The Effect of 1-MCP on the Expression of Carotenoid, Chlorophyll Degradation, and Ethylene Response Factors in ‘Qihong’ Kiwifruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials and Treatment
2.2. Measurement of Physiological Indices
2.3. Carotenoid and Chlorophyll Measurements
2.4. Gene Identification and Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.5. Correlation Analysis and Network Visualization
2.6. Promoter Analysis
2.7. Dual-Luciferase Assays
2.8. Statistical Analysis
3. Results and Discussion
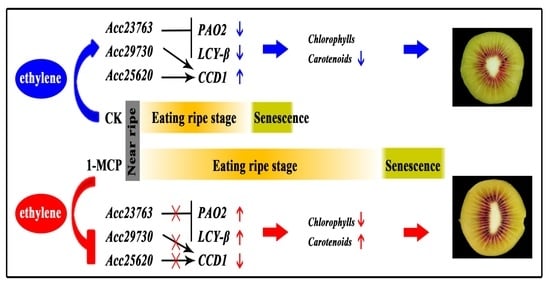
3.1. The Fruit Physiology Indicators and Flesh Color after 1-MCP Treatment
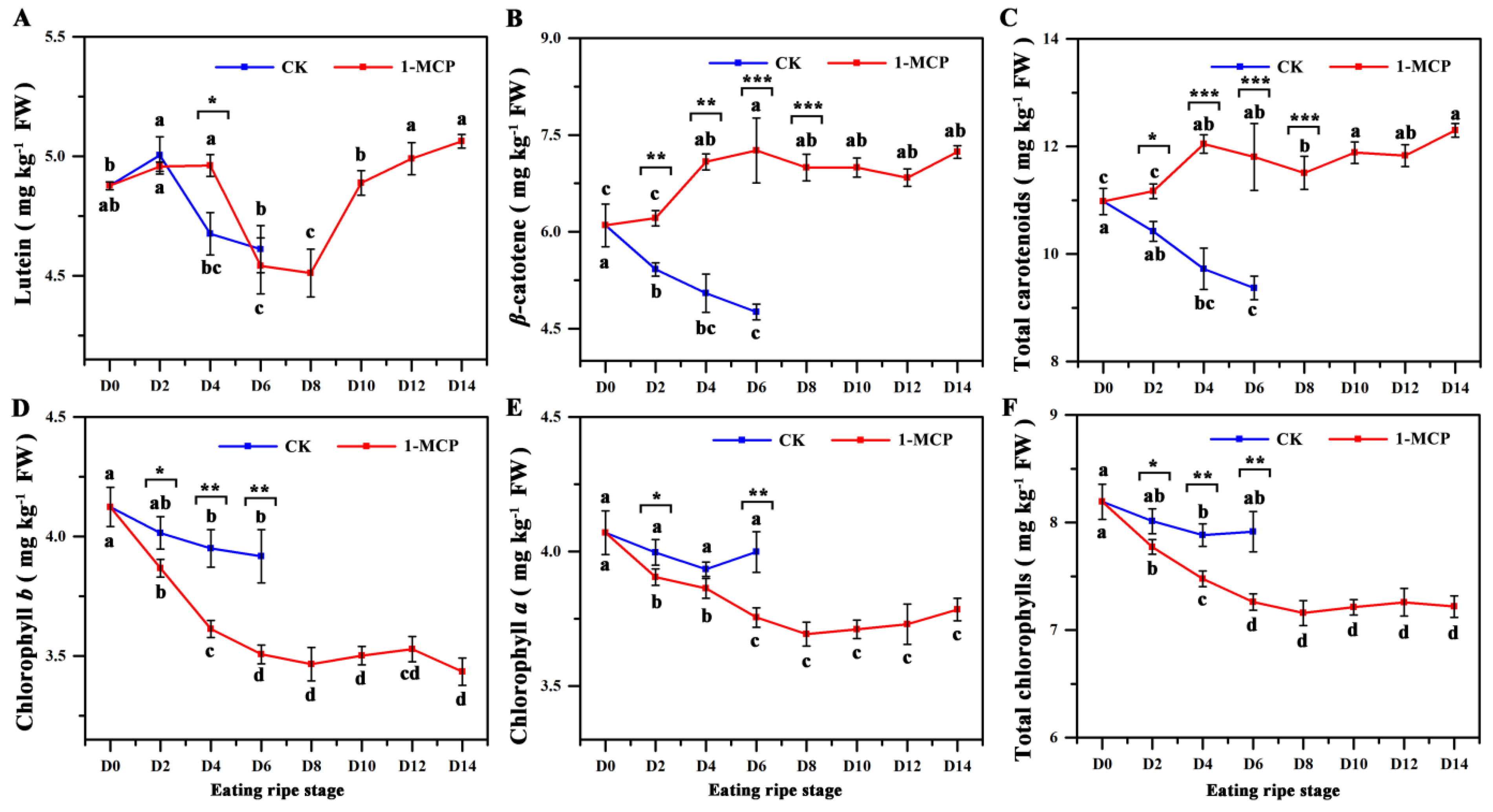
3.2. The Fruit Carotenoid Contents in 1-MCP Treated Fruit and Control Fruit
3.3. The Fruit Chlorophyll Contents in 1-MCP Treated and Control Fruit
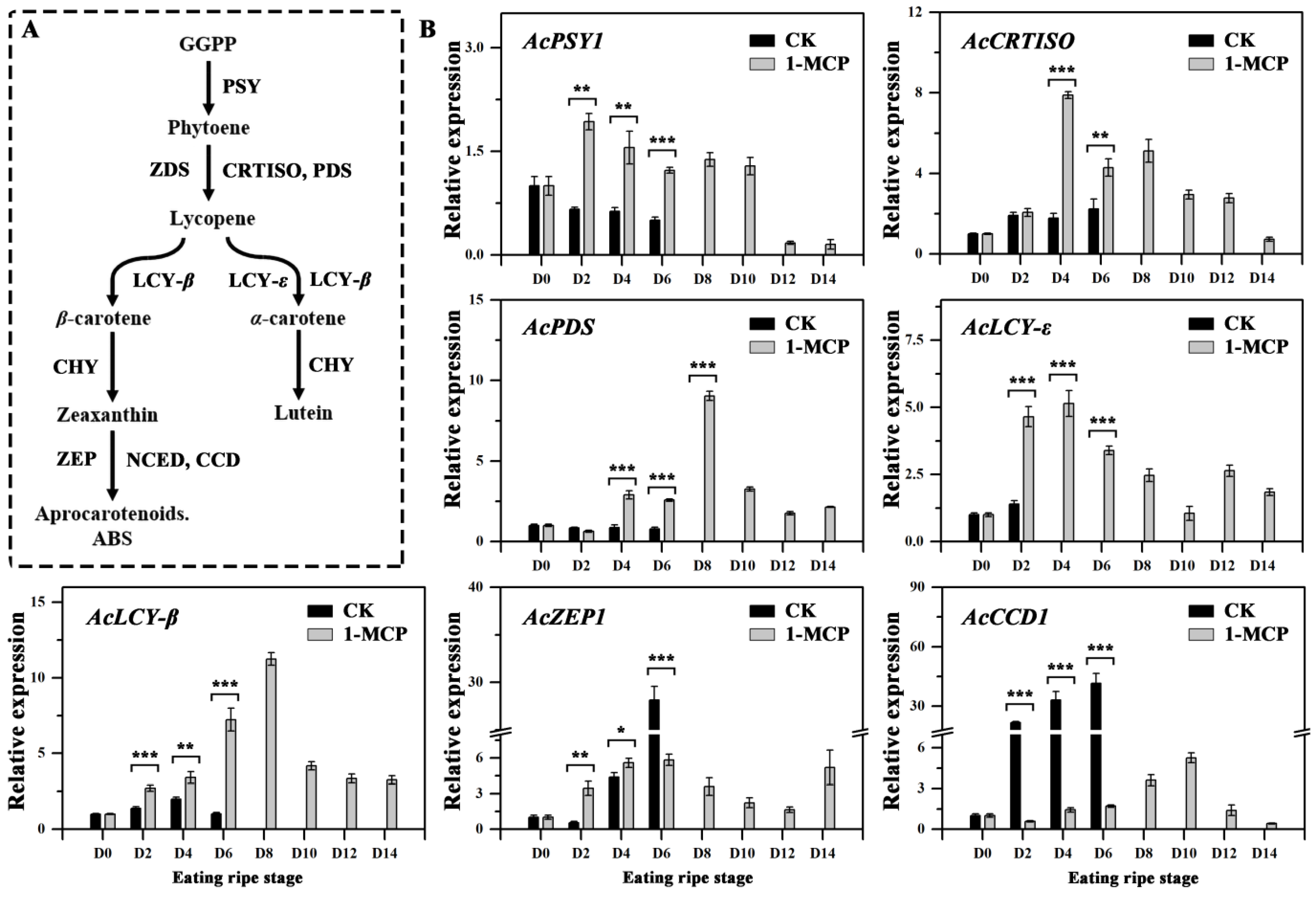
3.4. Expression of Genes Involved in Carotenoid Biosynthesis and Degradation
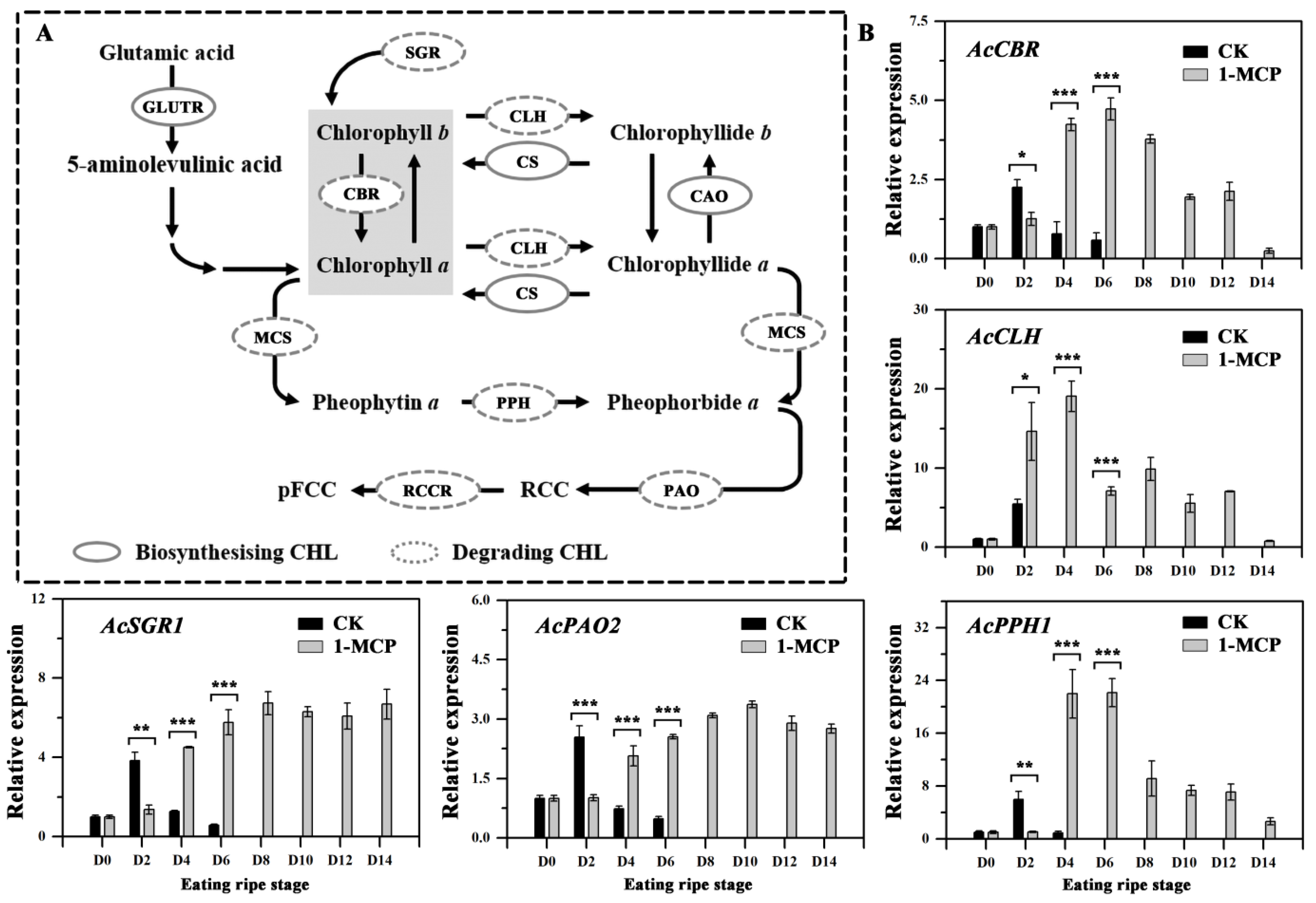
3.5. Expression of Genes Involved in Chlorophyll Degradation
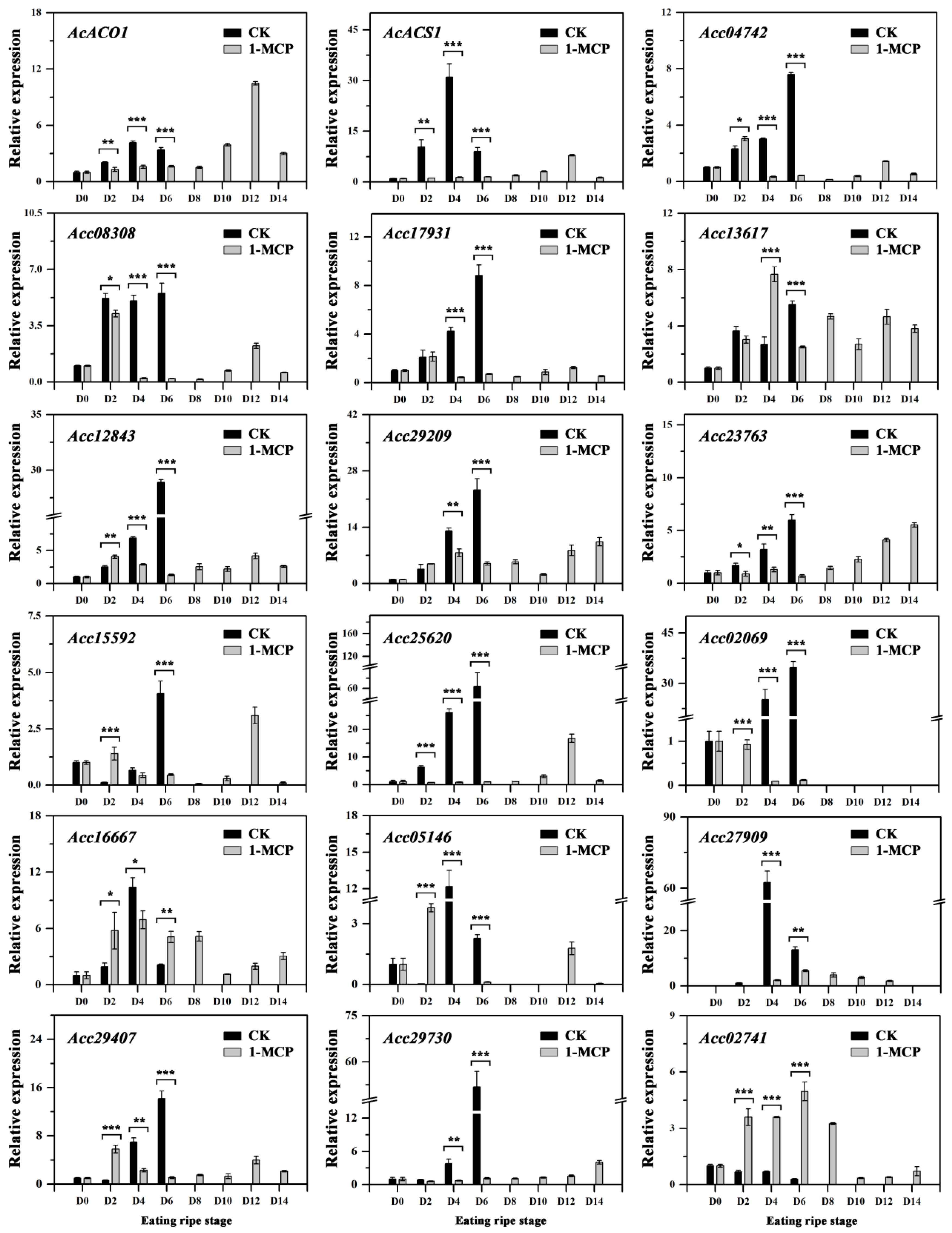
3.6. Expression of Ethylene Related Genes in Kiwifruit during Storage
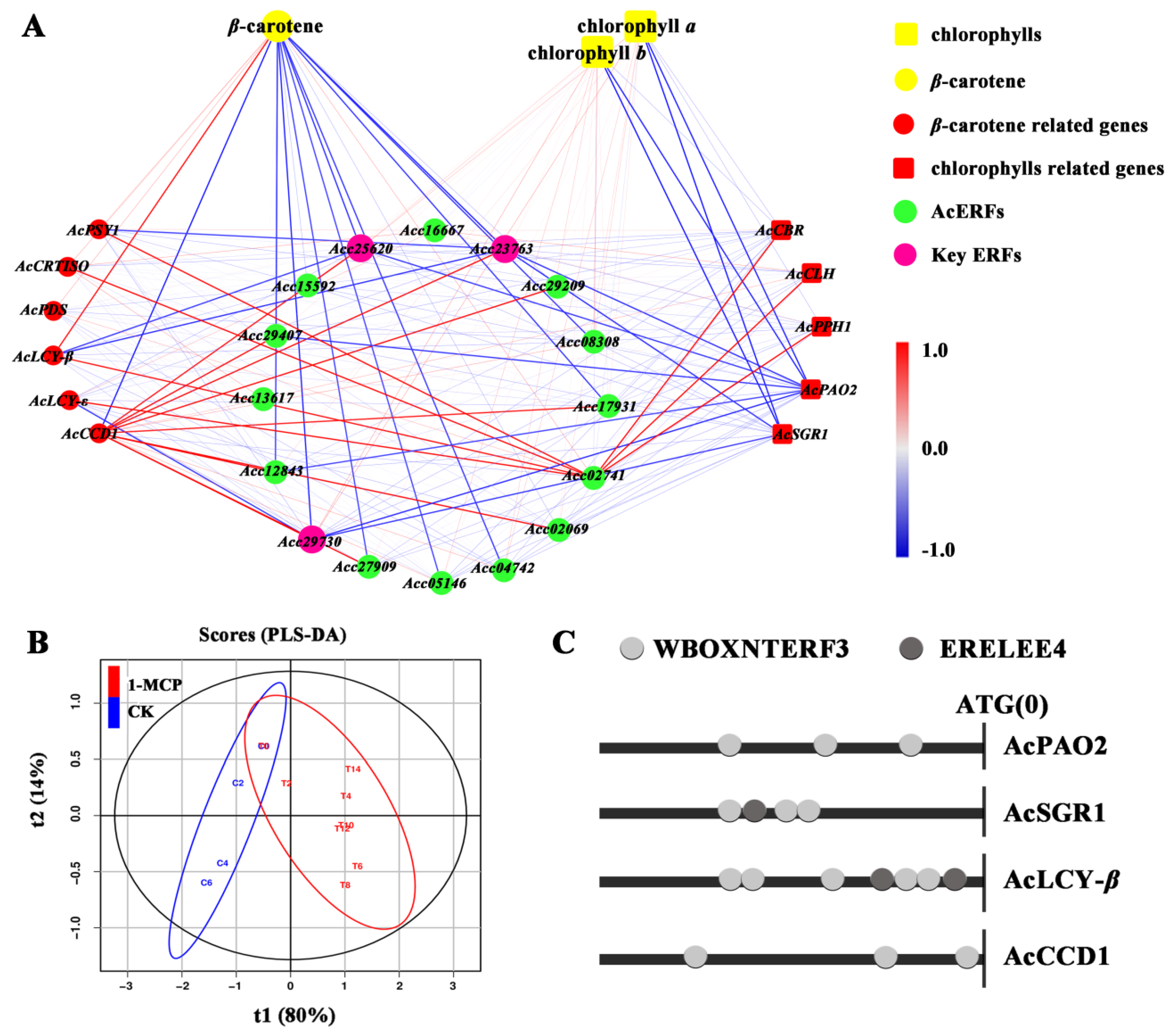
3.7. Correlation Analysis of Pigment Contents and Expression of Related Genes
3.8. The Activation of AcSGR1, AcPAO2, AcLCY-β and AcCCD1 Promoters by Acc25620, Acc29730 and Acc23763 after 1-MCP Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Qi, Y.; Chen, X.; He, H.; Liu, Z.; Zhang, Z.; Ren, Y.; Ren, X. Phenolic compounds and antioxidant activity in red- and in green-fleshed kiwifruits. Food Res. Int. 2019, 116, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Ampomah-Dwamena, C.; McGhie, T.; Wibisono, R.; Montefiori, M.; Hellens, R.P.; Allan, A.C. The kiwifruit lycopene beta-cyclase plays a significant role in carotenoid accumulation in fruit. J. Exp. Bot. 2009, 60, 3765–3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhou, B.; Qi, Y.; Chen, X.; Liu, C.; Liu, Z.; Ren, X. Expression Differences of Pigment Structural Genes and Transcription Factors Explain Flesh Coloration in Three Contrasting Kiwifruit Cultivars. Front. Plant Sci. 2017, 8, 1507. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, I.; Fukuda, T.; Oota, T. Genotypic differences in chlorophyll, lutein, and beta-carotene contents in the fruits of actinidia species. J. Agric. Food Chem. 2005, 53, 6403–6407. [Google Scholar] [CrossRef]
- Jacob-Wilk, D.; Holland, D.; Goldschmidt, E.E.; Riov, J.; Eyal, Y. Chlorophyll breakdown by chlorophyllase: Isolation and functional expression of the Chlase1 gene from ethylene-treated Citrus fruit and its regulation during development. Plant J. 1999, 20, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.R.; Xie, X.L.; Xia, X.J.; Yu, J.Q.; Ferguson, I.B.; Giovannoni, J.J.; Chen, K.S. Involvement of an ethylene response factor in chlorophyll degradation during citrus fruit degreening. Plant J. 2016, 86, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ye, L.; Ding, X.; Gao, Q.; Xiao, S.; Tan, Q.; Huang, J.; Chen, W.; Li, X. Transcriptomic analysis reveals key factors in fruit ripening and rubbery texture caused by 1-MCP in papaya. BMC Plant Biol. 2019, 19, 309. [Google Scholar] [CrossRef]
- Zhu, X.; Song, Z.; Li, Q.; Li, J.; Chen, W.; Li, X. Physiological and transcriptomic analysis reveals the roles of 1-MCP in the ripening and fruit aroma quality of banana fruit (Fenjiao). Food Res. Int. 2020, 130, 108968. [Google Scholar] [CrossRef] [PubMed]
- Amir-Shapira, D.; Goldschmidt, E.E.; Altman, A. Chlorophyll catabolism in senescing plant tissues: In vivo breakdown intermediates suggest different degradative pathways for Citrus fruit and parsley leaves. Proc. Natl. Acad. Sci. USA 1987, 84, 1901–1905. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, D.J.; Tadeo, F.R.; Legaz, F.; Primo-Millo, E.; Talon, M. In vivo sucrose stimulation of colour change in citrus fruit epicarps: Interactions between nutritional and hormonal signals. Physiol Plant. 2001, 112, 244–250. [Google Scholar] [CrossRef]
- Jeong, J.; Huber, D.J.; Sargent, S.A. Influence of 1-methylcyclopropene (1-MCP) on ripening and cell-wall matrix polysaccharides of avocado (Persea americana) fruit. Postharvest Biol. Technol. 2002, 25, 241–256. [Google Scholar] [CrossRef]
- Gong, Y.P.; Mattheis, J.P. Effect of ethylene and 1-methylcyclopropene on chlorophyll catabolism of broccoli florets. Plant Growth Regul. 2003, 40, 33–38. [Google Scholar] [CrossRef]
- Hershkovitz, V.; Saguy, S.I.; Pesis, E. Postharvest application of 1-MCP to improve the quality of various avocado cultivars. Postharvest Biol. Technol. 2005, 37, 252–264. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, M.; Bai, L.; Han, X.; Ge, Y.; Wang, W.; Li, J. Effects of 1-methylcyclopropene (1-MCP) on the expression of genes involved in the chlorophyll degradation pathway of apple fruit during storage. Food Chem. 2020, 308, 125707. [Google Scholar] [CrossRef] [PubMed]
- Hoeberichts, F.A.; Van der Plas, L.H.W.; Woltering, E.J. Ethylene perception is required for the expression of tomato ripening-related genes and associated physiological changes even at advanced stages of ripening. Postharvest Biol. Technol. 2002, 26, 125–133. [Google Scholar] [CrossRef]
- Marty, I.; Bureau, S.; Sarkissian, G.; Gouble, B.; Audergon, J.M.; Albagnac, G. Ethylene regulation of carotenoid accumulation and carotenogenic gene expression in colour-contrasted apricot varieties (Prunus armeniaca). J. Exp. Bot. 2005, 56, 1877–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisutiamonkul, A.; Ampomah-Dwamena, C.; Allan, A.C.; Ketsa, S. Carotenoid accumulation in durian (Durio zibethinus) fruit is affected by ethylene via modulation of carotenoid pathway gene expression. Plant Physiol. Biochem. 2017, 115, 308–319. [Google Scholar] [CrossRef]
- Kita, M.; Kato, M.; Ban, Y.; Honda, C.; Yaegaki, H.; Ikoma, Y.; Moriguchi, T. Carotenoid accumulation in Japanese apricot (Prunus mume Siebold & Zucc.): Molecular analysis of carotenogenic gene expression and ethylene regulation. J. Agric. Food Chem. 2007, 55, 3414–3420. [Google Scholar] [CrossRef]
- Barret, G.P.M.; Fabi, J.P.; De Rosso, V.V.; Cordenunsi, B.R.; Lajolo, F.M.; do Nascimento, J.R.O.; Mercadante, A.Z. Influence of ethylene on carotenoid biosynthesis during papaya postharvesting ripening. J. Food Compos. Anal. 2011, 24, 620–624. [Google Scholar] [CrossRef]
- Fabi, J.P.; Cordenunsi, B.R.; de Mattos Barreto, G.P.; Mercadante, A.Z.; Lajolo, F.M.; Oliveira do Nascimento, J.R. Papaya fruit ripening: Response to ethylene and 1-methylcyclopropene (1-MCP). J. Agric. Food Chem. 2007, 55, 6118–6123. [Google Scholar] [CrossRef]
- Ziliotto, F.; Begheldo, M.; Rasori, A.; Bonghi, C.; Tonutti, P. Transcriptome profiling of ripening nectarine (Prunus persica L. Batsch) fruit treated with 1-MCP. J. Exp. Bot. 2008, 59, 2781–2791. [Google Scholar] [CrossRef] [Green Version]
- Pratt, H.K.; Reid, M.S. Chinese Gooseberry: Seasonal Patterns in Fruit Growth and Maturation, Ripening, Respiration and the Role of Ethylene. J. Sci. Food Agric. 1974, 25, 747–757. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Gunaseelan, K.; Wang, M.Y.; Luo, L.K.; Wang, T.C.; Norling, C.L.; Johnston, S.L.; Maddumage, R.; Schroder, R.; Schaffer, R.J. Dissecting the role of climacteric ethylene in kiwifruit (Actinidia chinensis) ripening using a 1-aminocyclopropane-1-carboxylic acid oxidase knockdown line. J. Exp. Bot. 2011, 62, 3821–3835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.D.; Wang, W.Q.; Tong, Y.; Li, M.J.; Grierson, D.; Ferguson, I.; Chen, K.S.; Yin, X.R. Transcriptome Analysis Identifies a Zinc Finger Protein Regulating Starch Degradation in Kiwifruit. Plant Physiol. 2018, 178, 850–863. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Liu, S.; Liu, Y.; Xu, J.; Liu, T.; Dong, S. Effectiveness of lysozyme coatings and 1-MCP treatments on storage and preservation of kiwifruit. Food Chem. 2019, 288, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Q.; Wang, J.; Wu, Y.Y.; Li, D.W.; Allan, A.C.; Yin, X.R. Genome-wide analysis of coding and non-coding RNA reveals a conserved miR164-NAC regulatory pathway for fruit ripening. New Phytol. 2020, 225, 1618–1634. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Liu, X.F.; Fu, B.L.; Zhang, Q.Y.; Tong, Y.; Wang, J.; Wang, W.Q.; Grierson, D.; Yin, X.R. Methyl Jasmonate Enhances Ethylene Synthesis in Kiwifruit by Inducing NAC Genes That Activate ACS1. J. Agric. Food Chem. 2020, 68, 3267–3276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Q.; Li, J.; Gong, H.; Fan, X.; Yang, Y.; Liu, X.; Yin, X. Transcriptome co-expression network analysis identifies key genes and regulators of ripening kiwifruit ester biosynthesis. BMC Plant Biol. 2020, 20, 103. [Google Scholar] [CrossRef]
- Richardson, A.C.; Boldingh, H.L.; McAtee, P.A.; Gunaseelan, K.; Luo, Z.; Atkinson, R.G.; David, K.M.; Burdon, J.N.; Schaffer, R.J. Fruit development of the diploid kiwifruit, Actinidia chinensis ‘Hort16A’. BMC Plant. Biol. 2011, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, H.N.; Hall, A.J.; Burdon, J.; Lallu, N.; Connolly, P.; Amos, N. Modelling the Effect of Holding Temperature on Flesh De-Greening of‘Hort16A’ (ZESPRI™ GOLD) Kiwifruit. Acta Hort. 2007, 753, 769–776. [Google Scholar] [CrossRef]
- Chai, J.; Wang, Y.; Liu, Y.; Yong, K.; Liu, Z. 1-MCP extends the shelf life of ready-to-eat ‘Hayward’ and ‘Qihong’ kiwifruit stored at room temperature. Sci. Hortic. 2021, 289, 110437. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Karagiannis, E.; Belghazi, M.; Molassiotis, A. Coupling of Physiological and Proteomic Analysis to Understand the Ethylene- and Chilling-Induced Kiwifruit Ripening Syndrome. Front. Plant Sci. 2016, 7, 120. [Google Scholar] [CrossRef]
- Famiani, F.; Baldicchi, A.; Farinelli, D.; Cruz-Castillo, J.G.; Marocchi, F.; Mastroleo, M.; Moscatello, S.; Proietti, S.; Battistelli, A. Yield affects qualitative kiwifruit characteristics and dry matter content may be an indicator of both quality and storability. Sci. Hortic. 2012, 146, 124–130. [Google Scholar] [CrossRef]
- Hanbury, A.; Serra, J. Colour image analysis in 3D-polar coordinates. Lect. Notes Comput. Sci. 2003, 2781, 124–131. [Google Scholar]
- Huang, S.; Ding, J.; Deng, D.; Tang, W.; Sun, H.; Liu, D.; Zhang, L.; Niu, X.; Zhang, X.; Meng, M.; et al. Draft genome of the kiwifruit Actinidia chinensis. Nat. Commun. 2013, 4, 2640. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Crowhurst, R.; Hilario, E.; Nardozza, S.; Fraser, L.; Peng, Y.; Gunaseelan, K.; Simpson, R.; Tahir, J.; Deroles, S.C.; et al. A manually annotated Actinidia chinensis var. chinensis (kiwifruit) genome highlights the challenges associated with draft genomes and gene prediction in plants. BMC Genom. 2018, 19, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Liu, C.; Yan, D.; Wen, X.; Liu, Y.; Wang, H.; Dai, J.; Zhang, Y.; Liu, Y.; Zhou, B.; et al. MdHB1 down-regulation activates anthocyanin biosynthesis in the white-fleshed apple cultivar ‘Granny Smith’. J. Exp. Bot. 2017, 68, 1055–1069. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Dong, Y.; Yan, H.; Ge, W.; Shen, C.; Guan, J.; Liu, L.; Zhang, Y. Effects of 1-MCP on chlorophyll degradation pathway-associated genes expression and chloroplast ultrastructure during the peel yellowing of Chinese pear fruits in storage. Food Chem. 2012, 135, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Pongprasert, N.; Srilaong, V. A novel technique using 1-MCP microbubbles for delaying postharvest ripening of banana fruit. Postharvest Biol. Technol. 2014, 95, 42–45. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houben, M.; Van de Poel, B. 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef] [Green Version]
- Dandekar, A.M.; Teo, G.; Defilippi, B.G.; Uratsu, S.L.; Passey, A.J.; Kader, A.A.; Stow, J.R.; Colgan, R.J.; James, D.J. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 2004, 13, 373–384. [Google Scholar] [CrossRef]
- Gao, M.; Matsuta, N.; Murayama, H.; Toyomasu, T.; Mitsuhashi, W.; Dandekar, A.M.; Tao, R.; Nishimura, K. Gene expression and ethylene production in transgenic pear (Pyrus communis cv. ‘La France’) with sense or antisense cDNA encoding ACC oxidase. Plant Sci. 2007, 173, 32–42. [Google Scholar] [CrossRef]
- Ayub, R.; Guis, M.; BenAmor, M.; Gillot, L.; Roustan, J.P.; Latche, A.; Bouzayen, M.; Pech, J.C. Expression of ACC oxidase antisense gene inhibits ripening of cantaloupe melon fruits. Nat. Biotechnol. 1996, 14, 862–866. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.S.; Chen, M.; Li, L.C.; Ma, Y.Z. Functions of the ERF transcription factor family in plants. Botany 2008, 86, 969–977. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lv, G.; Chai, J.; Yang, Y.; Ma, F.; Liu, Z. The Effect of 1-MCP on the Expression of Carotenoid, Chlorophyll Degradation, and Ethylene Response Factors in ‘Qihong’ Kiwifruit. Foods 2021, 10, 3017. https://doi.org/10.3390/foods10123017
Liu Y, Lv G, Chai J, Yang Y, Ma F, Liu Z. The Effect of 1-MCP on the Expression of Carotenoid, Chlorophyll Degradation, and Ethylene Response Factors in ‘Qihong’ Kiwifruit. Foods. 2021; 10(12):3017. https://doi.org/10.3390/foods10123017
Chicago/Turabian StyleLiu, Yanfei, Guowen Lv, Jiaxin Chai, Yaqi Yang, Fengwang Ma, and Zhande Liu. 2021. "The Effect of 1-MCP on the Expression of Carotenoid, Chlorophyll Degradation, and Ethylene Response Factors in ‘Qihong’ Kiwifruit" Foods 10, no. 12: 3017. https://doi.org/10.3390/foods10123017
APA StyleLiu, Y., Lv, G., Chai, J., Yang, Y., Ma, F., & Liu, Z. (2021). The Effect of 1-MCP on the Expression of Carotenoid, Chlorophyll Degradation, and Ethylene Response Factors in ‘Qihong’ Kiwifruit. Foods, 10(12), 3017. https://doi.org/10.3390/foods10123017