Inactivation of Salmonella spp., Escherichia coli O157:H7 and Listeria monocytogenes in Tahini by Microwave Heating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture Preparation
2.2. Stressed Bacterial Suspension Preparation
2.2.1. Desiccation Stress
2.2.2. Starvation
2.3. Tahini Samples and Bacterial Inoculation
2.4. Microwave Heating
2.5. Bacterial Enumeration
2.6. D-Value Calculation
2.7. Chemical and Physical Analysis of Tahini Samples
2.7.1. Proximate Analysis and aw
2.7.2. Acid Value, Peroxide Value, and p-Anisidine Value Determination
2.7.3. Color Measurement
2.8. Statistical Analysis
3. Results
3.1. Microwave Heating Impact on Stressed and Unstressed Salmonella spp.
3.2. Microwave Heating Impact on Stressed and Unstressed E. coli O157:H7
3.3. Microwave Heating Impact on Stressed and Unstressed Listeria Monocytogenes
3.4. Stress-Induced Changes in z-Values
3.5. Thermal Profile of Tahini during Microwave Heating at Different Power Levels
3.6. Proximate Composition and aw of Tahini
3.7. Color Analysis of Control and Microwave-Treated Tahini
3.8. Acid, Peroxide and p-Anisidine Values of Microwave-Heated Tahini
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abu-Jdayil, B.; Al-Malah, K.; Asoud, H. Rheological characterization of milled sesame (tehineh). Food Hydrocoll. 2002, 16, 55–61. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Mansour, E.H. Nutritional and physicochemical evaluations of tahina (sesame butter) pre-pared from heat-treated sesame seeds. J. Sci. Food Agric. 2000, 80, 2005–2011. [Google Scholar] [CrossRef]
- Lake, R.; King, N.; Cressey, P.; Gilbert, S. Salmonella (Non-Typhoidal) in High Lipid Foods Made from Sesame Seeds, Peanuts or Cocoa Beans; European Society of Radiology: Vienna, Austria, 2010. [Google Scholar]
- Unicomb, L.E.; Simmons, G.; Merritt, T.; Gregory, J.; Nicol, C.; Jelfs, P.; Kirk, M.; Tan, A.; Thomson, R.; Adamopoulos, J.; et al. Sesame seed products contaminated with Salmonella: Three outbreaks associated with tahini. Epidemiol. Infect. 2005, 133, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- WHO. Hazard Analysis and Critical Control Point Generic Models for Some Traditional Foods: A Manual for the Eastern Mediterranean Region. 2008. Available online: https://apps.who.int/iris/handle/10665/119885 (accessed on 20 December 2020).
- Olaimat, A.N.; Osaili, T.M.; Al-Holy, M.A.; Al-Nabulsi, A.A.; Obaid, R.S.; Alaboudi, A.R.; Ayyash, M.; Holley, R. Microbial safety of oily, low water activity food products: A review. Food Microbiol. 2020, 92, 103571. [Google Scholar] [CrossRef]
- Yamani, M.I.; Isa, J.K. Microbiological quality of tehena and development of a generic HACCP plan for its production. World J. Agric. Sci. 2006, 2, 290–297. [Google Scholar]
- Karam, L.A. The Determination and Validation of Microbial Criteria for Tahini and Halawi Products. Ph.D. Thesis, American University of Beirut, Beirut, Lebanon, 2010. [Google Scholar]
- QUALEB. GMP Guide for Halawa and Tahini Production. 2015. Available online: https://www.economy.gov.lb/media/10349/qualeb-gmp-tahina-halawa-guide-english.pdf (accessed on 20 December 2020).
- Torlak, E.; Sert, D.; Serin, P. Fate of Salmonella during sesame seeds roasting and storage of tahini. Int. J. Food Microbiol. 2013, 163, 214–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Keller, E.S.; Grasso-Kelley, E.M. Fate of Salmonella throughout production and refrigerated stor-age of tahini. J. Food Prot. 2017, 80, 940–946. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Olaimat, A.N.; Osaili, T.M.; Shaker, R.R.; Elabedeen, N.Z.; Jaradat, Z.W.; Abushelaibi, A.; Holley, R.A. Use of acetic and citric acids to control Salmonella Typhimurium in tahini (sesame paste). Food Microbiol. 2014, 42, 102–108. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Nabulsi, A. Inactivation of stressed Escherichia coli O157:H7 in tahini (sesame seeds paste) by gamma irradiation. Food Control. 2016, 69, 221–226. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Vadivambal, R.; Jayas, D.S. Non-uniform temperature distribution during microwave heating of food mate-rials—A review. Food Bioprocess Technol. Int. J. 2010, 3, 161–171. [Google Scholar] [CrossRef]
- Lakins, D.G.; Alvarado, C.Z.; Thompson, L.D.; Brashears, M.T.; Brooks, J.C.; Brashears, M.M. Reduction of Salmonella Enteritidis in Shell Eggs Using Directional Microwave Technology. Poult. Sci. 2008, 87, 985–991. [Google Scholar] [CrossRef]
- Valero, A.; Cejudo, M.; Garcia-Gimeno, R.M. Inactivation kinetics for Salmonella Enteritidis in potato ome-let using microwave heating treatments. Food Control 2014, 43, 175–182. [Google Scholar] [CrossRef]
- Sung, H.-J.; Kang, D.-H. Effect of a 915 MHz microwave system on inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in salsa. LWT 2014, 59, 754–759. [Google Scholar] [CrossRef]
- Song, W.-J.; Kang, D.-H. Inactivation of Salmonella Senftenberg, Salmonella Typhimurium and Salmonella Tennessee in peanut butter by 915 MHz microwave heating. Food Microbiol. 2016, 53, 48–52. [Google Scholar] [CrossRef]
- Song, W.-J.; Kang, D.-H. Influence of water activity on inactivation of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes in peanut butter by microwave heating. Food Microbiol. 2016, 60, 104–111. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Nabulsi, A.A.; Osaili, T.M.; Al-Holy, M.; Ayyash, M.M.; Mehyar, G.F.; Jaradat, Z.W.; Abu Ghoush, M. Survival and inhibition of Staphylococcus aureus in commercial and hydrated tahini using acetic and citric acids. Food Control. 2017, 77, 179–186. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.; Osaili, T.; Olaimat, A.; Almasri, W.; Al-Holy, M.; Jaradat, Z.; Ayyash, M.; Awaisheh, S.; Holley, R. Inhibitory effect of thyme and cinnamon essential oils against E. coli O157:H7 in Tahini. Food Sci. Technol. 2020, 40, 885–893. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, G.; Gerner-Smidt, P.; Mantripragada, V.; Ezeoke, I.; Doyle, M.P. Thermal Inactivation of Salmonella in Peanut Butter. J. Food Prot. 2009, 72, 1596–1601. [Google Scholar] [CrossRef]
- Syamaladevi, R.; Tang, J.; Villa-Rojas, R.; Sablani, S.; Carter, B.; Campbell, G. Influence of Water Activity on Thermal Resistance of Microorganisms in Low-Moisture Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 353–370. [Google Scholar] [CrossRef] [Green Version]
- Reeve, C.A.; Bockman, A.T.; Matin, A. Role of protein degradation in the survival of carbon-starved Esche-richia coli and Salmonella typhimurium. J. Bacteriol. 1984, 157, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Osaili, T.M.; Al-Nabulsi, A.A.; Aljaafreh, T.F. Use of gamma radiation for inactivating Salmonella spp., Esch-erichia coli O157:H7 and Listeria monocytogenes in tahini halva. Int. J. Food Microbiol. 2018, 278, 20–25. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Nabulsi, A.A.; Aljaafreh, T.F.; Olaimat, A.N. Use of gamma radiation to inactivate stressed Salmonella spp., Escherichia coli O157:H7 and Listeria monocytogenes in tahini halva. LWT 2018, 98, 438–443. [Google Scholar] [CrossRef]
- ISO. ISO 11290-1: Microbiology of Food and Animal Feeding Stuffs—Horizontal Methods for the Detection and Enumeration of Listeria Monocytogenes, Part 1: Detection Method; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- ISO. ISO 16654/2001. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Escherichia Coli O157; International Organization for Standardization: Geneva, Switzerland, 2001. [Google Scholar]
- ISO. Microbiology of Food and Animal Feeding Stuffs: Horizontal method for the detection of Salmonella spp. Detection of Salmonella spp. In Animal Faeces and in Environmental Samples from the Primary Production Stage. Amendment 1, Annex D; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- AOAC. Official Method of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1984. [Google Scholar]
- Osaili, T.; Al-Nabulsi, A.; Nazzal, D.; Al-Holy, M.; Olaimat, A.; Obaid, R.; Holley, R. Effect of water activity and storage of tahini on the viability of stressed Salmonella serovars. Food Sci. Technol. 2021, 41, 144–150. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the AOCS; American Oil Chemists’ Society: Champaign, IL, USA, 1998. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the AOCS; American Oil Chemists’ Society: Champaign, IL, USA, 1993. [Google Scholar]
- Osaili, T.M.; Al-Nabulsi, A.A.; Shaker, N.R.R. Effect of storage temperatures and stresses on the survival of Salmonella spp. in halva. Lett. Appl. Microbiol. 2017, 65, 403–409. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Osaili, T.M.; Shaker, R.R.; Olaimat, A.N.; Attlee, A.; Al-Holy, M.A.; Elabedeen, N.Z.; Jaradar, Z.W.; Holley, R.A. Survival of E. coli O157: H7 and Listeria innocua in tahini (sesame paste). J. Food Agric. Environ. 2013, 11, 303–306. [Google Scholar]
- Alaouie, Z.; Hallal, N.; AlKhatib, A.; Khachfe, H.M. Assessing the microbial quality of tahini (sesame paste) in Lebanon. Glob. Health 2017, 6, 20–25. [Google Scholar]
- Benlloch-Tinoco, M.; Pina-Perez, M.C.; Martinez-Navarrete, N.; Rodrigo, D. Listeria monocytogenes inactivation kinetics under microwave and conventional thermal processing in a kiwi fruit puree. Innov. Food Sci. Emerg. Technol. 2014, 22, 131–136. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Demetriou, D.; Panagiou, A.; Levidiotou, S.; Gessouli, H.; Ionnides, K.; Antoniades, G. Survival of enterobacteria in liquid cultures during microwave radiation and conventional heating. Microbiol. Res. 1995, 150, 305–309. [Google Scholar] [CrossRef]
- Kozempel, M.F.; Annous, B.A.; Cook, R.D.; Scullen, O.J.; Whiting, R.C. Inactivation of Microorganisms with Microwaves at Reduced Temperaturas. J. Food Prot. 1998, 61, 582–585. [Google Scholar] [CrossRef]
- Lu, Y.; Turley, A.; Dong, X.; Wu, C. Reduction of Salmonella enterica on grape tomatoes using microwave heating. Int. J. Food Microbiol. 2010, 145, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Fung, D.Y.C.; Cunningham, F.E. Effect of Microwaves on Microorganisms in Foods. J. Food Prot. 1980, 43, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.E. Microwave Cooking: An Overview. J. Food Prot. 1983, 46, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Heddleson, R.A.; Doores, S. Injury of Salmonella Species Heated by Microwave Energy. J. Food Prot. 1994, 57, 1068–1073. [Google Scholar] [CrossRef]
- Dev, S.; Raghavan, G.; Gariepy, Y. Dielectric properties of egg components and microwave heating for in-shell pasteurization of eggs. J. Food Eng. 2008, 86, 207–214. [Google Scholar] [CrossRef]
- Oliveira, M.; Franca, A. Microwave heating of foodstuffs. J. Food Eng. 2002, 53, 347–359. [Google Scholar] [CrossRef]
- Heddleson, R.A.; Doores, S. Factors affecting microwave heating of foods and microwave induced destruc-tion of foodborne pathogens–a review. J. Food Prot. 1994, 57, 1025–1037. [Google Scholar] [CrossRef]
- Cañumir, J.A.; Celis, J.E.; de Bruijn, J.; Vidal, L.V. Pasteurisation of Apple Juice by Using Microwaves. LWT 2002, 35, 389–392. [Google Scholar] [CrossRef]
- Picouet, P.A.; Landl, A.; Abadias, M.; Castellari, M.; Viñas, I. Minimal processing of a Granny Smith apple purée by microwave heating. Innov. Food Sci. Emerg. Technol. 2009, 10, 545–550. [Google Scholar] [CrossRef]
- Gundavarapu, S.; Hung, Y.-C.; Brackett, R.E.; Mallikarjunan, P. Evaluation of Microbiological Safety of Shrimp Cooked in a Microwave Oven. J. Food Prot. 1995, 58, 742–747. [Google Scholar] [CrossRef]
- Osaili, T.; Griffis, C.L.; Martin, E.M.; Beard, B.L.; Keener, A.; Marcy, J.A. Thermal inactivation studies of Escherichia coli O157:H7, Salmonella, and Listeria monocyto-genes in ready-to-eat chicken-fried beef patties. J. Food Prot. 2006, 69, 1080–1086. [Google Scholar] [CrossRef]
- Griffis, C.L.; Osaili, T.M. Control of Thermal Meat Processing. In Safety of Meat and Processed Meat; Springer: Berlin/Heidelberg, Germany, 2009; pp. 229–253. [Google Scholar]
- Tajchakavit, S.; Ramaswamy, H.; Fustier, P. Enhanced destruction of spoilage microorganisms in apple juice during continuous flow microwave heating. Food Res. Int. 1998, 31, 713–722. [Google Scholar] [CrossRef]
- Salazar, J.K.; Natarajan, V.; Stewart, D.; Warren, J.; Gonsalves, L.J.; Mhetras, T.; Tortorello, M.L. Fate of Listeria monocytogenes in Ready-to-Eat Refrigerated Dips Treated with High Pressure Processing. J. Food Prot. 2019, 82, 1320–1325. [Google Scholar] [CrossRef]
- Russell, N.; Evans, R.; ter Steeg, P.; Hellemons, J.; Verheul, A.; Abee, T. Membranes as a target for stress adaptation. Int. J. Food Microbiol. 1995, 28, 255–261. [Google Scholar] [CrossRef]
- Shaker, R.R.; Osaili, T.M.; Al-Hasan, A.S.A.; Ayyash, M.M.; Forsythe, S.J. Effect of desiccation, starvation, heat, and cold stresses on the thermal resistance of En-terobacter sakazakii in rehydrated infant milk formula. J. Food Sci. 2008, 73, 354–359. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Nabulsi, A.A.; Shaker, R.R.; Ayyash, M.M.; Olaimat, A.N.; Al-Hasan, A.S.A.; Kadora, K.M.; Holley, R.A. Effects of extended dry storage of powdered infant milk formula on susceptibility of Enter-obacter sakazakii to hot water and ionizing radiation. J. Food Prot. 2008, 71, 934–939. [Google Scholar] [CrossRef]
- Koseki, S.; Nakamura, N.; Shiina, T. Comparison of Desiccation Tolerance among Listeria monocytogenes, Escherichia coli O157:H7, Salmonella enterica, and Cronobacter sakazakii in Powdered Infant Formula. J. Food Prot. 2015, 78, 104–110. [Google Scholar] [CrossRef]
- Leenanon, B.; Drake, M.A. Acid Stress, Starvation, and Cold Stress Affect Poststress Behavior of Escherichia coli O157:H7 and Nonpathogenic Escherichia coli. J. Food Prot. 2001, 64, 970–974. [Google Scholar] [CrossRef]
- Lou, Y.; Yousef, A.E. Resistance of Listeria monocytogenes to Heat after Adaptation to Environmental Stresses. J. Food Prot. 1996, 59, 465–471. [Google Scholar] [CrossRef]
- Shen, C.; Geornaras, I.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Thermal inactivation of acid, cold, heat, starvation, and desiccation stress-adapted Escherichia coli O157:H7 in moisture-enhanced nonintact beef. J. Food Prot. 2011, 74, 531–538. [Google Scholar] [CrossRef]
- White, A.P.; Gibson, D.L.; Kim, W.; Kay, W.W.; Surette, M.G. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Sal-monella. J. Bacteriol. 2006, 188, 3219–3227. [Google Scholar] [CrossRef] [Green Version]
- Podolak, R.; Enache, E.; Stone, W.; Black, D.G.; Elliott, P.H. Sources and Risk Factors for Contamination, Survival, Persistence, and Heat Resistance of Salmonella in Low-Moisture Foods. J. Food Prot. 2010, 73, 1919–1936. [Google Scholar] [CrossRef]
- Anriany, Y.A.; Weiner, R.M.; Johnson, J.A.; De Rezende, C.E.; Joseph, S.W. Salmonella enterica Serovar Typhimurium DT104 Displays a Rugose Phenotype. Appl. Environ. Microbiol. 2001, 67, 4048–4056. [Google Scholar] [CrossRef] [Green Version]
- Spector, M.P.; Kenyon, W.J. Resistance and survival strategies of Salmonella enterica to environmental stresses. Food Res. Int. 2012, 45, 455–481. [Google Scholar] [CrossRef]
- Billi, D.; Potts, M. Life and death of dried prokaryotes. Res. Microbiol. 2002, 153, 7–12. [Google Scholar] [CrossRef]
- Kaushik, J.K.; Bhat, R. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J. Biol. Chem. 2003, 278, 26458–26465. [Google Scholar] [CrossRef] [Green Version]
- Potts, M. Desiccation tolerance of prokaryotes. Microbiol. Rev. 1994, 58, 755–805. [Google Scholar] [CrossRef]
- Welsh, D.T.; Herbert, R.A. Osmotically induced intracellular trehalose, but not glycine betaine accumulation promotes desiccation tolerance in Escherichia coli. FEMS Microbiol. Lett. 1999, 174, 57–63. [Google Scholar] [CrossRef] [PubMed]
- De La Vega-Miranda, B.; Santiesteban-López, N.; López-Malo, A.; Sosa-Morales, M. Inactivation of Salmonella Typhimurium in fresh vegetables using water-assisted microwave heating. Food Control 2012, 26, 19–22. [Google Scholar] [CrossRef]
- Ling, B.; Tang, J.; Kong, F.; Mitcham, E.J.; Wang, S. Kinetics of Food Quality Changes During Thermal Processing: A Review. Food Bioprocess Technol. 2014, 8, 343–358. [Google Scholar] [CrossRef]
- Nam, K.; Ahn, D. Effects of Ascorbic Acid and Antioxidants on the Color of Irradiated Ground Beef. J. Food Sci. 2003, 68, 1686–1690. [Google Scholar] [CrossRef]
- Zeng, X.-A.; Han, Z.; Zi, Z.-H. Effects of pulsed electric field treatments on quality of peanut oil. Food Control 2010, 21, 611–614. [Google Scholar] [CrossRef]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef]
- Mohammed, K.; Koko, M.; Obadi, M.; Letsididi, K.S.; Cao, P.; Liu, Y. Effects of microwave roasting process and time on chemical composition and oxidative stability of sunflower oil. J. Food Nutr. Res. 2017, 5, 659–667. [Google Scholar]
- Ali, M.A.; Nargis, A.; Othman, N.H.; Noor, A.F.; Sadik, G.; Hossen, J. Oxidation stability and compositional characteristics of oils from microwave roasted pumpkin seeds during thermal oxidation. Int. J. Food Prop. 2017, 20, 2569–2580. [Google Scholar] [CrossRef]
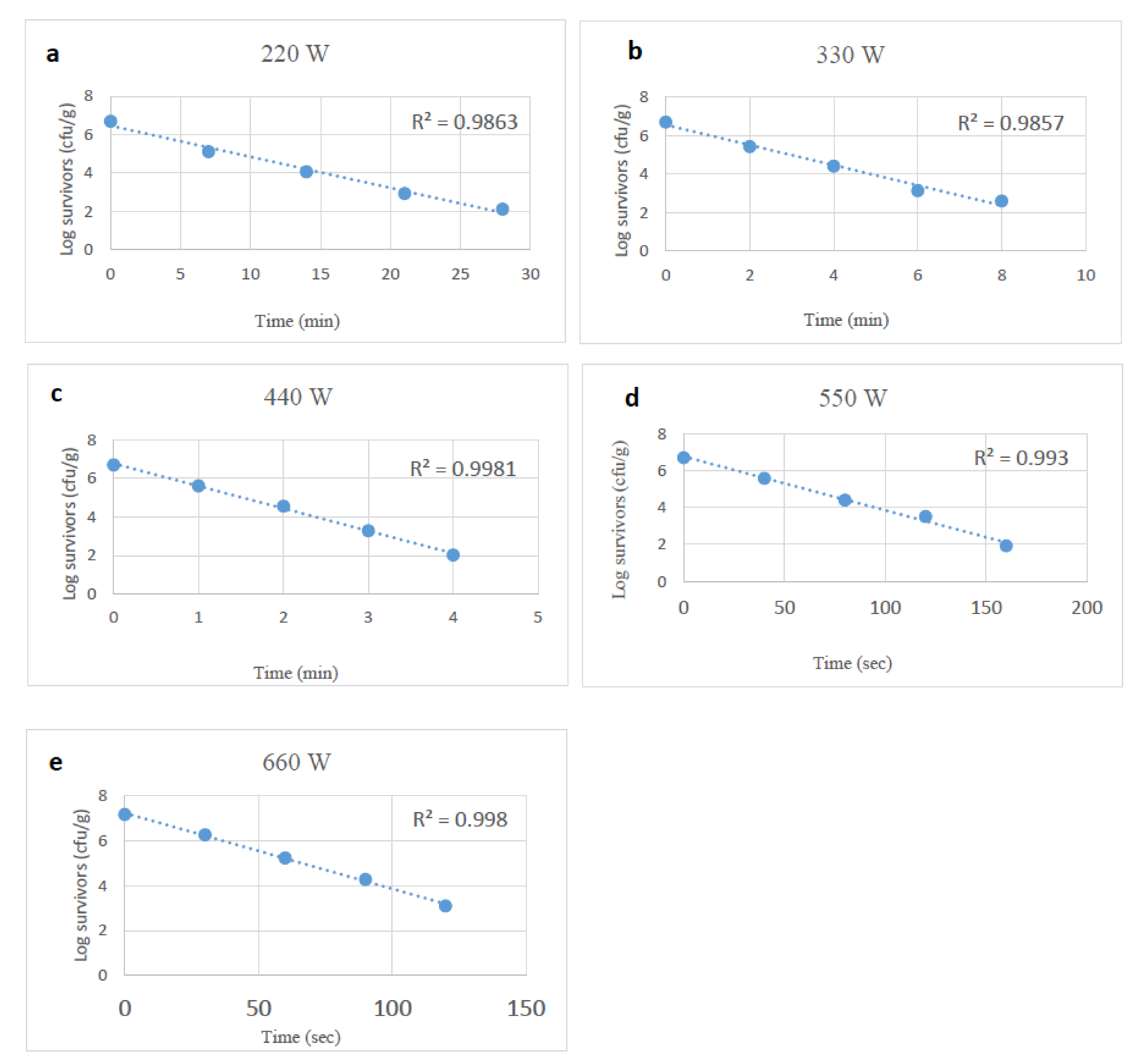

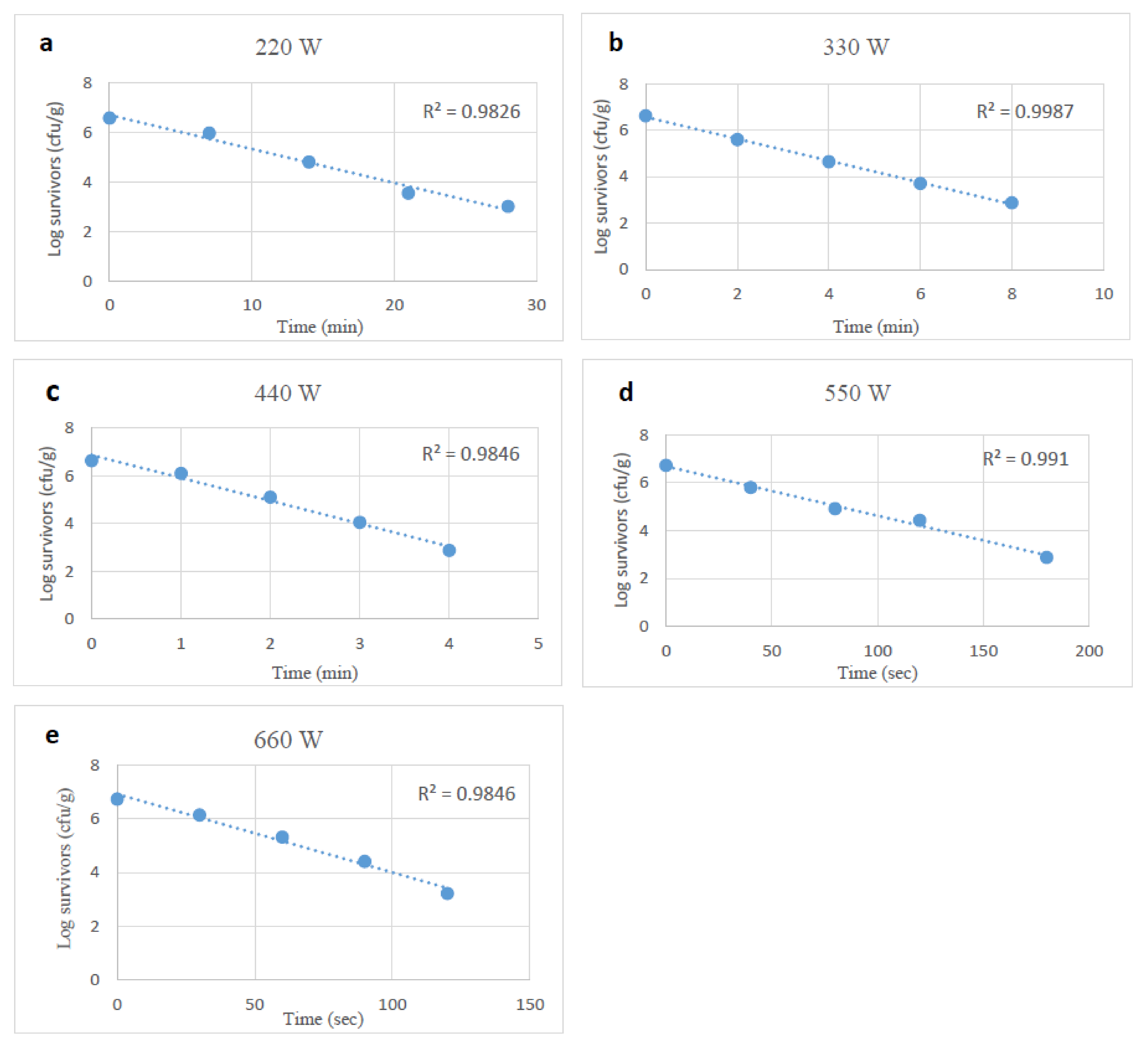







| D-Values (min) | |||||
|---|---|---|---|---|---|
| Watts | 220 | 330 | 440 | 550 | 660 |
| Control | 6.18 ± 0.24 †,A | 1.91 ± 0.03 B | 0.86 ± 0.01 C | 0.57 ± 0.01 D | 0.50 ± 0.02 D |
| Desiccation | 5.64 ± 0.19 A,* | 2.01 ± 0.08 B | 0.99 ± 0.03 C,* | 0.79 ± 0.05 D,* | 0.60 ± 0.05 E,* |
| Starvation | 7.29 ± 0.04 A,* | 2.13 ± 0.09 B,* | 1.05 ± 0.04 C,* | 0.81 ± 0.02 D,* | 0.57 ± 0.02 E,* |
| D-Values (min) | |||||
|---|---|---|---|---|---|
| Watts | 220 | 330 | 440 | 550 | 660 |
| Control | 6.08 ± 0.54 †,A | 1.80 ± 0.20 B | 1.04 ± 0.11 C | 0.84 ± 0.03 C,D | 0.50 ± 0.01 D |
| Desiccation | 5.76 ± 0.54 A | 1.93 ± 0.10 B | 1.14 ± 0.12 C | 0.86 ± 0.12 C,D | 0.60 ± 0.05 D,* |
| Starvation | 6.91 ± 0.75 A | 1.93 ± 0.10 B | 1.28 ± 0.12 C | 0.83 ± 0.08 C,D | 0.60 ± 0.05 D,* |
| D-Values (min) | |||||
|---|---|---|---|---|---|
| Watts | 220 | 330 | 440 | 550 | 660 |
| Control | 4.69 ± 0.21 †,A | 1.93 ± 0.20 B | 0.91 ± 0.10 C | 0.72 ± 0.04 C,D | 0.48 ±0.02 D |
| Desiccation | 6.17 ± 0.48 A,* | 2.10 ± 0.08 B | 1.31± 0.07 C,* | 0.92± 0.01 C,D,* | 0.63 ± 0.04 D,* |
| Starvation | 7.15 ± 0.28 A,* | 2.38 ± 0.23 B | 1.28 ± 0.11 C,* | 0.88 ± 0.05 D,* | 0.59 ± 0.04 D,* |
| Z-Values (Watts) † | |||
|---|---|---|---|
| Microorganism | Control | Desiccation | Starvation |
| Salmonella | 410 † ± 9.6 | 470 ± 12.4 * | 420 ± 10.0 |
| Escherichia coli O157:H7 | 440 ± 18.9 | 480 ± 22.7 | 460 ± 12.5 |
| Listeria monocytogenes | 460 ± 12.5 | 470 ± 12.5 | 420 ± 10.5 * |
| Temperature (°C) | L * | a * | b * |
|---|---|---|---|
| 23 | 57.87 ± 0.32 ††,A | 1.07 ± 0.07 A | 29.37 ± 0.13 A |
| 60 | 57.56 ± 0.07 A,B | 1.05 ± 0.06 A | 29.74 ± 0.21 A,B |
| 90 | 57.43 ± 0.04 A,B | 1.14 ± 0.08 A | 29.77 ± 0.93 A,B |
| 120 | 57.24 ± 0.08 A,B | 1.52 ± 0.40 B | 30.50 ± 0.09 A,B |
| 150 | 57.00 ± 0.92 B | 1.54 ± 0.13 B | 30.57 ± 0.42 B |
| Temperature (°C) | Acid Value (mg KOH/g) | Peroxide Value (meq O2/kg oil) | p-Anisidine Value |
|---|---|---|---|
| 23 | 0.82 ± 0.01 †,A | 1.52 ± 0.02 A | 1.64 ± 0.01 A |
| 60 | 0.84 ± 0.01 A | 1.58 ± 0.02 A,B | 1.66 ± 0.01 A,B |
| 90 | 0.88 ± 0.02 B | 1.60 ± 0.05 A,B | 1.87 ± 0.09 B,C |
| 120 | 0.95 ± 0.01 C | 1.63 ± 0.05 A,B | 2.04 ± 0.12 C |
| 150 | 1.00 ± 0.01 D | 1.65 ± 0.07 B | 2.80 ± 0.11 D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osaili, T.M.; Al-Nabulsi, A.A.; Al Sheikh, Y.M.; Alaboudi, A.R.; Olaimat, A.N.; Al-Holy, M.; Al-Rousan, W.M.; Holley, R. Inactivation of Salmonella spp., Escherichia coli O157:H7 and Listeria monocytogenes in Tahini by Microwave Heating. Foods 2021, 10, 2972. https://doi.org/10.3390/foods10122972
Osaili TM, Al-Nabulsi AA, Al Sheikh YM, Alaboudi AR, Olaimat AN, Al-Holy M, Al-Rousan WM, Holley R. Inactivation of Salmonella spp., Escherichia coli O157:H7 and Listeria monocytogenes in Tahini by Microwave Heating. Foods. 2021; 10(12):2972. https://doi.org/10.3390/foods10122972
Chicago/Turabian StyleOsaili, Tareq M., Anas A. Al-Nabulsi, Yasmeen M. Al Sheikh, Akram R. Alaboudi, Amin N. Olaimat, Murad Al-Holy, Walid M. Al-Rousan, and Richard Holley. 2021. "Inactivation of Salmonella spp., Escherichia coli O157:H7 and Listeria monocytogenes in Tahini by Microwave Heating" Foods 10, no. 12: 2972. https://doi.org/10.3390/foods10122972
APA StyleOsaili, T. M., Al-Nabulsi, A. A., Al Sheikh, Y. M., Alaboudi, A. R., Olaimat, A. N., Al-Holy, M., Al-Rousan, W. M., & Holley, R. (2021). Inactivation of Salmonella spp., Escherichia coli O157:H7 and Listeria monocytogenes in Tahini by Microwave Heating. Foods, 10(12), 2972. https://doi.org/10.3390/foods10122972







