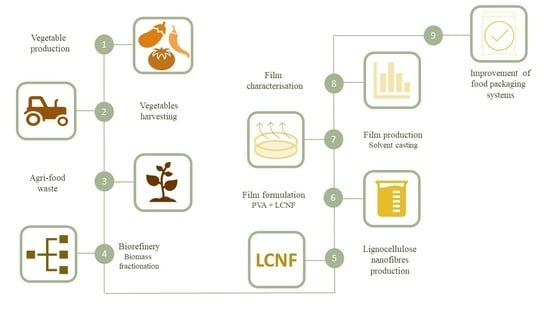
Lignocellulose Nanofibre Obtained from Agricultural Wastes of Tomato, Pepper and Eggplants Improves the Performance of Films of Polyvinyl Alcohol (PVA) for Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cellulose Pulp Obtention
2.3. Lignocellulose Nanofibres Production
2.3.1. Mechanical Pre-Treatment
2.3.2. TEMPO-Mediated Oxidation Pre-Treatment
2.3.3. High-Pressure Homogenization
2.4. Film Formulation and Production
2.5. Film Characterisation
2.5.1. Mechanical Properties
2.5.2. X-ray Diffraction (XRD) Analysis
2.5.3. Thermogravimetric Analysis (TGA)
2.5.4. Spectroscopy Analysis
2.5.5. Antioxidant Activity
2.5.6. Water Permeability
2.5.7. Optical Properties
2.5.8. Statistical Analysis
3. Results
3.1. Mechanical Properties
3.2. X-ray Diffraction Analysis
3.3. Thermogravimetric Analysis
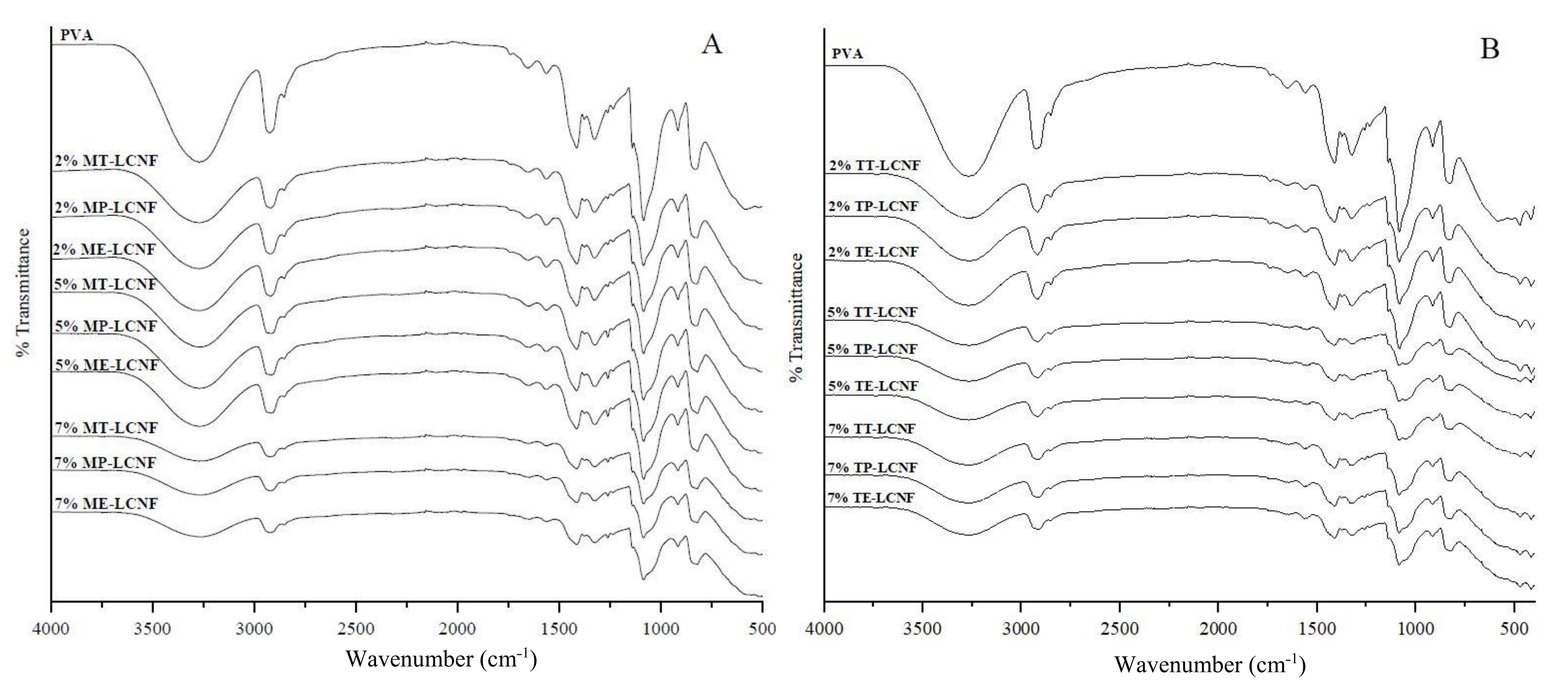
3.4. Spectroscopy Analysis
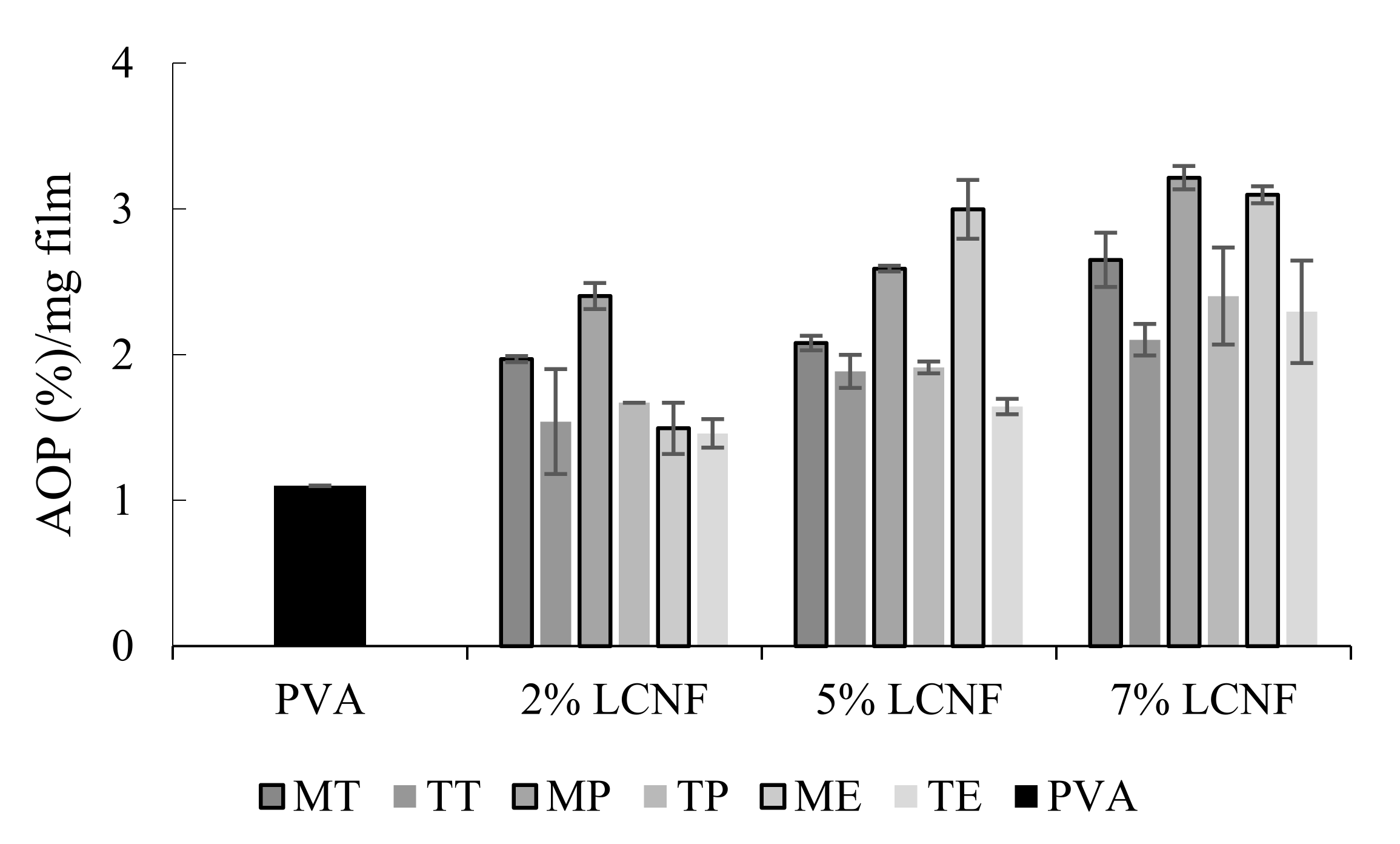
3.5. Antioxidant Activity
3.6. Water Permeability
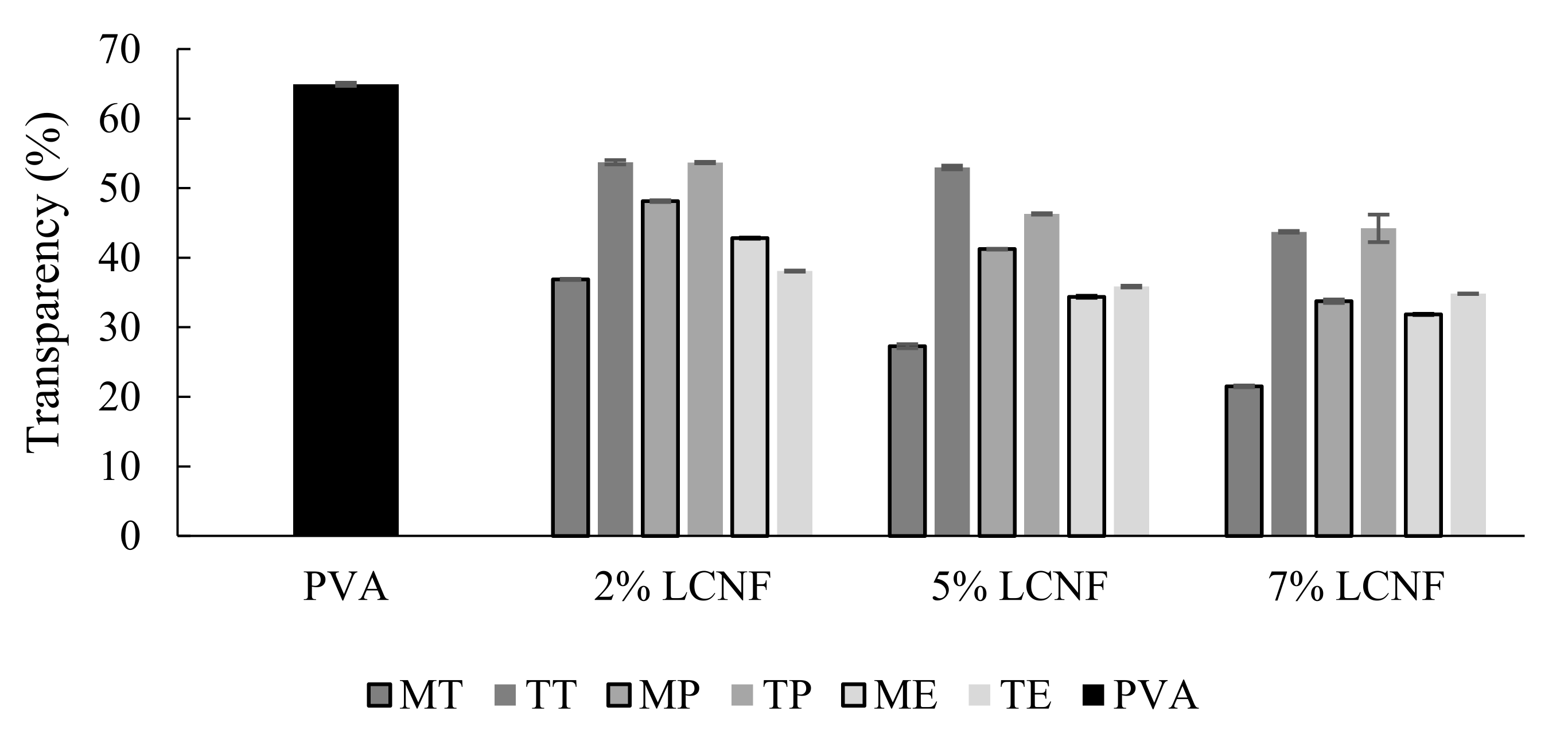
3.7. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [Green Version]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Plastic Europe. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.mdpi.com/journal/foods/instructions#preparation (accessed on 12 September 2021).
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef]
- Bilal, M.; Gul, I.; Basharat, A.; Qamar, S.A. Polysaccharides-based bio-nanostructures and their potential food applications. Int. J. Biol. Macromol. 2021, 176, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Othman, S.H. Bio-nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-sized Filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef] [Green Version]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, Q.; Li, L. Dehydration of water-plasticized poly(vinyl alcohol) systems: Particular behavior of isothermal mass transfer. Polym. Int. 2009, 58, 97–104. [Google Scholar] [CrossRef]
- Rudra, R.; Kumar, V.; Kundu, P.P. Acid catalysed cross-linking of poly vinyl alcohol (PVA) by glutaraldehyde: Effect of crosslink density on the characteristics of PVA membranes used in single chambered microbial fuel cells. RSC Adv. 2015, 5, 83436–83447. [Google Scholar] [CrossRef]
- Paralikar, S.A.; Simonsen, J.; Lombardi, J. Poly(vinyl alcohol)/cellulose nanocrystal barrier membranes. J. Membr. Sci. 2008, 320, 248–258. [Google Scholar] [CrossRef]
- Musetti, A.; Paderni, K.; Fabbri, P.; Pulvirenti, A.; Al-Moghazy, M.; Fava, P. Poly(vinyl alcohol)-Based Film Potentially Suitable for Antimicrobial Packaging Applications. J. Food Sci. 2014, 79. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Gohil, J.M.; Bhattacharya, A.; Ray, P. Studies on the crosslinking of poly(vinyl alcohol). J. Polym. Res. 2006, 13, 161–169. [Google Scholar] [CrossRef]
- Lu, J.; Wang, T.; Drzal, L.T. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos. Part. A Appl. Sci. Manuf. 2008, 39, 738–746. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Amara, C.; El Mahdi, A.; Medimagh, R.; Khwaldia, K. Nanocellulose-based composites for packaging applications. Curr. Opin. Green Sustain. Chem. 2021, 31, 100512. [Google Scholar] [CrossRef]
- Dubief, D.; Samain, E.; Dufresne, A. Polysaccharide Microcrystals Reinforced Amorphous Poly(β-hydroxyoctanoate) Nanocomposite Materials. Macromolecules 1999, 32, 5765–5771. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Espinosa, E.; Carrasco, E.; Pérez-Rodríguez, F.; Rodríguez, A. Cellulose Nanofibers from Olive Tree Pruning as Food Packaging Additive of a Biodegradable Film. Foods 2021, 10, 1584. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación. Cifras del Sector de Frutas y Hortalizas. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/cifrasdelsectorfyhactualizado2020definitivo-junio2021_tcm30-563965.pdf (accessed on 12 October 2021).
- Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of Cellulose Nanofibers from Olive Tree Harvest—A Residue with Wide Applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Bascón-Villegas, I.; Espinosa, E.; Sánchez, R.; Tarrés, Q.; Pérez-Rodríguez, F.; Rodríguez, A. Horticultural Plant Residues as New Source for Lignocellulose Nanofibers Isolation: Application on the Recycling Paperboard Process. Molecules 2020, 25, 3275. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-Robles, J.; Rodríguez, A. A comparative study of the suitability of different cereal straws for lignocellulose nanofibers isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; González, Z.; Domínguez-Robles, J.; Ferrari, B.; Rodríguez, A. Rapidly growing vegetables as new sources for lignocellulose nanofibre isolation: Physicochemical, thermal and rheological characterisation. Carbohydr. Polym. 2017, 175, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nesic, A.; Seslija, S.I. The influence of nanofillers on physical–chemical properties of polysaccharide-based film intended for food packaging. Food Packag. 2017, 637–697. [Google Scholar] [CrossRef]
- Markus Schmid, K.M. Chapter 11—Whey Protein-Based Packaging Films and Coatings. En Whey Proteins from Milk to Medicine; Deeth, H.C., Bansal, N., Eds.; Academic Press: Cambridge, UK, 2019; pp. 407–437. ISBN 9780128121245. [Google Scholar]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Antimicrobial and antioxidant properties of polyvinyl alcohol bio composite films containing seaweed extracted cellulose nano-crystal and basil leaves extract. Int. J. Biol. Macromol. 2018, 107, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte films based on chitosan/olive oil and reinforced with cellulose nanocrystals. Carbohydr. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef]
- Assender, H.E.; Windle, A.H. Crystallinity in poly(vinyl alcohol) 1. An X-ray diffraction study of atactic PVOH. Polymer 1998, 39, 4295–4302. [Google Scholar] [CrossRef]
- Popescu, M.-C. Structure and sorption properties of CNC reinforced PVA films. Int. J. Biol. Macromol. 2017, 101, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; You, J.; Jin, H.-J.; Kwak, H.W. Chemical and physical reinforcement behavior of dialdehyde nanocellulose in PVA composite film: A comparison of nanofiber and nanocrystal. Carbohydr. Polym. 2020, 232, 115771. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Fang, G.; Huang, C.; Rojas, O.J.; Pan, H. Highly Transparent, Strong, and Flexible Films with Modified Cellulose Nanofiber Bearing UV Shielding Property. Biomacromolecules 2018, 19, 4565–4575. [Google Scholar] [CrossRef]
- Othman, S.H.; Nordin, N.; Azman, N.A.A.; Tawakkal, I.S.M.A.; Basha, R.K. Effects of nanocellulose fiber and thymol on mechanical, thermal, and barrier properties of corn starch films. Int. J. Biol. Macromol. 2021, 183, 1352–1361. [Google Scholar] [CrossRef]
- Nair, S.S.; Yan, N. Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulose 2015, 22, 3137–3150. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, D.; Han, Y.; Lyu, S.; Lu, Y.; Li, G.; Jiang, F.; Wang, S. Effect of high residual lignin on the properties of cellulose nanofibrils/films. Cellulose 2018, 25, 6421–6431. [Google Scholar] [CrossRef]
- Ingrao, C.; Bacenetti, J.; Bezama, A.; Blok, V.; Goglio, P.; Koukios, E.G.; Lindner, M.; Nemecek, T.; Siracusa, V.; Zabaniotou, A.; et al. The potential roles of bio-economy in the transition to equitable, sustainable, post fossil-carbon societies: Findings from this virtual special issue. J. Clean. Prod. 2018, 204, 471–488. [Google Scholar] [CrossRef]
- Mathijs, E.; Brunori, G.; Carus, M.; Griffon, M.; Last, L. Sustainable Agriculture, Forestry and Fisheries in the Bioeconomy. In A Challenge for Europe. 4th SCAR Foresight Exercise; Barna, K., Ed.; European Commission: Brussels, Belgium, 2015; ISBN 978-92-79-47538-2. [Google Scholar]
- Mandal, A.; Chakrabarty, D. Studies on the mechanical, thermal, morphological and barrier properties of nanocomposites based on poly(vinyl alcohol) and nanocellulose from sugarcane bagasse. J. Ind. Eng. Chem. 2014, 20, 462–473. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Wang, X.; Bian, H.; Ni, S.; Sun, S.; Jiao, L.; Dai, H. BNNS/PVA bilayer composite film with multiple-improved properties by the synergistic actions of cellulose nanofibrils and lignin nanoparticles. Int. J. Biol. Macromol. 2020, 157, 259–266. [Google Scholar] [CrossRef]
- Thá, E.L.; Matos, M.; Avelino, F.; Lomonaco, D.; Rodrigues-Souza, I.; Gagosian, V.S.C.; Cestari, M.M.; Magalhães, W.L.E.; Leme, D.M. Safety aspects of kraft lignin fractions: Discussions on the in chemico antioxidant activity and the induction of oxidative stress on a cell-based in vitro model. Int. J. Biol. Macromol. 2021, 182, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Naidu, D.S.; John, M.J. Cellulose nanofibrils reinforced xylan-alginate composites: Mechanical, thermal and barrier properties. Int. J. Biol. Macromol. 2021, 179, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Water interactions and physical properties for packaging applications. Cellulose 2010, 17, 835–848. [Google Scholar] [CrossRef]






| Raw Material | Pre-Treatment | Codification | PVA–LCNF Concentration (%) | Codification |
|---|---|---|---|---|
| Polyvinyl alcohol | PVA | 100 | PVA | |
| Tomato plant | Mechanical | Mechanical tomato (MT) | 98:2 | 2% MT-LCNF |
| 95:5 | 5% MT-LCNF | |||
| 93:7 | 7% MT-LCNF | |||
| TEMPO-mediated oxidation | TEMPO tomato (TT) | 98:2 | 2% TT-LCNF | |
| 95:5 | 5% TT-LCNF | |||
| 93:7 | 7% TT-LCNF | |||
| Pepper plant | Mechanical | Mechanical pepper (MP) | 98:2 | 2% MP-LCNF |
| 95:5 | 5% MP-LCNF | |||
| 93:7 | 7% MP-LCNF | |||
| TEMPO-mediated oxidation | TEMPO pepper (TP) | 98:2 | 2% TP-LCNF | |
| 95:5 | 5% TP-LCNF | |||
| 93:7 | 7% TP-LCNF | |||
| Eggplant plant | Mechanical | Mechanical eggplant (ME) | 98:2 | 2% ME-LCNF |
| 95:5 | 5% ME-LCNF | |||
| 93:7 | 7% ME-LCNF | |||
| TEMPO-mediated oxidation | TEMPO eggplant (TE) | 98:2 | 2% TE-LCNF | |
| 95:5 | 5% TE-LCNF | |||
| 93:7 | 7% TE-LCNF |
| Formulation | LCNF Concentration (%) | Tonset (°C) a | Tmax (°C) b | |
|---|---|---|---|---|
| PVA | 210.0 | 250.32 | ||
| Mechanical pre-treatment | Tomato | 2 | 213.5 | 256.14 |
| 5 | 217.4 | 262.88 | ||
| 7 | 217.6 | 269.55 | ||
| Pepper | 2 | 209.7 | 261.87 | |
| 5 | 212.4 | 266.14 | ||
| 7 | 217.6 | 271.74 | ||
| Eggplant | 2 | 216.0 | 263.76 | |
| 5 | 221.5 | 268.01 | ||
| 7 | 231.3 | 277.36 | ||
| TEMPO pre-treatment | Tomato | 2 | 214.0 | 259.19 |
| 5 | 218.1 | 262.06 | ||
| 7 | 221.2 | 269.14 | ||
| Pepper | 2 | 208.7 | 256.11 | |
| 5 | 211.2 | 264.33 | ||
| 7 | 224.1 | 269.73 | ||
| Eggplant | 2 | 218.5 | 258.20 | |
| 5 | 220.5 | 261.76 | ||
| 7 | 223.6 | 264.14 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bascón-Villegas, I.; Sánchez-Gutiérrez, M.; Pérez-Rodríguez, F.; Espinosa, E.; Rodríguez, A. Lignocellulose Nanofibre Obtained from Agricultural Wastes of Tomato, Pepper and Eggplants Improves the Performance of Films of Polyvinyl Alcohol (PVA) for Food Packaging. Foods 2021, 10, 3043. https://doi.org/10.3390/foods10123043
Bascón-Villegas I, Sánchez-Gutiérrez M, Pérez-Rodríguez F, Espinosa E, Rodríguez A. Lignocellulose Nanofibre Obtained from Agricultural Wastes of Tomato, Pepper and Eggplants Improves the Performance of Films of Polyvinyl Alcohol (PVA) for Food Packaging. Foods. 2021; 10(12):3043. https://doi.org/10.3390/foods10123043
Chicago/Turabian StyleBascón-Villegas, Isabel, Mónica Sánchez-Gutiérrez, Fernando Pérez-Rodríguez, Eduardo Espinosa, and Alejandro Rodríguez. 2021. "Lignocellulose Nanofibre Obtained from Agricultural Wastes of Tomato, Pepper and Eggplants Improves the Performance of Films of Polyvinyl Alcohol (PVA) for Food Packaging" Foods 10, no. 12: 3043. https://doi.org/10.3390/foods10123043
APA StyleBascón-Villegas, I., Sánchez-Gutiérrez, M., Pérez-Rodríguez, F., Espinosa, E., & Rodríguez, A. (2021). Lignocellulose Nanofibre Obtained from Agricultural Wastes of Tomato, Pepper and Eggplants Improves the Performance of Films of Polyvinyl Alcohol (PVA) for Food Packaging. Foods, 10(12), 3043. https://doi.org/10.3390/foods10123043