1. Introduction
More than 50% of fishery products are discarded as wastes [
1,
2]. The use of fish by-products involves using zero-cost raw materials that can be converted into low-market-value products, such as meals for animal nutrition or fertilizers, or into high-market-value products, such as functional and bioactive protein hydrolysates for human foods. The fish by-products, which include head, skin, frames, and viscera, are rich sources of proteins, such as collagen. Salmon frames (SF) are the leftovers from salmon fillet production. SF contain 9–15% of the total salmon weight and consist of proteins, lipids, and bones [
3]. Compared to the chemical hydrolysis, the enzymatic hydrolysis of fish by-products is an efficient valorization alternative that converts the original proteins into peptides exhibiting various functional properties [
4,
5]. The enzymatic hydrolysis of SF proteins exhibits high-quality peptides [
3], high essential amino acid content [
6], and relevant bioactive properties [
6,
7,
8,
9]. SF hydrolysates have been tested as food supplements in formulations such as wheat crackers [
10] and biscuits [
11].
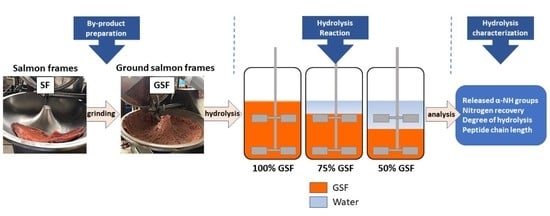
The enzymatic hydrolysis of fish proteins involves a series of unitary operations, as follows: (i) raw-material homogenization, (ii) enzymatic reaction, (iii) inactivation/termination of hydrolysis, and (iv) separation/dehydration [
12]. The first step is performed by mixing a certain amount of raw material with water to allow the mixture to be homogenized and, later, agitated during the enzymatic reaction step. Once the reaction stops, the water content is eliminated to obtain a protein hydrolysate in powder format. Technically, the water, which was added in the first step, should be removed in the last step. Thus, the first goal of this study was to compare the performance of SF hydrolysis with and without added water. The novelty of our study is the enzymatic hydrolysis of SF in the condition without added water, the main challenge of which is to keep the system properly mixed. However, as the fish raw material has a high-water content (approximately 60%), it seems plausible to proceed with the enzymatic hydrolysis. Benjakul and Morrisey [
13] observed that an increase in the by-product/water ratio results in an increase in α-amino groups released and nitrogen recovery [
13]. Vega and Brennan [
14] showed that the hydrolysis of cod offal by papain does not require extra water. Results indicated that hydrolysis performance and reaction kinetics are not affected by the high viscosity of the reaction mixture nor by the slow stirring rate [
14]. The effect of the SF/water ratio on the hydrolysis performance has been studied by some authors [
6,
15,
16]. In these and other works, the authors used different by-product/water proportions, such as 1:1–1:3 solid/liquid ratio [
6], 1:1 salmon co-products/water ratio [
8,
9], 0.71–1.21 frames/water ratio [
15], and 1:10 SF/water (
w/
v) ratio [
16]. The hydrolysis of SF proteins without added water has not been tested yet (100% SF). The increase in the by-product concentration in the reaction mixture will reduce the drying cost because this is the most expensive stage of hydrolysates production [
17]. In addition, in many enzymatic hydrolysis processes, NaOH has been used frequently to maintain the pH at the optimum levels for catalytic activity of the enzyme. The comparison of controlled and uncontrolled pH regimes has not been studied before. The second goal of this study was comparing uncontrolled with controlled pH conditions in terms of reaction efficiency. A quantitative comparison of the enzymatic hydrolysis at different water contents, including the condition without added water, and in different pH control regimes was made in this work. The objective was to evaluate the performance of the SF protein hydrolysis, involving no ideal but economically convenient conditions. The condition without added water and uncontrolled pH corresponds to the main novelty of this study.
3. Results
Ground SF samples were hydrolyzed by subtilisin in different SF/water mixtures under different pH regimes in an agitated batch reactor. Pictures of reaction mixtures can be observed in
Supplementary Material. The results of the reaction progress are shown in
Figure 1.
In general, the concentration of released α-NH groups was higher in the conditions with less added water. The impact generated by the SF/water ratio is more significant than the effect of pH regimes. This is a significant finding considering that the protease subtilisin expresses more activity in alkaline pH. The initial and final pH values obtained in the experiments are shown in
Table 2. In the uncontrolled pH condition R3, the reaction began at pH 6.4–6.5 (native pH) and dropped by 0.3 pH units after 60 min of reaction. However, under the pH regime R2, the pH dropped by between 1.2 and 1.4 pH units from the initial pH 8. The pH drop depended on the SF proportion and the initial pH value. Higher SF/water ratios and a lower initial pH were correlated to lower pH drops. A higher protein concentration in the reaction mixture buffered the pH during the reaction progress.
The effect of the protease dose was evaluated at 100% SF and 55 °C under the pH regime R3, which corresponds to the combination of both non-ideal conditions tested in this work. The results are shown in
Figure 2. Three protease doses were tested: 6, 13, and 20 AU per kg of SF. The results indicated that an increase in the protease dose from 6 to 13 AU/kg increased the hydrolysis efficiency. However, a new increase, from 13 to 20 AU/kg, did not cause an increase in reaction efficiency. The decision of using 13 AU of subtilisin per kg of SF was based on this result. This dose of subtilisin concords with the study of Liaset et al. [
15], where E/S ratios between 30 and 90 AU of Protamex per kg of crude protein were used. Considering that the protein content was 16.2% of crude protein in SF, they used between 4.7 and 14.6 AU of Protamex per kg of SF. In other published works, Idowu et al. [
16] used between 8.6 and 26.0 AU of Alcalase per kg of SF, while He et al. [
8] used between 12 and 72 AU per kg of salmon co-products. In all the cases, the protease dose was within the same range of Anson units used in our study. Differences in reaction performance could depend on the protein content of the SF or co-products.
A saturation of cleavage sites on the superficial area of SF particles could explain the lack of increase in reaction efficiency when using more than 13 AU/kg of SF. This is a major concern because protease additions over 13 AU/kg do not increase the reaction efficiency. In addition, since protease is the highest cost issue during hydrolysis operation, this is a key point to focus on in a cost-efficiency analysis of the process. The adsorption of subtilisin on the protein was suggested by O’Meara and Munro [
19] during a study of lean meat protein by Alcalase.
A high SF concentration was feasible and convenient in terms of the high concentration of α-NH groups released, the buffering of pH drop, and the lowest amount of water. Nevertheless, knowing the complexity of the enzymatic hydrolysis of proteins, a deep insight is needed. A wide set of parameters were evaluated when the reaction stopped. These included the released water from the SF tissue, the α-NH group yield, the total nitrogen released, nitrogen recovery (NR), the degree of hydrolysis (
DH’), and peptide size estimation. The necessity of adding water has been based on improving mixing during the reaction. However, considering that the water content of SF is 53%, the amount of water in the reaction mixture is half of the by-product mass in the 100% m/m SF condition. In our experiments, the agitation never presented a challenge because even with 100% m/m SF, the reaction mixture could be mixed and behaved as a viscous fluid. After the addition of protease, the mixture evolved to a fluid suspension during the first minute of reaction (data not shown). The water contained in SF was released during the hydrolysis of proteins due to the degradation of large proteins. The amounts of water involved in hydrolysis reactions are plotted in
Figure 3. In general, around 50% of the water contained in SF was released after 60 min of the hydrolysis reaction. The amount of released water was affected by both the SF proportion and the pH regime. The higher SF proportions and the more alkaline pH conditions generated higher water release. However, although higher amounts of water were released, it corresponded to a lower percentage of the total water contained in SF tissue (
Figure 3b). The pH regimes R2 and R3 achieved more similar percentages of released water, around 50%.
The results of all experiments were plotted to observe the correlation of released water with the concentration and number of α-NH groups in
Figure 4. High concentrations of α-NH groups did not mean a high number of α-NH groups (mmoles in
Figure 4b). The experiments with different protease doses than 13 AU/kg were included. The amount of released water was interestingly correlated with the concentration of α-NH groups, independently of the reaction conditions, with Pearson’s R = 0.884. The correlation depended on the reaction conditions in the case of the number of α-NH groups (
Figure 4b). Pearson’s R was 0.998, 0.954, and 0.929 for 50, 75, and 100% SF conditions, respectively. It is well established that water and protein are associated at the molecular level. Thus, the release of α-NH groups from SF proteins inevitably involves the release of the linked water and its transfer, along with peptides, toward the soluble phase. As mentioned above, the number of released α-NH groups was not necessarily higher in the more concentrated SF reactions. Some batches in the 50 and 75% SF condition overmatched the number of α-NH groups released in the 100% SF condition. However, as the amount of added and released water was higher in the 50 and 75% SF conditions, the resulting concentrations of α-NH groups were lower than that in the 100% SF. A high number of α-NH groups and a low water volume are convenient results. However, the number of α-NH groups corresponds to an estimation of the peptide concentration in the soluble phase and it does not consider the size of the peptides.
Another parameter considered to evaluate the reaction productivity is the amount of nitrogen extracted from the SF and transferred to the soluble phase. This value was used to calculate the nitrogen recovery (NR), according to Equation (1), and the specific yield of nitrogen, according to Equation (2). These parameters were plotted in
Figure 5.
The total nitrogen amount transferred to the soluble phase was around 200 mmoles in the different reaction conditions except for the 75% SF under pH regimes R1 and R2 (
Figure 5a). These conditions achieved 280 and 246 mmoles of total nitrogen extracted in the soluble phase, respectively. The profile of total nitrogen through the different reaction conditions was not clear. However, the percentage of nitrogen extracted was clearly decreased when SF increased (
Figure 5b). A more dramatic profile was obtained with the nitrogen-specific yield plotted against the different reaction conditions. The highest specific yield was 300 mmoles/kg·AU, while the lowest value was 73 mmoles/kg·AU, for the 50 and 100% SF, respectively. The most convenient condition can be established by aiming to obtain the highest NR, the lowest volume of water, and the lowest protease dose. A plot of total water versus total nitrogen is presented in
Figure 6. The total water obtained after the SF hydrolysis was not correlated with the total nitrogen transferred to the soluble phase (no statistical analysis) and just depended on the amount of added water. The total nitrogen transferred to the soluble phase was between 183 and 280 mmoles and did not correlate with the percentage of SF in the reaction mixture. The 100% SF batch will achieve the lowest cost of water evaporation during the drying stage. Twice the mass of water and, of course, drying cost is obtained in the 50% SF condition. Thus, the economic convenience will be between the 75 and 100% SF conditions depending on the gain from the nitrogen obtained and the cost of the drying process. However, these high-SF conditions involve high protease doses, which should be included in the economic evaluation.
In addition to the process characterization, the degree of hydrolysis (
DH) and the peptide chain length (
PCL) were determined for each condition. The
DH was estimated from the free α-NH groups/total nitrogen ratio considering that it should be calculated from the α-NH groups/total peptide bonds ratio. Thus, an underestimation of the
DH was obtained and denominated
DH’. The
PCL corresponds to a characterization of peptides through the calculation of the total nitrogen/free α-NH groups ratio, both quantified in the soluble phase. The
DH’ and
PCL obtained in each reaction condition were plotted in
Figure 7.
The results showed a decrease of the DH’ when increasing the SF/water ratio and when uncontrolled pH regimes were applied. The effect on PCL was exactly contrary due to the inverse relationship between DH’ and PCL (Equations (3) and (4)). Larger peptides were produced at higher SF/water ratios under uncontrolled pH regimes. We can infer that the addition of water to the reaction mixture is a modulating parameter to modify the characteristics of the protein hydrolysate. As DH’ and PCL are molecular characteristics that modulate hydrolysates’ functional properties, we have found that different operating conditions will generate different hydrolysates in terms of these properties. Thus, the desired hydrolysate properties can be aimed through the manipulation of the reaction conditions.
4. Discussion
The study of the by-product/water ratio effect on the hydrolysis performance has been assessed in some publications. However, the hydrolysis of fish by-products without added water has been only published by Vega and Brennan (1988) [
14]. This is, to the best of our knowledge, the only previous research on this issue. Our findings agree with that publication in terms that the agitation and mixing in the batch reactor are feasible and, definitively, not a problem at all. The viscosity of the ground SF is not enough to impede proper mixing. Furthermore, the hydrolysis of large proteins decreases rapidly and enormously the initial agitation resistance. We can declare that the addition of water is no longer an argument to increase the mixing properties of fish by-product hydrolysis. This clearly decreases the energy costs during the drying stage. The effect of water addition was not only limited to the mixing properties but also affected the reaction performance and hydrolysates properties by changing the by-product concentration and the volume of the soluble phase. The main effects observed among the different reaction conditions considering the SF/water ratio and pH regimes were in terms of the number and concentration of α-NH groups released. The higher the SF/water ratio, the higher the concentration of α-NH groups released. However, these higher concentrations did not mean a higher number of α-NH groups or higher total nitrogen extracted. The protease subtilisin was chosen because it is the most cost efficient compared with other commercial and extracted proteases [
20]. The results showed lower subtilisin activity under the pH regime R3 compared to that in regimes R1 and R2. It was evidenced in the lower concentrations (
Figure 4a) and the number of α-NH groups released (
Figure 4b). However, the activity was good enough to produce a similar number of α-NH groups with controlled pH as with the uncontrolled pH regimes (
Figure 4b). Despite these findings, new studies are needed to evaluate the hydrolysis performance using neutral proteases according to the pH of the ground SF (pH 6.4–6.5).
At this point, an economic analysis is needed to establish which condition is the most convenient, considering that higher SF/water ratios and uncontrolled pH regimes are low-cost but less productive. The reaction conditions studied also affected the characteristics of the hydrolysates, which is a significant concern in terms of functional properties. The DH’ and PCL were affected by the SF/water ratios and the pH regimes. We observed that the larger peptide sizes were obtained at higher SF/water ratios under uncontrolled pH regimes. We can infer that the proportion between water and by-product affected the protease distribution between both phases, the surface of by-product particles (muscle and bones) and the soluble phase, containing hydrolyzed protein and peptides. According to this hypothesis, the catalytic action of protease would be distributed as an adsorbed protease and a free protease; thus, the cleavage of peptide bonds will occur on the surface of particulate material and the already hydrolyzed peptides dissolved in the soluble phase. The predominating catalytic action will depend on the distribution of the protease molecules. We have now formulated the hypothesis that the protease distribution can be modulated by the operating conditions. In addition, this modulation will affect the molecular size of peptides and their functional properties. Future research aims to apply the knowledge obtained in this study to improve the hydrolysis performance and to evaluate the protease distribution in both the particulate material (insoluble phase) and the soluble aqueous phase. Furthermore, an economic evaluation of the process can be made. The operating costs at different SF/water ratios under different pH regimes can be evaluated considering the drying stage and its associated energy costs.