaris in Pig Manure Anaerobic Digestate Effluent by the Pretreatment of Indigenous Bacteria. Bioresour. Technol. 2021, 328, 124846. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. The Antioxidant Capacity and Polyphenol Content of Organic and Conventional Retail Vegetables after Domestic Cooking. Food Res. Int. 2009, 42, 210–215. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sdiri, S.; Cuenca, J.; Navarro, P.; Salvador, A.; Bermejo, A. New Triploids Late-Maturing Mandarins as a Rich Source of Antioxidant Compounds. Eur. Food Res. Technol. 2020, 246, 225–237. [Google Scholar] [CrossRef]
- Manthong, N.; Wilairat, P.; Nacapricha, D.; Chaneam, S. Simultaneous Colorimetric Measurements of Antioxidant Capacity by Flow Injection Analysis with Paired Emitter Detector Diode. Anal. Sci. 2019, 35, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, A.; Delaviz, H.; Mohammadi, H. The Effects of Cooking Methods on Antioxidant Activity and Phenol Content in Vegetables. World J. Pharm. Pharm. Sci. 2014, 3, 242–252. [Google Scholar]
- Lu, X.; Ma, S.; Li, S.; Zhang, C.; Bao, J.; Zhang, X. Effect of Cooking Time on Glucoraphanin and Sulforaphane Contents in Broccoli Cooked by Different Cooking Methods. Foood Sci. 2020, 41, 41–47. [Google Scholar] [CrossRef]
- Kmiecik, W.; Lisiewska, Z.; Słupski, J. Effects of Freezing and Storing of Frozen Products on the Content of Nitrates, Nitrites, and Oxalates in Dill (Anethum graveolens L.). Food Chem. 2004, 86, 105–111. [Google Scholar] [CrossRef]
- Chetty, A.A.; Prasad, S. Flow Injection Analysis of Nitrate-N Determination in Root Vegetables: Study of the Effects of Cooking. Food Chem. 2009, 116, 561–566. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; van Loey, A.M.; Hendrickx, M.E. Comparative Study of the Cell Wall Composition of Broccoli, Carrot, and Tomato: Structural Characterization of the Extractable Pectins and Hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef]
- Jaworska, G. Nitrates, Nitrites, and Oxalates in Products of Spinach and New Zealand Spinach. Food Chem. 2005, 93, 395–401. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Zhuang, Q.; Scholz, F.; Pragst, F. The Voltammetric Behaviour of Solid 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Microparticles. Electrochem. Commun. 1999, 1, 406–410. [Google Scholar] [CrossRef]
- Kim, D.-B.; Shin, G.-H.; Lee, Y.-J.; Lee, J.S.; Cho, J.-H.; Baik, S.-O.; Lee, O.-H. Assessment and Comparison of the Antioxidant Activities and Nitrite Scavenging Activity of Commonly Consumed Beverages in Korea. Food Chem. 2014, 151, 58–64. [Google Scholar] [CrossRef]
- Bellail, A.A.; Shaltout, O.E.; Youssef, M.M.; Gamal, A.M.A.E. Effect of Home-Cooking Methods on Phenolic Composition and Antioxidant Activity of Sweetpotato (Ipomoea Batatas (L.) Lam.) Cultivars Grown in Egypt. Food Nutr. Sci. 2012, 3, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A.; Nisar, P. Effects of High Temperature Frying of Spinach Leaves in Sunflower Oil on Carotenoids, Chlorophylls, and Tocopherol Composition. Front. Chem. 2017, 5, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.M. Effect of food processing methods on the bioactive compound of cauliflower. Egypt. J. Agric. Res. 2015, 93, 117–131. [Google Scholar] [CrossRef]
- del Pilar Ramírez-Anaya, J.; Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; de la Serrana, H.L.-G. Phenols and the Antioxidant Capacity of Mediterranean Vegetables Prepared with Extra Virgin Olive Oil Using Different Domestic Cooking Techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Rufián-Henares, J.Á.; Pastoriza, S. Effect of Home Cooking on the Antioxidant Capacity of Vegetables: Relationship with Maillard Reaction Indicators. Food Res. Int. 2019, 121, 514–523. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of Different Cooking Methods on Nutritional and Physicochemical Characteristics of Selected Vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Ashour, M.M.; El-Hamzy, E.M. Influence of Different Cooking Methods on Physicochemical Characteristics, Phytochemical Profile, Antioxidant Capacity and Chromatic Parameters of Selected Vegetables. Sciences 2017, 7, 1127–1147. [Google Scholar]
- Mazzeo, T.; N’Dri, D.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effect of Two Cooking Procedures on Phytochemical Compounds, Total Antioxidant Capacity and Colour of Selected Frozen Vegetables. Food Chem. 2011, 128, 627–633. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolics, Ascorbic Acid, Carotenoids and Antioxidant Activity of Broccoli and Their Changes during Conventional and Microwave Cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
- Chung, J.-C.; Chou, S.-S.; Hwang, D.-F. Changes in Nitrate and Nitrite Content of Four Vegetables during Storage at Refrigerated and Ambient Temperatures. Food Addit. Contam. 2004, 21, 317–322. [Google Scholar] [CrossRef]
- Chen, G.; Tang, Y.; Zhao, P.; Fan, Z.; He, Y.; Zhang, Q.; Luo, Y.; Wang, D.; Deng, W.; Fan, Y.; et al. Study on Nitrite Content and Its N2-NH4+ Transforming Theory in Paocai and Zhacai. Food Ferment. Sci. Technol. 2021, 57, 1–13. [Google Scholar]
- Martínez, S.; Armesto, J.; Gómez-Limia, L.; Carballo, J. Impact of Processing and Storage on the Nutritional and Sensory Properties and Bioactive Components of Brassica Spp. A Review. Food Chem. 2020, 313, 126065. [Google Scholar] [CrossRef] [PubMed]
- Balan, D.; Israel-Roming, F.; Luta, G.; Gherghina, E. Changes in the Nutrients Content of Some Green Vegetables during Storage and Thermal Processing. Rom. Biotechnol. Lett. 2016, 21, 11857–11865. [Google Scholar]
- Prabhu, S.; Barrett, D.M. Effects of Storage Condition and Domestic Cooking on the Quality and Nutrient Content of African Leafy Vegetables (Cassia tora and Corchorus tridens): Quality and Nutrient Content of African Leafy Vegetables. J. Sci. Food Agric. 2009, 89, 1709–1721. [Google Scholar] [CrossRef]





| Vegetable | Nitrate Content (g/kg DW) a | Nitrite Content (mg/kg DW) a | Antioxidant Capacity (FRAP) d (mmoL AAE/kg DW) a,b | Moisture Content (%) |
|---|---|---|---|---|
| Water spinach | 54.4 ± 2.4 | 18.2 ± 1.1 | 40.7 ± 2.3 | 94.2 ± 1.5 |
| Cabbage | 8.0 ± 0.3 | 6.1 ± 0.6 | 16.8 ± 0.4 | 93.4 ± 0.3 |
| Sunflower seed oil c | ND | ND | <0.1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Liu, Y.; Cui, X.; Zhang, Q.; Wang, Y.; Cao, L.; Luo, X.; Xiong, J.; Ruan, R. Assessment of Potential Nitrite Safety Risk of Leafy Vegetables after Domestic Cooking. Foods 2021, 10, 2953. https://doi.org/10.3390/foods10122953
Wu S, Liu Y, Cui X, Zhang Q, Wang Y, Cao L, Luo X, Xiong J, Ruan R. Assessment of Potential Nitrite Safety Risk of Leafy Vegetables after Domestic Cooking. Foods. 2021; 10(12):2953. https://doi.org/10.3390/foods10122953
Chicago/Turabian StyleWu, Songheng, Yuhuan Liu, Xian Cui, Qi Zhang, Yunpu Wang, Leipeng Cao, Xuan Luo, Jianghua Xiong, and Roger Ruan. 2021. "Assessment of Potential Nitrite Safety Risk of Leafy Vegetables after Domestic Cooking" Foods 10, no. 12: 2953. https://doi.org/10.3390/foods10122953
APA StyleWu, S., Liu, Y., Cui, X., Zhang, Q., Wang, Y., Cao, L., Luo, X., Xiong, J., & Ruan, R. (2021). Assessment of Potential Nitrite Safety Risk of Leafy Vegetables after Domestic Cooking. Foods, 10(12), 2953. https://doi.org/10.3390/foods10122953