Effect of Chitosan Coatings with Cinnamon Essential Oil on Postharvest Quality of Mangoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coating Solutions
- Control: distilled water;
- E: 0.1% Tween 80 (w/v) + 0.2% CEO (v/v);
- PE: 0.1% CNC (w/v) + 0.2% CEO (v/v);
- CH: 1% CH (w/v);
- CH-E: 1% CH (w/v) + 0.1% Tween 80 (w/v) + 0.2% CEO (v/v);
- CH-PE: 1% CH (w/v) + 0.1% CNC (w/v) + 0.2% CEO (v/v).
2.3. Electrical Charges of the Emulsions
2.4. Characterization of Edible Coatings
2.4.1. Water Solubility (WS)
2.4.2. Water Vapor Permeability (WVP)
2.4.3. Migration of CEO in Different Food Simulants
2.5. Application of Coating in Mango Fresh Storage
2.6. Weight Loss
2.7. Hardness
2.8. Total Soluble Solid (TSS)
2.9. Titratable Acidity (TA)
2.10. Ascorbic Acid Content
2.11. Malondialdehyde (MDA) Content
2.12. Polyphenol Oxidase (PPO) and Peroxidase (POD) Activity Assay
2.13. Phenylalanine Ammonialyase (PAL) Activity Assay
2.14. Pectin methyl Esterase (PME) Activity Assay
2.15. Polygalacturonase (PG) Activity Assay
2.16. Data Analysis
3. Results and Discussion
3.1. Zeta-Potential of Emulsions and Physical Properties of Coatings
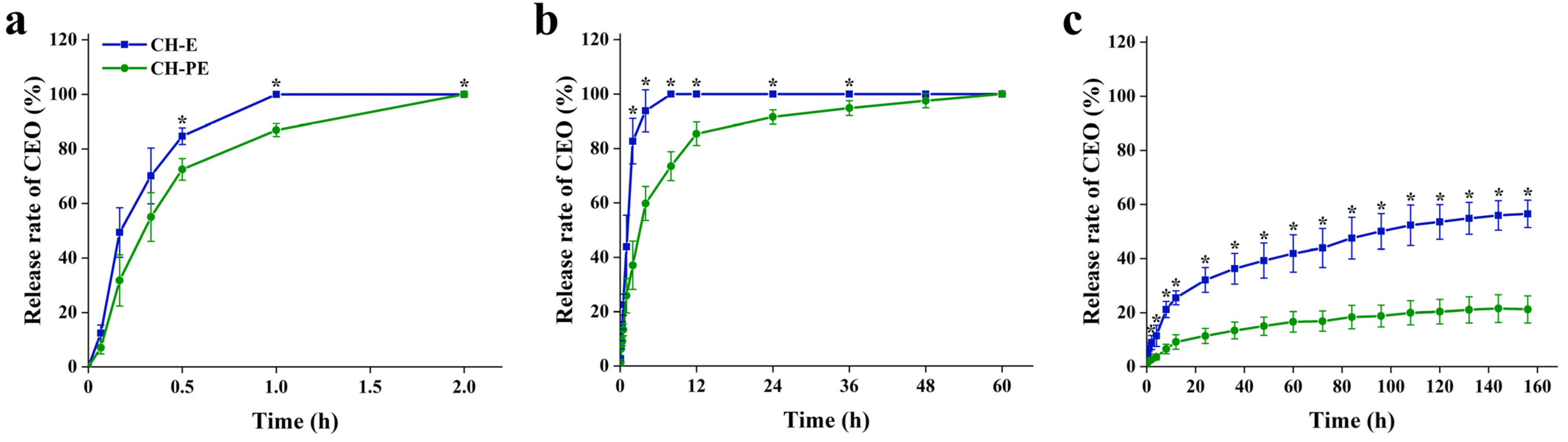
3.2. Migration of CEO in Different Food Simulants
3.3. Effects of The Coatings on Mango Sensory and Nutrient Qualities
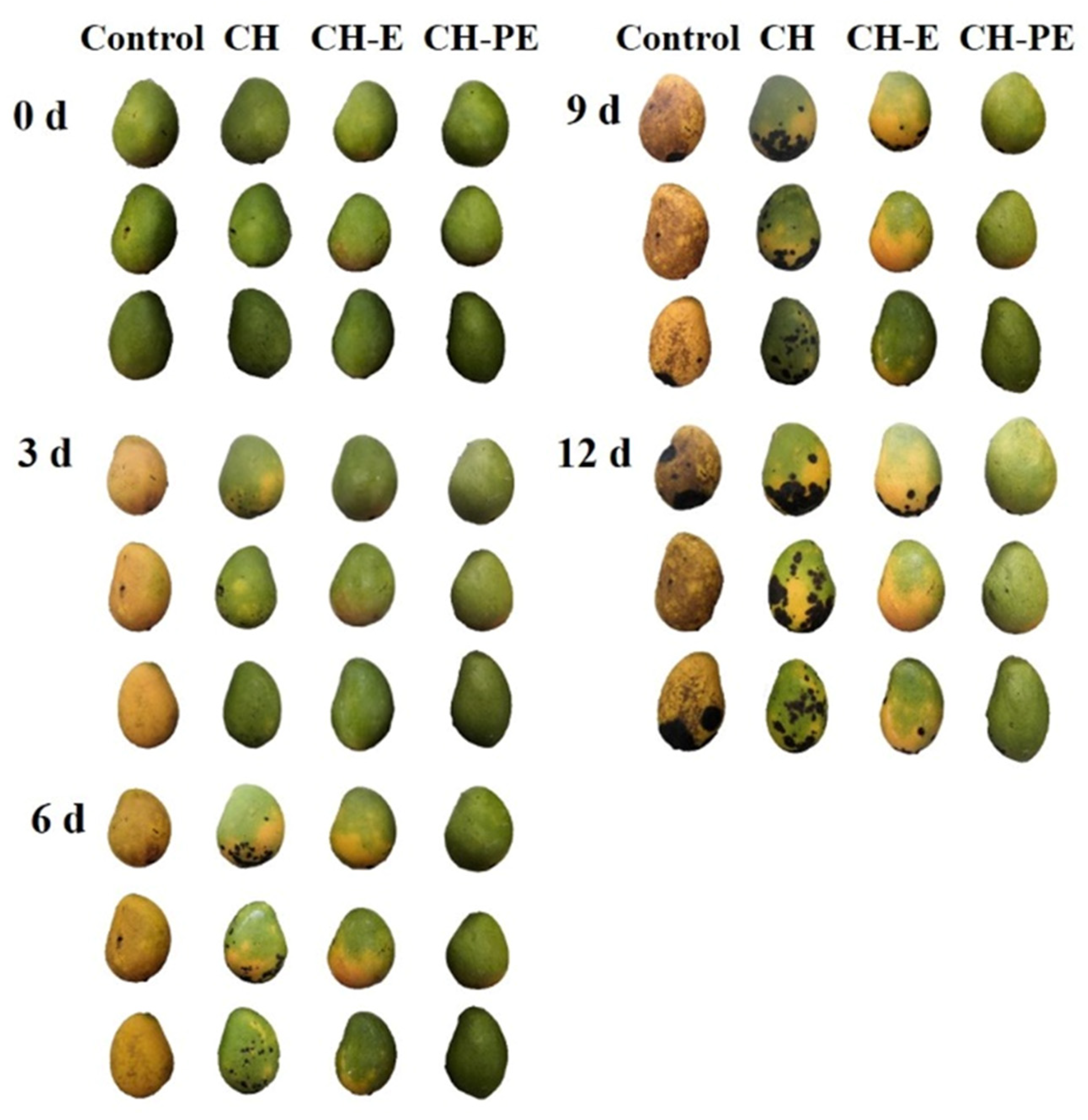
3.3.1. Visual Appearance
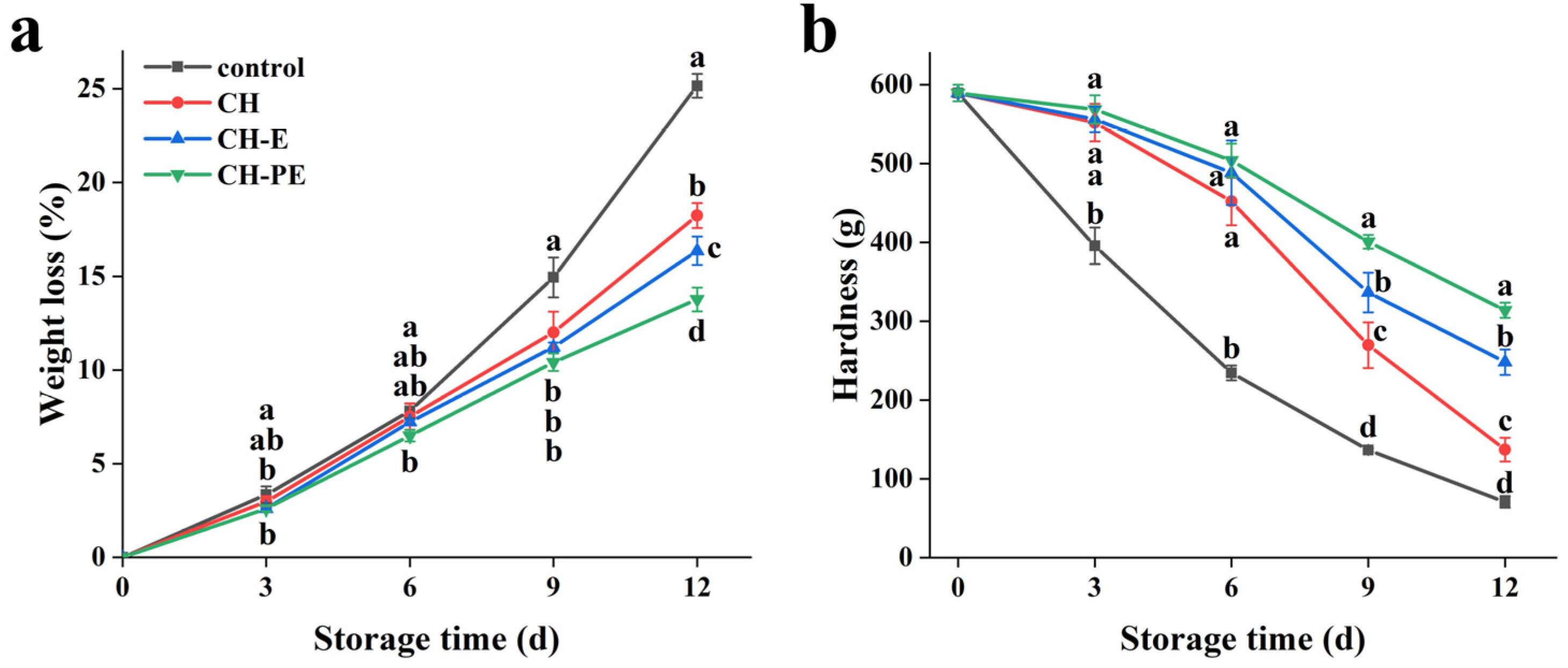
3.3.2. Weight Loss and Hardness
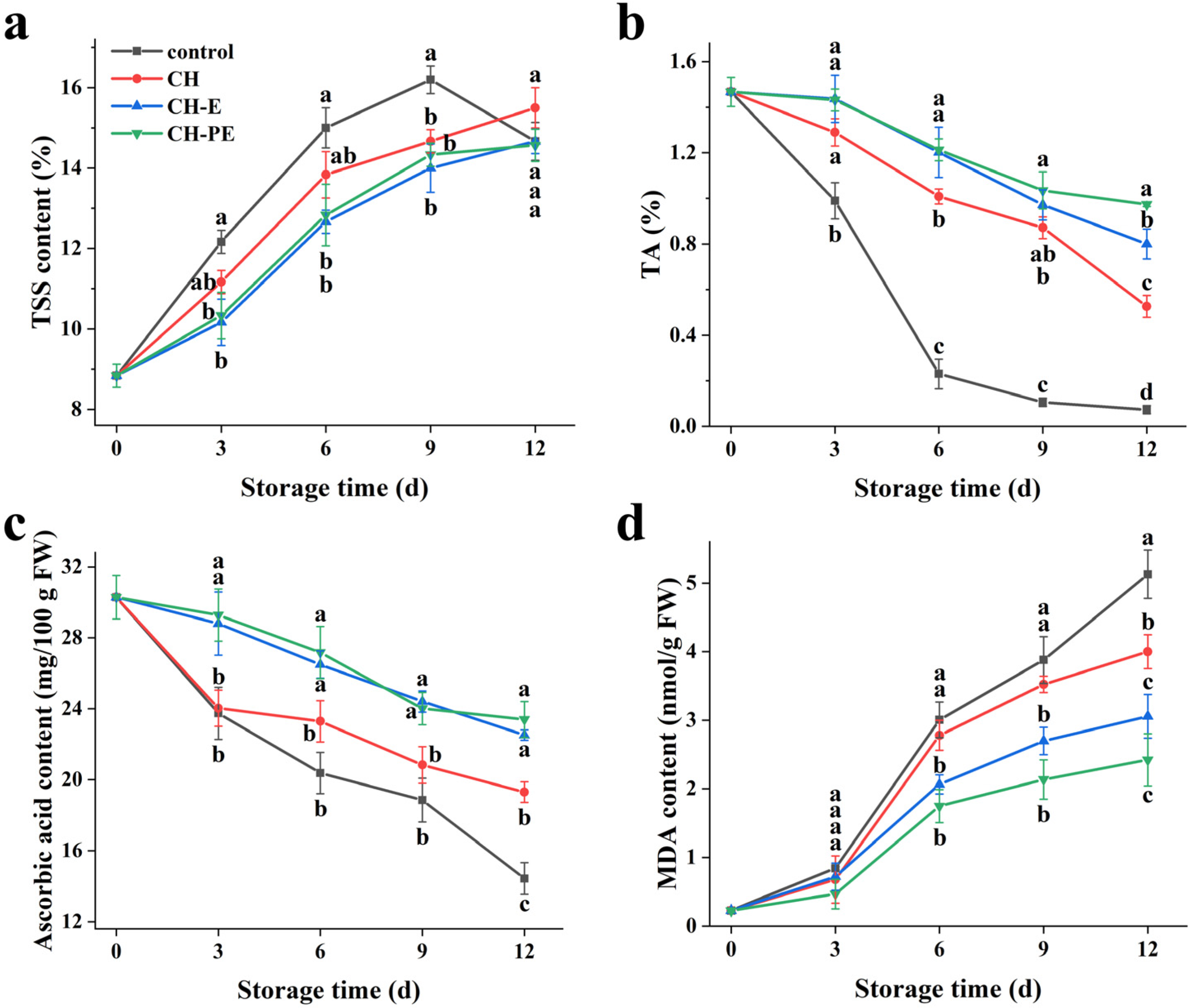
3.3.3. TSS, TA, Ascorbic Acid and MDA
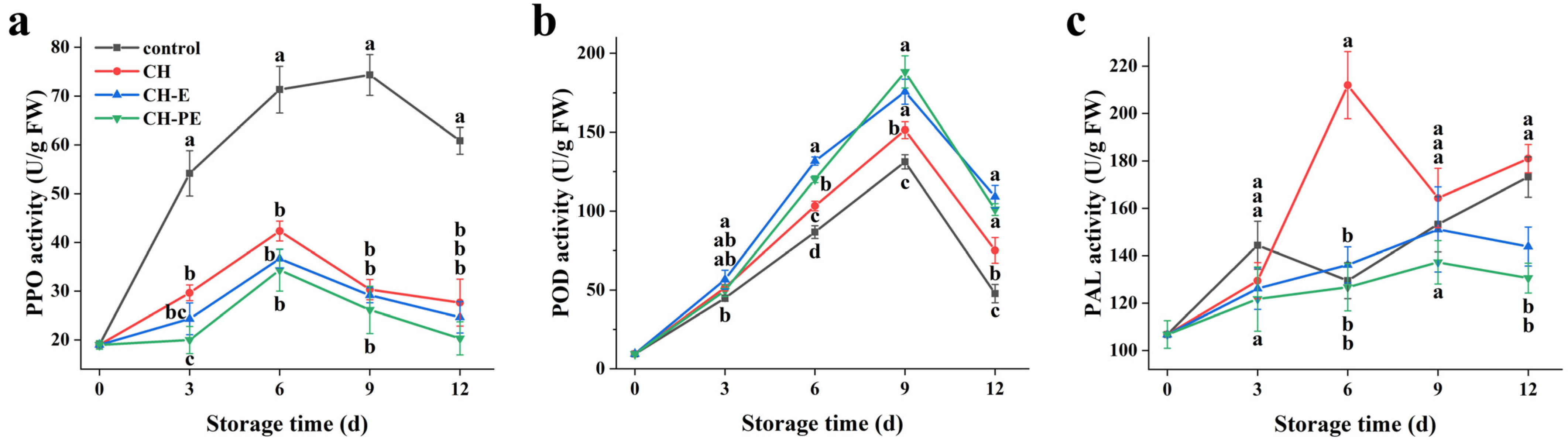
3.4. Effects of the Coatings on Activities of Mango PPO, POD and PAL
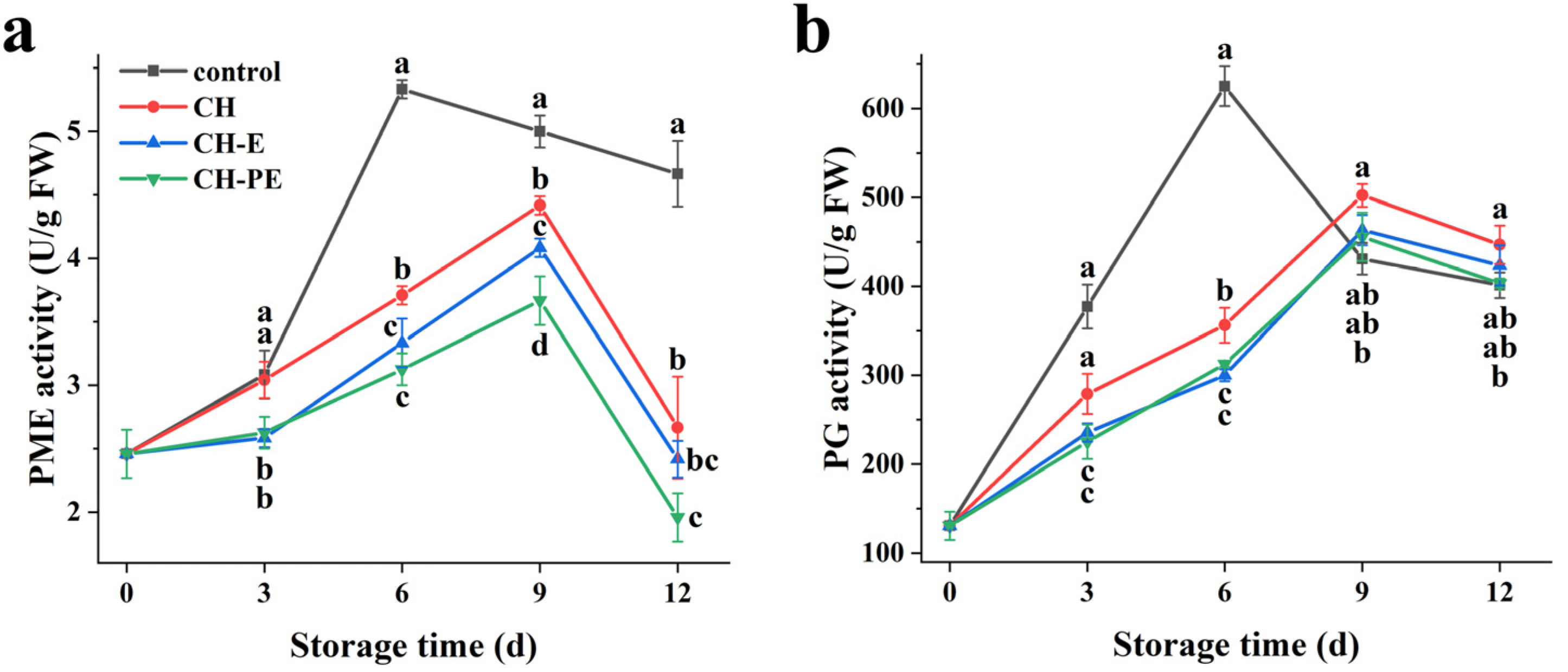
3.5. Effects of The Coatings on Activities of Mango PME and PG
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Mangos and their bioactive components: Adding variety to the fruit plate for health. Food Funct. 2017, 8, 3010–3032. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, K.Á.R.; da Conceição, M.L.; de Oliveira, S.P.A.; Lima, M.D.; Galvão, M.D.; Madruga, M.S.; Magnani, M.; de Souza, E.L. Postharvest quality improvements in mango cultivar Tommy Atkins by chitosan coating with Mentha piperita L. essential oil. J. Horticult. Sci. Biotechnol. 2020, 95, 260–272. [Google Scholar] [CrossRef]
- Bambalele, N.L.; Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. Recent advances on postharvest technologies of mango fruit: A review. Int. J. Fruit Sci. 2021, 21, 565–586. [Google Scholar] [CrossRef]
- de Oliveira, K.Á.R.; Fernandes, K.F.D.; de Souza, E.L. Current advances on the development and application of probiotic-loaded edible films and coatings for the bioprotection of fresh and minimally processed fruit and vegetables. Foods 2021, 10, 2207. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Gamage, M.V.; Dumée, L.F.; Kong, L.X.; Zhao, S.F. Edible films and coatings for shelf life extension of mango: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–29. [Google Scholar] [CrossRef]
- Pirozzi, A.; Grosso, V.D.; Ferrari, G.; Donsì, F. Edible coatings containing oregano essential oil nanoemulsion for improving postharvest quality and shelf life of tomatoes. Foods 2020, 9, 1605. [Google Scholar] [CrossRef]
- Lan, W.J.; Wang, S.Y.; Chen, M.R.; Sameen, D.E.; Lee, K.; Liu, Y.W. Developing poly(vinyl alcohol)/chitosan films incorporate with D-limonene: Study of structural, antibacterial, and fruit preservation properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- de Souza, E.L.; Lundgren, G.A.; de Oliveira, K.Á.R.; Berger, L.R.R.; Magnani, M. An analysis of the published literature on the effects of edible coatings formed by polysaccharides and essential oils on postharvest microbial control and overall quality of fruit. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 1947–1967. [Google Scholar] [CrossRef] [Green Version]
- Sarıcaoglu, F.T.; Turhan, S. Physicochemical, antioxidant and antimicrobial properties of mechanically deboned chicken meat protein films enriched with various essential oils. Food Packag. Shelf Life 2020, 25, 100527. [Google Scholar] [CrossRef]
- Xing, Y.G.; Lin, H.B.; Cao, D.; Xu, Q.L.; Han, W.F.; Wang, R.R.; Che, Z.M.; Li, X.H. Effect of chitosan coating with cinnamon oil on the quality and physiological attributes of China jujube fruits. Biomed. Res. Int. 2015, 2015, 835151. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.B.; Wang, N.; Wei, Y.Y.; Zou, X.R.; Jiang, S.; Xu, F.; Wang, H.F.; Shao, X.F. Terpinen-4-ol enhances disease resistance of postharvest strawberry fruit more effectively than tea tree oil by activating the phenylpropanoid metabolism pathway. J. Agric. Food Chem. 2020, 68, 6739–6747. [Google Scholar] [CrossRef]
- Solís-Contreras, G.A.; Rodríguez-Guillermo, M.C.; Reyes-Vega, M.D.L.L.; Aguilar, C.N.; Rebolloso-Padilla, O.N.; Corona-Flores, J.; Soriano-Melgar, L.D.A.A.; Ruelas-Chacon, X. Extending shelf-life and quality of minimally processed Golden Delicious apples with three bioactive coatings combined with cinnamon essential oil. Foods 2021, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.K.; Bhargava, K.; Kotturi, H. Antimicrobial activity of cinnamon oil nanoemulsion against Listeria monocytogenes and Salmonella spp. on melons. LWT-Food Sci. Technol. 2019, 111, 682–687. [Google Scholar] [CrossRef]
- Fathi, M.; Vinceković, M.; Jurić, S.; Viskić, M.; Jambrak, A.R.; Donsì, F. Food-Grade colloidal systems for the delivery of essential oils. Food Rev. Int. 2021, 37, 1–45. [Google Scholar] [CrossRef]
- Pakdel, A.S.; Niinivaara, E.; Cranston, E.D.; Berry, R.M.; Dubé, M.A. Cellulose nanocrystal (CNC)-latex nanocomposites: Effect of CNC hydrophilicity and charge on rheological, mechanical, and adhesive properties. Macromol. Rapid Commun. 2021, 42, 2000448. [Google Scholar] [CrossRef]
- Zhang, H.C.; Jung, J.; Zhao, Y.Y. Preparation and characterization of cellulose nanocrystals films incorporated with essential oil loaded β-chitosan beads. Food Hydrocolloids 2017, 69, 164–172. [Google Scholar] [CrossRef]
- Deng, Z.L.; Jung, J.; Simonsen, J.; Zhao, Y.Y. Cellulose nanocrystals Pickering emulsion incorporated chitosan coatings for improving storability of postharvest Bartlett pears (Pyrus communis) during long-term cold storage. Food Hydrocolloids 2018, 84, 229–337. [Google Scholar] [CrossRef]
- Lian, H.; Peng, Y.; Shi, J.Y.; Wang, Q.G. Effect of emulsifier hydrophilic-lipophilic balance (HLB) on the release of thyme essential oil from chitosan films. Food Hydrocolloids 2019, 97, 105213. [Google Scholar] [CrossRef]
- Vázquez-Celestino, D.; Ramos-Sotelo, H.; Rivera-Pastrana, D.M.; Vázquez-Barrios, M.E.; Mercado-Silva, E.M. Effects of waxing, microperforated polyethylene bag, 1-methylcyclopropene and nitric oxide on firmness and shrivel and weight loss of ‘Manila’ mango fruit during ripening. Postharvest Biol. Technol. 2016, 111, 398–405. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.X.; Wang, J.; Liu, Y.; Lu, P.; Huang, L.J. Effect of chitosan- and alginate-based coatings enriched with cinnamon essential oil microcapsules to improve the postharvest quality of mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.D.; Huang, C.X.; Zhang, L.Y.; Wang, J.; Huang, X.Q.; Zhao, Y.; Liu, Y.; Li, C.C. Application of chlorine dioxide microcapsule sustained-release antibacterial films for preservation of mangos. J. Food Sci. Technol.-Mysore 2019, 56, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Q.; Zhao, S.; Gong, J.; Huang, M.; Yu, W.W.; Zhang, K.K.; Hu, D.Y. Dissipation and the effects of thidiazuron on antioxidant enzyme activity and malondialdehyde content in strawberry. J. Sci. Food Agric. 2019, 99, 4331–4337. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Stockmann, R.; Terefe, N.S. Effect of citric acid and high pressure thermal processing on enzyme activity and related quality attributes of pear puree. Innov. Food Sci. Emerg. Technol. 2018, 45, 196–207. [Google Scholar] [CrossRef]
- Fan, X.G.; Jiang, W.B.; Gong, H.S.; Yang, Y.Q.; Zhang, A.D.; Liu, H.; Cao, J.K.; Guo, F.J.; Cui, K.B. Cell wall polysaccharides degradation and ultrastructure modification of apricot during storage at a near freezing temperature. Food Chem. 2019, 300, 125194. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sui, J.; Yu, B.; Yuan, C.; Guo, L.; Abd El-Aty, A.M.; Cui, B. Physicochemical properties and antibacterial activity of corn starch-based films incorporated with Zanthoxylum bungeanum essential oil. Carbohydr. Polym. 2021, 254, 117314. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Wu, M.; Wang, C.X.; Yan, S.; Lu, P.; Wang, S.F. Developed chitosan/oregano essential oil biocomposite packaging film enhanced by cellulose nanofibril. Polymers 2020, 12, 1780. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; González-Martínez, C.; Chiralt, A.; Desobry, S. Study of the release of limonene present in chitosan films enriched with bergamot oil in food simulants. J. Food Eng. 2011, 105, 138–143. [Google Scholar] [CrossRef]
- Ma, J.J.; Zhou, Z.Q.; Li, K.; Li, K.; Liu, L.X.; Zhang, W.W.; Xu, J.; Tu, X.H.; Du, L.Q.; Zhang, H. Novel edible coating based on shellac and tannic acid for prolonging postharvest shelf life and improving overall quality of mango. Food Chem. 2021, 354, 129510. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.L.; Zhou, G.X.; Yuan, M.L.; Qin, Y.Y. Biodegradable poly(lactic-acid)/poly(trimethylene-carbonate)/laponite composite film: Development and application to the packaging of mushrooms (Agaricus bisporus). Polym. Adv. Technol. 2015, 26, 1600–1607. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.X.; Liu, F.; Liao, L.K.; Huang, X.B.; Li, R.Y.; Zou, Y.; Zhou, L.; Zou, L.Q.; Liu, Y.H.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.Q.; Wang, Z.D.; Duan, Y.Q. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef]
- Xing, Y.G.; Yang, H.; Guo, X.L.; Bi, X.F.; Liu, X.C.; Xu, Q.L.; Wang, Q.; Li, W.X.; Li, X.L.; Shui, Y.R.; et al. Effect of chitosan/Nano-TiO2 composite coatings on the postharvest quality and physicochemical characteristics of mango fruits. Sci. Hortic. 2020, 263, 109135. [Google Scholar] [CrossRef]
- Jung, J.; Deng, Z.L.; Zhao, Y.Y. Mechanisms and performance of cellulose nanocrystals Pickering emulsion chitosan coatings for reducing ethylene production and physiological disorders in postharvest ‘Bartlett’ pears (Pyrus communis L.) during cold storage. Food Chem. 2020, 309, 125693. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, T.; Liu, W.; Zou, L.Q.; Liu, C.M.; Terefe, N.S. Inhibitory effects of organic acids on polyphenol oxidase: From model systems to food systems. Crit. Rev. Food Sci. Nutr. 2020, 60, 3594–3621. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, L.; Miao, J.Y.; Yu, W.Z.; Zou, L.Q.; Zhou, W.; Liu, C.M.; Liu, W. Effect of cinnamon essential oil nanoemulsion combined with ascorbic acid on enzymatic browning of cloudy apple juice. Food Bioprocess Technol. 2020, 13, 860–870. [Google Scholar] [CrossRef]
- Gong, D.Q.; Zhu, S.J.; Gu, H.; Zhang, L.B.; Hong, K.Q.; Xie, J.H. Disease resistance of ‘Zill’ and ‘Keitt’ mango fruit to anthracnose in relation to defence enzyme activities and the content of anti-fungal substances. J. Horticult. Sci. Biotechnol. 2013, 88, 243–250. [Google Scholar] [CrossRef]
- Chen, C.Y.; Nie, Z.P.; Wan, C.P.; Chen, J.Y. Preservation of Xinyu tangerines with an edible coating using Ficus hirta Vahl. fruits extract-incorporated chitosan. Biomolecules 2019, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Y.; Wang, N.; Jiang, S.H.; Xu, H.F.; Wang, Y.C.; Wang, C.Z.; Li, M.; Liu, J.X.; Qu, C.Z.; Liu, W.; et al. Analysis of the xyloglucan endotransglucosylase/hydrolase gene family during apple fruit ripening and softening. J. Agric. Food Chem. 2017, 65, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol.Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.M.; Ma, F.W.; Shi, S.G.; Qi, X.D.; Zhu, X.Q.; Yuan, J.W. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]
| E | PE | CH | CH-E | CH-PE | |
|---|---|---|---|---|---|
| Zeta-potential (mV) | 19.167 ± 0.551 c | −55.133 ± 3.844 d | 34.033 ± 4.255 b | 67.367 ± 6.882 a | 56.000 ± 7.672 a |
| WVP (×10−11 g/m·s·Pa) | - | - | 11.869 ± 0.379 a | 7.518 ± 0.724 b | 5.222 ± 0.100 c |
| WS (%) | - | - | 31.829 ± 0.438 a | 17.463 ± 0.797 b | 13.911 ± 0.985 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Xu, J.; Zhou, L.; Zou, L.; Liu, W. Effect of Chitosan Coatings with Cinnamon Essential Oil on Postharvest Quality of Mangoes. Foods 2021, 10, 3003. https://doi.org/10.3390/foods10123003
Yu K, Xu J, Zhou L, Zou L, Liu W. Effect of Chitosan Coatings with Cinnamon Essential Oil on Postharvest Quality of Mangoes. Foods. 2021; 10(12):3003. https://doi.org/10.3390/foods10123003
Chicago/Turabian StyleYu, Kaibo, Jing Xu, Lei Zhou, Liqiang Zou, and Wei Liu. 2021. "Effect of Chitosan Coatings with Cinnamon Essential Oil on Postharvest Quality of Mangoes" Foods 10, no. 12: 3003. https://doi.org/10.3390/foods10123003
APA StyleYu, K., Xu, J., Zhou, L., Zou, L., & Liu, W. (2021). Effect of Chitosan Coatings with Cinnamon Essential Oil on Postharvest Quality of Mangoes. Foods, 10(12), 3003. https://doi.org/10.3390/foods10123003






