Plant-Based Diet as a Strategy for Weight Control
Abstract
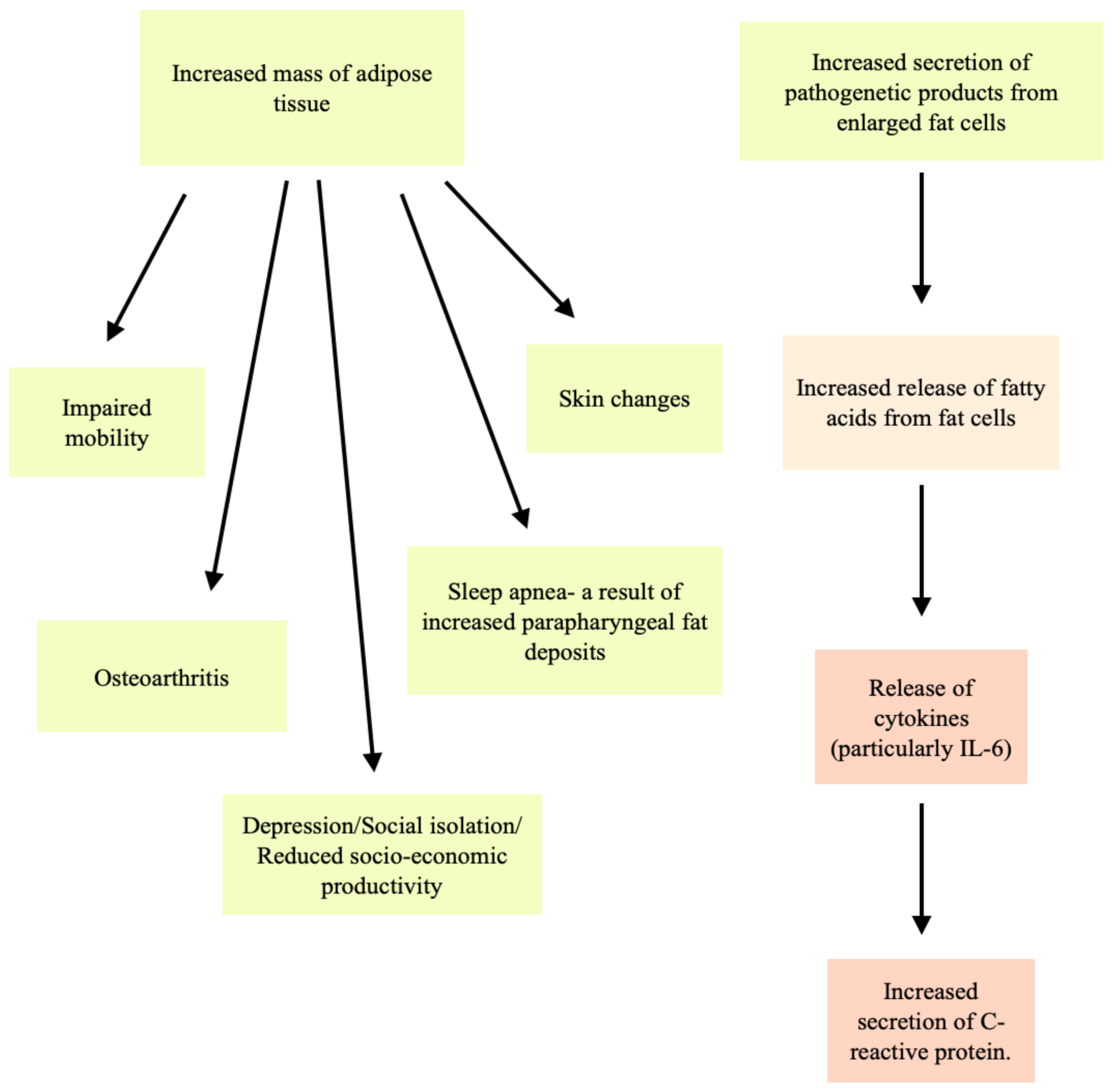
:1. Introduction
2. Materials and Methods
2.1. Databases and Keywords
2.2. Eligibility Criteria
3. Results and Discussion
3.1. Studies Involving Vegan Diets
- Lower calorie density compared to other diets—individuals can eat bigger portions because plants have a lower calorie density than foods of animal origin (Table 1).
- Antioxidants—vegan and plant-based diets, if well balanced, contain plentiful fruits and vegetables, which are rich in antioxidants. The consumption of raw fruits, vegetables, roots, nuts, and germinated seeds provides an intake of carotenoids, vitamin C, vitamin E, and other compounds that have an antioxidant effect.
- Lipid-lowering effects—the absence [23,67] or limited intake of dietary cholesterol [68]. Moreover, some plants that are rich in sterols and stanols may lower serum low-density lipoprotein cholesterol concentrations and improve endothelial dysfunction [37,38]. Foods rich in sterols/stanols are nuts, flaxseed, fresh cauliflower (200 mg/100 g), avocado (75 mg/100 g), raspberry, lingonberry, grapes, apple, blueberry, and others [38]. Potatoes are an example of a poor source of sterols/stanols [38].
3.2. Studies Involving Vegetarian Diets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 March 2021).
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Medical Consequences of Obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.M. The Globesity Epidemic. In The Obesity Epidemic; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–20. ISBN 978-3-319-68977-7. [Google Scholar]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin. Infect. Dis. 2020, 71, 896–897. [Google Scholar] [CrossRef] [Green Version]
- Ellulu, M.S.; Patimah, I.; Khaza’Ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Grasemann, H.; Holguin, F. Oxidative stress and obesity-related asthma. Paediatr. Respir. Rev. 2020, 37, 18–21. [Google Scholar] [CrossRef]
- Bosque-Varela, P.; Moscote-Salazar, L.R.; Agrawal, A. Obesity and stroke in the COVID19 era. Clin. Neurol. Neurosurg. 2020, 196, 105969. [Google Scholar] [CrossRef]
- Bello, N.T. Update on drug safety evaluation of naltrexone/bupropion for the treatment of obesity. Expert Opin. Drug Saf. 2019, 18, 549–552. [Google Scholar] [CrossRef]
- Ballinger, A.; Peikin, S.R. Orlistat: Its current status as an anti-obesity drug. Eur. J. Pharmacol. 2002, 440, 109–117. [Google Scholar] [CrossRef]
- Suleiman, J.B.; Nna, V.; Zakaria, Z.; Othman, Z.A.; Abu Bakar, A.B.; Mohamed, M. Obesity-induced testicular oxidative stress, inflammation and apoptosis: Protective and therapeutic effects of orlistat. Reprod. Toxicol. 2020, 95, 113–122. [Google Scholar] [CrossRef]
- Barrea, L.; Pugliese, G.; Muscogiuri, G.; Laudisio, D.; Colao, A.; Savastano, S. New-generation anti-obesity drugs: Naltrexone/bupropion and liraglutide. An update for endocrinologists and nutritionists. Minerva Endocrinol. 2020, 45, 127–137. [Google Scholar] [CrossRef]
- Najjar, R.S.; Feresin, R.G. Plant-Based Diets in the Reduction of Body Fat: Physiological Effects and Biochemical Insights. Nutrients 2019, 11, 2712. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.-Y.; Huang, C.-C.; Hu, F.B.; Chavarro, J.E. Vegetarian Diets and Weight Reduction: A Meta-Analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2015, 31, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Orlich, M.J.; Fraser, G.E. Vegetarian diets in the Adventist Health Study 2: A review of initial published findings. Am. J. Clin. Nutr. 2014, 100, 353S–358S. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian Diets and Blood Pressure: A Meta-Analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan Diet Health Benefits in Metabolic Syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef]
- Hopwood, C.J.; Bleidorn, W.; Schwaba, T.; Chen, S. Health, environmental, and animal rights motives for vegetarian eating. PLoS ONE 2020, 15, e0230609. [Google Scholar] [CrossRef] [Green Version]
- Cramer, H.; Kessler, C.S.; Sundberg, T.; Leach, M.J.; Schumann, D.; Adams, J.; Lauche, R. Characteristics of Americans Choosing Vegetarian and Vegan Diets for Health Reasons. J. Nutr. Educ. Behav. 2017, 49, 561–567.e1. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A Low-Fat Vegan Diet Improves Glycemic Control and Cardiovascular Risk Factors in a Randomized Clinical Trial in Individuals with Type 2 Diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Poole-Wilson, P.; Langer, G. Effect of pH on ionic exchange and function in rat and rabbit myocardium. Am. J. Physiol. Content 1975, 229, 570–581. [Google Scholar] [CrossRef]
- Kabeerdoss, J.; Devi, R.S.; Mary, R.R.; Ramakrishna, B.S. Faecal microbiota composition in vegetarians: Comparison with omnivores in a cohort of young women in southern India. Br. J. Nutr. 2011, 108, 953–957. [Google Scholar] [CrossRef] [Green Version]
- Miquel, S.; Martin, R.; Rossi, O.; Humaran, L.G.B.; Chatel, J.; Sokol, H.; Thomas, M.; Wells, J.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Kim, M.-S.; Hwang, S.-S.; Park, E.-J.; Bae, J.-W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation: Diet therapy, gut microbiota and metabolic diseases. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar] [CrossRef]
- Barnard, N.D.; Alwarith, J.; Rembert, E.; Brandon, L.; Nguyen, M.; Goergen, A.; Horne, T.; Nascimento, G.F.D.; Lakkadi, K.; Tura, A.; et al. A Mediterranean Diet and Low-Fat Vegan Diet to Improve Body Weight and Cardiometabolic Risk Factors: A Randomized, Cross-over Trial. J. Am. Coll. Nutr. 2021, 1–13. Available online: https://www.tandfonline.com/doi/full/10.1080/07315724.2020.1869625?scroll=top&needAccess=true (accessed on 10 March 2021). [CrossRef]
- Mihrshahi, S.; Ding, D.; Gale, J.; Allman-Farinelli, M.; Banks, E.; Bauman, A.E. Vegetarian diet and all-cause mortality: Evidence from a large population-based Australian cohort—The 45 and Up Study. Prev. Med. 2017, 97, 1–7. [Google Scholar] [CrossRef]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Kim, S.-A.; Lee, I.-K.; Kim, J.-G.; Park, K.-G.; Jeong, J.-Y.; Jeon, J.-H.; Shin, J.-Y.; Lee, D.-H. Effect of a Brown Rice Based Vegan Diet and Conventional Diabetic Diet on Glycemic Control of Patients with Type 2 Diabetes: A 12-Week Randomized Clinical Trial. PLoS ONE 2016, 11, e0155918. [Google Scholar] [CrossRef] [Green Version]
- Kjeldsen-Kragh, J.; Borchgrevink, C.; Laerum, E.; Haugen, M.; Eek, M.; Førre, O.; Mowinkel, P.; Hovi, K. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Hafstrom, I.; Ringertz, B.; Spångberg, A.; Von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001, 40, 1175–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nenonen, M.T.; Helve, T.A.; Rauma, A.L.; Hanninen, O.O. Uncooked, lactobacilli-rich, vegan food and rheumatoid arthritis. Rheumatology 1998, 37, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltonen, R.; Nenonen, M.; Helve, T.; Hanninen, O.; Toivanen, P.; Eerola, E. Faecal microbial flora and disease activity in rheumatoid arthritis during a vegan diet. Rheumatology 1997, 36, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef]
- Piironen, V.; Toivo, J.; Lampi, A.-M. Natural Sources of Dietary Plant Sterols. J. Food Compos. Anal. 2000, 13, 619–624. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Barnard, N.D.; Scialli, A.R. A Two-Year Randomized Weight Loss Trial Comparing a Vegan Diet to a More Moderate Low-Fat Diet. Obesity 2007, 15, 2276–2281. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-J.; Han, P.; Sun, S.-Y.; Wang, L.-Y.; Yan, B.; Zhang, J.-H.; Zhang, W.; Yang, S.-Y.; Li, X.-J. Attenuated associations between increasing BMI and unfavorable lipid profiles in Chinese Buddhist vegetarians. Asia Pac. J. Clin. Nutr. 2013, 22, 249–256. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Davidson, C.; Wingard, E.E.; Wilcox, S.; Frongillo, E.A. Comparative effectiveness of plant-based diets for weight loss: A randomized controlled trial of five different diets. Nutrition 2015, 31, 350–358. [Google Scholar] [CrossRef]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association—Recommended Diet in Coronary Artery Disease Trial. J. Am. Hear. Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.A.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588S–1596S. [Google Scholar] [CrossRef]
- Barnard, N.D.; Gloede, L.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Green, A.A.; Ferdowsian, H. A Low-Fat Vegan Diet Elicits Greater Macronutrient Changes, but Is Comparable in Adherence and Acceptability, Compared with a More Conventional Diabetes Diet among Individuals with Type 2 Diabetes. J. Am. Diet. Assoc. 2009, 109, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; Fleeman, R.; Hlozkova, A.; Holubkov, R.; Barnard, N.D. A plant-based diet in overweight individuals in a 16-week randomized clinical trial: Metabolic benefits of plant protein. Nutr. Diabetes 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; Dort, S.; Holubkov, R.; Barnard, N.D. A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: The Role of Carbohydrates. Nutrients 2018, 10, 1302. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; Hlozkova, A.; Fleeman, R.; Fletcher, K.; Holubkov, R.; Barnard, N.D. Fat Quantity and Quality, as Part of a Low-Fat, Vegan Diet, Are Associated with Changes in Body Composition, Insulin Resistance, and Insulin Secretion. A 16-Week Randomized Controlled Trial. Nutrients 2019, 11, 615. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef]
- Mishra, S.; Xu, J.; Agarwal, U.; Gonzales, J.R.; Levin, S.A.; Barnard, N.D. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: The GEICO study. Eur. J. Clin. Nutr. 2013, 67, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.A.; Wong, J.M.W.; Kendall, C.W.C.; Esfahani, A.; Ng, V.W.Y.; Leong, T.C.K.; Faulkner, D.A.; Vidgen, E.; Paul, G.; Mukherjea, R.; et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: A randomised controlled trial. BMJ Open 2014, 4, e003505. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Davidson, C.R.; Wingard, E.E.; Billings, D.L. Low glycemic index vegan or low-calorie weight loss diets for women with polycystic ovary syndrome: A randomized controlled feasibility study. Nutr. Res. 2014, 34, 552–558. [Google Scholar] [CrossRef]
- Lederer, A.-K.; Maul-Pavicic, A.; Hannibal, L.; Hettich, M.; Steinborn, C.; Gründemann, C.; Zimmermann-Klemd, A.M.; Müller, A.; Sehnert, B.; Salzer, U.; et al. Vegan diet reduces neutrophils, monocytes and platelets related to branched-chain amino acids —A randomized, controlled trial. Clin. Nutr. 2020, 39, 3241–3250. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.; Barnard, N.D.; Scialli, A.R.; Lanou, A.J. Effects of a low-fat vegan diet and a Step II diet on macro- and micronutrient intakes in overweight postmenopausal women. Nutrition 2004, 20, 738–746. [Google Scholar] [CrossRef]
- Cui, X.; Wang, B.; Wu, Y.; Xie, L.; Xun, P.; Tang, Q.; Cai, W.; Shen, X. Vegetarians have a lower fasting insulin level and higher insulin sensitivity than matched omnivores: A cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 467–473. [Google Scholar] [CrossRef]
- Kahleova, H.; Tintera, J.; Thieme, L.; Veleba, J.; Klementova, M.; Kudlackova, M.; Malinska, H.; Oliyarnyk, O.; Markova, I.; Haluzik, M.; et al. A plant-based meal affects thalamus perfusion differently than an energy- and macronutrient-matched conventional meal in men with type 2 diabetes, overweight/obese, and healthy men: A three-group randomized crossover study. Clin. Nutr. 2021, 40, 1822–1833. [Google Scholar] [CrossRef]
- Olabi, A.; Levitsky, D.; Hunter, J.; Spies, R.; Rovers, A.; Abdouni, L. Food and mood: A nutritional and mood assessment of a 30-day vegan space diet. Food Qual. Prefer. 2015, 40, 110–115. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention with Vegetarian Diet). Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef]
- Burke, L.E.; Hudson, A.G.; Warziski, M.T.; Styn, M.A.; Music, E.; Elci, O.U.; Sereika, S.M. Effects of a vegetarian diet and treatment preference on biochemical and dietary variables in overweight and obese adults: A randomized clinical trial. Am. J. Clin. Nutr. 2007, 86, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Ornish, D.; Brown, S.; Billings, J.; Scherwitz, L.; Armstrong, W.; Ports, T.; McLanahan, S.; Kirkeeide, R.; Gould, K.; Brand, R. Can lifestyle changes reverse coronary heart disease? The lifestyle heart trial. Lancet 1990, 336, 129–133. [Google Scholar] [CrossRef]
- Chainani-Wu, N.; Weidner, G.; Purnell, D.M.; Frenda, S.; Merritt-Worden, T.; Pischke, C.; Campo, R.; Kemp, C.; Kersh, E.S.; Ornish, D. Changes in Emerging Cardiac Biomarkers after an Intensive Lifestyle Intervention. Am. J. Cardiol. 2011, 108, 498–507. [Google Scholar] [CrossRef]
- Dod, H.S.; Bhardwaj, R.; Sajja, V.; Weidner, G.; Hobbs, G.R.; Konat, G.W.; Manivannan, S.; Gharib, W.; Warden, B.E.; Nanda, N.C.; et al. Effect of Intensive Lifestyle Changes on Endothelial Function and on Inflammatory Markers of Atherosclerosis. Am. J. Cardiol. 2010, 105, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Marracci, G.; Kim, E.; Spain, R.; Cameron, M.; Overs, S.; Riddehough, A.; Li, D.K.; McDougall, J.; Lovera, J.; et al. Low-fat, plant-based diet in multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2016, 9, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets with Whey, Vegetable, or Animal Protein in Patients with Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.D.; Brooks, M.M.; Evans, R.W.; Linkov, F.; Burke, L.E. Weight Loss Is More Important than the Diet Type in Improving Adiponectin Levels among Overweight/Obese Adults. J. Am. Coll. Nutr. 2013, 32, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.S.; Sklar, M.; Barnard, N.D.; Gore, S.; Sullivan, R.; Browning, S. Toward Improved Management of NIDDM: A Randomized, Controlled, Pilot Intervention Using a Lowfat, Vegetarian Diet. Prev. Med. 1999, 29, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Bunner, A.; Wells, C.L.; Gonzales, J.C.; Agarwal, U.; Bayat, E.; Barnard, N.D. A dietary intervention for chronic diabetic neuropathy pain: A randomized controlled pilot study. Nutr. Diabetes 2015, 5, e158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USA Department of Agriculture. Available online: https://fdc.nal.usda.gov/fdc-app.html#/?query= (accessed on 10 September 2021).
- Behrman, E.D.; Gopalan, V.E. Cholesterol and Plants. J. Chem. Educ. 2005, 82, 1791–1793. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; et al. Major Lipids, Apolipoproteins, and Risk of Vascular Disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef] [Green Version]
- Prospective Studies Collaboration; Lewington, S.A.; Whitlock, G.A.; Clarke, R.O.; Sherliker, P.A.; Emberson, J.O.; Halsey, J.I.; Qizilbash, N.A.; Peto, R.I.; Collins, R.O. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration Cardiovascular Disease, Chronic Kidney Disease, and Diabetes Mortality Burden of Cardiometabolic Risk Factors from 1980 to 2010: A Comparative Risk Assessment. Lancet Diabetes Endocrinol. 2014, 2, 634–647. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC); Taddei, C.; Zhou, B.; Bixby, H.; Carrillo-Larco, R.M.; Danaei, G.; Jackson, R.T.; Farzadfar, F.; Sophiea, M.K.; Di Cesare, M.; et al. Repositioning of the global epicentre of non-optimal cholesterol. Nature 2020, 582, 73–77. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration; Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef]
- Wu, Y.; Potempa, L.A.; El Kebir, D.; Filep, J.G. C-reactive protein and inflammation: Conformational changes affect function. Biol. Chem. 2015, 396, 1181–1197. [Google Scholar] [CrossRef]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.R.; Renlund, D.G.; Muhlestein, J.B. Which White Blood Cell Subtypes Predict Increased Cardiovascular Risk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Tzoulaki, I.; Murray, G.D.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G.R. Relative Value of Inflammatory, Hemostatic, and Rheological Factors for Incident Myocardial Infarction and Stroke. Circulation 2007, 115, 2119–2127. [Google Scholar] [CrossRef] [Green Version]
- Kwaijtaal, M.; van Diest, R.; Bär, F.W.; van der Ven, A.J.; Bruggeman, C.A.; de Baets, M.H.; Appels, A. Inflammatory markers predict late cardiac events in patients who are exhausted after percutaneous coronary intervention. Atherosclerosis 2005, 182, 341–348. [Google Scholar] [CrossRef]
- Buffon, A.; Liuzzo, G.; Biasucci, L.M.; Pasqualetti, P.; Ramazzotti, V.; Rebuzzi, A.G.; Crea, F.; Maseri, A. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J. Am. Coll. Cardiol. 1999, 34, 1512–1521. [Google Scholar] [CrossRef] [Green Version]
- Walter, D.H.; Fichtlscherer, S.; Sellwig, M.; Auch-Schwelk, W.; Schächinger, V.; Zeiher, A.M. Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J. Am. Coll. Cardiol. 2001, 37, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Brennan, M.-L.; Fu, X.; Aviles, R.J.; Pearce, G.L.; Penn, M.S.; Topol, E.; Sprecher, D.L.; Hazen, S.L. Association between Myeloperoxidase Levels and Risk of Coronary Artery Disease. JAMA 2001, 286, 2136–2142. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Everett, B.M.; Libby, P.; Thuren, T.; Glynn, R.J.; Ridker, P.M.; MacFadyen, J.G.; Everett, B.M.; Libby, P.; et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the CANTOS randomised controlled trial. Lancet 2017, 391, 319–328. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.C.; La Ferrera, G.M.G.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef] [Green Version]
- Woo, K.S.; Kwok, T.C.; Celermajer, D.S. Vegan Diet, Subnormal Vitamin B-12 Status and Cardiovascular Health. Nutrients 2014, 6, 3259–3273. [Google Scholar] [CrossRef] [Green Version]


| Food | Average Kilocalories (kcal) Per 100 g | Average Cholesterol Content mg/100 g | Average Protein Content g/100 g | Omnivorous Diet | Plant Based Diet | Vegan Diet |
|---|---|---|---|---|---|---|
| Ground beef | 260 | 87 | 25.54 | + | − | − |
| Beef (roast) | 219 | 82 | 27.45 | + | − | − |
| Beef sausage | 328 | 61 | 13.3 | + | − | − |
| Chicken feet | 215 | 84 | 19.4 | + | − | − |
| Chicken (back) | 298 | 87 | 25.73 | + | − | − |
| Pork hash | 185 | 56 | 12.96 | + | − | − |
| Pork sausage | 325 | 86 | 18.53 | + | − | − |
| Fish | 188 | 90 | 21.74 | + | − | − |
| Eggs* (hard-boiled) | 155 | 373 | 12.6 | + | + | − |
| Hard cheese | 157 | 69 | 13.83 | + | + | − |
| Cheese dip | 160 | 8 | 3.24 | + | + | − |
| Feta cheese | 265 | 89 | 14.21 | + | + | − |
| Roquefort cheese | 353 | 75 | 21.4 | + | + | − |
| Brie cheese | 334 | 100 | 20.75 | + | + | − |
| Cow milk (whole) | 67 | 15 | 3.3 | + | + | − |
| Cow butter, light | 499 | 106 | 3.3 | + | + | − |
| Peanut butter | 597 | 0 | 22.5 | + | + | + |
| Almond butter | 614 | 0 | 20.94 | + | + | + |
| Corn oil | 900 | 0 | 0 | + | + | + |
| Coconut oil | 883 | 0 | 0 | + | + | + |
| Olive oil | 884 | 0 | 0 | + | + | + |
| Sesame oil | 884 | 0 | 0 | + | + | + |
| Sunflower oils | 884 | 0 | 0 | + | + | + |
| Soy milk (unsweetened) | 38 | 0 | 3.53 | ± | ± | + |
| Oat milk | 50 | 0 | 1.25 | ± | ± | + |
| Beyond meet R- beyond burger | 230 | 0 | 17.7 | ± | + | + |
| Beyond meet R- beyond chicken grilled strips | 141 | 0 | 23.53 | ± | + | + |
| Beyond meet R- plant-based brat | 280 | 0 | 18.67 | ± | + | + |
| Mushrooms (raw) | 22 | 0 | 3.09 | + | + | + |
| Potatoes, baked | 93 | 0 | 1.95 | + | + | + |
| Chickpeas (canned) | 128 | 0 | 8 | + | + | + |
| Green peas (frozen) | 79 | 0 | 5.62 | + | + | + |
| Arugula (raw) | 25 | 0 | 2.58 | + | + | + |
| Tomatoes (raw) | 22 | 0 | 0.88 | + | + | + |
| Red peppers (raw) | 17 | 0 | 0.68 | + | + | + |
| Spinach (raw) | 23 | 0 | 2.86 | + | + | + |
| Cauliflower (raw) | 25 | 0 | 1.92 | + | + | + |
| Cucumbers (raw) | 10 | 0 | 0.59 | + | + | + |
| Avocado (raw) | 160 | 0 | 2 | + | + | + |
| Melon (honeydew, raw) | 36 | 0 | 0.54 | + | + | + |
| Watermelon (raw) | 30 | 0 | 0.61 | + | + | + |
| Blueberries (raw) | 57 | 0 | 0.74 | + | + | + |
| Strawberries (raw) | 32 | 0 | 0.67 | + | + | + |
| Rose-apples (raw) | 25 | 0 | 0.6 | + | + | + |
| Study Design | No of P. * | Study Duration | Diets That Were Studied | Main Results | Ref. |
|---|---|---|---|---|---|
| Participants with type 2 diabetes were randomly assigned to a low-fat vegan diet or a diet following the American Diabetes Association (ADA) guidelines. | 99 | 22 weeks | Vegan (n = 49)/American Diabetes Association (ADA) guidelines (n = 50) | A total of 43% of the vegan group and 26% of the ADA group participants reduced usage of diabetes medications. Both diets improved glycemic and lipid control. However, these improvements were reported to be better with a vegan diet. | [23] |
| A randomized crossover trial, which included overweight adults assigned to 2 groups. | 62 | 16 weeks | Vegan diet (n = 30)/Mediterranean diet (n = 32) | Significant reduction in body weight in the vegan group. | [28] |
| A randomized trial, which included participants diagnosed with type 2 diabetes randomly assigned into 2 groups. | 92 | 12 weeks | Vegan diet (n = 46)/Korean Diabetes Association Diet (n = 46) | A significant reduction in HbA1C levels was reported for both groups. However, glycemic control was found to be better with the vegan diet than with the conventional diet. | [32] |
| A randomized trial which included overweight, postmenopausal women randomly assigned into 2 groups. | 62 | 2 years | A low-fat, vegan diet (n = 28)/National Cholesterol Education Program diet (n = 34) | Individuals in the vegan group lost significantly more weight than those in the National Cholesterol Education Program group at 1 year (−4.9 (−0.5, −8.0) kg vs. −1.8 (0.8, −4.3); p < 0.05) and at 2 years (−3.1 (0.0, −6.0) kg vs. −0.8 (3.1, −4.2) kg; p < 0.05). | [39] |
| The study analyzed the interaction between BMI and vegetarian status. This was tested using a multivariable regression analysis adjusting for age, education, smoking, alcohol drinking, and physical activity. | 170 | No specific period | Vegetarian (n = 170)/omnivore diet (n = 120) | Compared with omnivores, vegetarians had significantly lower mean levels of BMI, blood pressure, total cholesterol, LDL cholesterol, and triglycerides. The researchers suggested a lower predicted probability of coronary heart disease for vegetarians. | [40] |
| A five-arm, randomized controlled trial, in which participants were overweight adults (BMI: 25.0–49.9), 18–65 years old. All tested diets were low-fat, which included limited amounts of nuts, butters, avocado, seeds, and olives. | 63 | 6 months | Vegan (n = 12)/vegetarian (n = 13)/pesco-vegetarian (n = 13)/semi-vegetarian (n = 13)/omnivorous (n = 12) | At the 6th month, the weight loss in the vegan group (−7.5% ± 4.5%) was significantly different from the other groups. | [41] |
| Open-label, blinded end-point randomized trial that included participants with coronary artery disease. The participants were randomized into 2 groups. | 100 | 8 weeks | Vegan (n = 50)/American Heart Association diet (n = 50) | A vegan diet resulted in a significantly (32%) lower high-sensitivity C-reactive protein compared with the American Heart Association diet. The degree of reduction in body mass did not significantly differ between the 2 diet groups. | [42] |
| A controlled trial in which participants were individuals with type 2 diabetes. Participants were randomly assigned into 2 groups. | 99 | 74 weeks | Vegan (n = 50)/American Diabetes Association guidelines (ADA) (n = 50) | Both groups reported reduced hunger and reduced disinhibition. The mean weight loss was reported to be 22% for the vegan group in week 74 and 20% for the ADA group. | [43,44] |
| A randomized clinical trial. Dual-energy X-ray absorptiometry was used to measure body composition. Insulin resistance was assessed with the Homeostasis Model Assessment index. | 75 | 16 weeks | Vegan (n = 38)/omnivorous diet (n = 37) | Weight decreased significantly in the vegan group. The mean weight loss for the vegan group was 5.8 kg compared to 3.8 kg for the omnivorous group. | [45,46,47] |
| A single-center, randomized, open, parallel design. All participants had a BMI between 28 and 40 kg/m2. Gut microbiota composition was assessed using uBiome Explorer™ kits; body composition and insulin sensitivity were also measured. | 168 | 16 weeks | Vegan diet (n = 84)/omnivorous diet (n = 84) | The data suggested that the low-fat vegan diet led to an increased relative abundance of Faecalibacterium prausnitzii and a smaller decrease, compared to the control group, in the relative abundance of Bacteroides fragilis. Changes in the relative abundance of Bacteroides fragilis were found to correlate positively with changes in insulin sensitivity. Body weight was significantly reduced in the vegan group (treatment effect: –5.9 kg). | [48] |
| A multicenter randomized control trial. | 291 | 18 weeks | Vegan diet (n = 142)/omnivorous diet (n = 149) | Authors reported improved body weight, plasma lipids, and glycemic status for the vegan group. | [49] |
| A parallel design study in which participants were overweight hyperlipidaemic men and postmenopausal women. | 39 | 6 months | Vegan diet (n = 20)/vegetarian diet (n = 19) | The relative LDL cholesterol and triglyceride reductions were found to be greater in the vegan group. | [50] |
| A randomized study that only included overweight/obese women with polycystic ovary syndrome. | 18 | 6 months | Vegan diet (n = 9)/low-cal. diet (n = 9) | The participants in the vegan group lost significantly more weight by the third month. However, there was no difference between groups at 6 months. | [51] |
| A randomized, controlled trial. The participants were randomized into 2 groups. All participants had a pre-treatment phase consisting of a 1-week, controlled, mixed diet. | 53 | 4 weeks | Vegan diet (n = 26)/meat-rich diet (n = 27) | In the vegan group, the total leukocyte, neutrophil, monocyte, and platelet counts decreased, and after 4 weeks, they were significantly lower than the other group. | [52] |
| A randomized, controlled trial that included overweight postmenopausal women. | 59 | 14 weeks | Low-fat vegan diet (n = 29)/National Cholesterol Education Program Step II diet (n = 30) | The low-fat vegan diet was associated with greater decreases in fat, saturated fat, protein, and cholesterol intake than the other diet. In both groups, there was a significant reduction in BMI. There was a significant difference between the groups. | [53] |
| The study compared some parameters of vegan/vegetarians to omnivores. Laboratory tests were performed for fasting blood glucose and fasting insulin concentrations.* The vegan/vegetarian participants were people who had followed this diet for at least 1 year. | 558 | No specific duration | Vegetarian diet (n = 206) and vegan diet (n = 73)/omnivore diet (n = 279) | Authors reported that a vegan diet was associated with lower fasting blood glucose, fasting insulin, and insulin resistance. | [54] |
| A randomized crossover study. The participants were men diagnosed with type 2 diabetes, overweight/obese men, and healthy men as the control. Participants with type 2 diabetes were instructed to skip their diabetes medication in the evening and morning before the assessments. The meals consisted of either a conventional meat cheeseburger or a plant-based tofu burger. | 60 | No specific duration | Vegan meal/meat-containing meal | Authors reported higher postprandial GLP-1 secretion after the vegan meal in men with type 2 diabetes, greater satiety, and changes in thalamus perfusion. Authors suggested a potential use of plant-based meals in addressing the key pathophysiologic mechanisms of food intake regulation. | [55] |
| The researchers studied the effect of a vegan diet on nutrient intake, body weight, and mood. No control group. | 16 | 30 days | Vegan diet | The authors reported average weight loss of 1.7 kg. | [56] |
| A randomized dietary intervention trial. The participants were divided into 2 groups. The dietary profile of both groups included the average intake of 2071.3± 548.4 kcal/day. | 118 | 3 months | Vegetarian diet (n = 60)/Mediterranean diet (n = 58) | LDL levels were significantly reduced in the vegetarian group. Both diets were effective in reducing body weight, BMI, and fat mass. However, there were no significant differences between the groups for these parameters. | [57] |
| A randomized clinical trial. Both diets were calorie-restricted and low-fat. | 176 | 1 year + 6 months maintenance phase | A vegetarian diet (n = 96)/standard diet (n = 80) | All participants had a reduction in total energy and fat intake and an increase in energy expenditure. This was reflected in reduced body weight. An insignificant decrease in LDL cholesterol levels for the vegetarian group was reported (p = 0.06). | [58] |
| A randomized, controlled trial that aimed to determine whether comprehensive lifestyle changes can affect coronary atherosclerosis after 1 year. Participants were divided into two groups. The first group was called “the experimental group” and had a low-fat vegetarian diet and some other lifestyle changes, including stopping smoking, stress management training, and moderate exercise. The control group had no lifestyle changes. | 48 | 1 year | A low-fat vegetarian diet (n = 28)/omnivorous diet (20) | In the experimental group, the total cholesterol levels were reduced from 5.88 mmol/L to 4.45 mmol/L; the LDL levels were reduced from 3.92 mmol/L to 2.46 mmol/L. In the control group, the total cholesterol levels were reduced from 6.34 mmol/L to 6.00 mmol/L, while the LDL levels were reduced from 4.32 mmol/L to 4.07 mmol/L. The mean weight of the experimental group was 91.1 kg at the beginning of the study and was reduced to 81 kg. The mean weight of the control group was 80.4 kg at the beginning of the study and 81.8 kg at the end of the study. | [59] |
| A prospective cohort study. The participants were people with coronary heart disease (CHD) or individuals at high risk with >3 CHD risk factors and/or diabetes. The intervention included a plant-based diet, moderate physical activity, and stress management. | 131 | 3 months | Plant-based diet/no control group | All participants had improved health status after 3 months of the study. Researchers reported significant reduction in: BMI, systolic and diastolic blood pressure, waist/hip ratio, C-reactive protein, insulin, LDL, and total cholesterol. The mean BMI was reduced from 33.6 to 31.8 kg/m2 (p-value < 0.001). The quality of life and cognitive functioning were also improved. | [60] |
| Multisite cardiac lifestyle intervention program. The participants were individuals with coronary artery disease (CAD) and/or risk factors for CAD. The control group had the usual standard of care, while the experimental group had a lifestyle intervention: a plant-based diet, moderate physical activity, stress management, and smoking cessation. | 47 | 12 weeks | Plant-based diet (n = 27)/control group (n = 20) | The mean body weight of the experimental group was 96.2 ± 3.8 kg at the baseline and 90.7 ± 3.6 kg at the end of the study. The mean body weight of the control group was 90.7 ±3.5 kg at the baseline and 91.2 ± 3.4 kg at the third month of the study. A significant decrease in C-reactive protein and interleukin-6 was reported in the experimental group. | [61] |
| A randomized, controlled trial. Participants were individuals with multiple sclerosis. The mean BMI was 28.4 ± 6.76 kg/m2 for the control group and 29.3 ± 7.42 for the diet group. | 61 | 1 year | A very-low-fat, plant-based diet (n = 32)/control group (n = 29) | Authors reported a significant reduction in BMI in the diet group, which was an average of 0.18 kg/m2 per month or 0.5 kg per month. The plant-based diet intervention also benefited the self-reported outcome of fatigue and reductions in LDL cholesterol, total cholesterol, and fasting insulin levels. | [62] |
| A randomized pilot study that evaluated the effect of 45-days of 3 types of isocaloric very-low-calorie ketogenic diets on the microbiota in patients with obesity and insulin resistance. The mean BMI of the participants was 35.9 ± 4.1 kg/m2. The participants were randomly assigned to 3 groups. The diets included 780 kcal/day. | 48 | 45 days | A diet with whey protein (n = 16)/a diet with vegetable protein (n = 16)/a diet with animal protein (n = 16) | Authors reported a significant reduction in initial body weight both in the whey protein group and in the vegetable protein group. Although a decreasing trend in total fat and trunk fat mass was observed in the three groups, a significant difference was observed only in the whey protein group and vegetable protein group. It was reported that following a plant-based very-low-calorie ketogenic diet is associated with healthier microbiota composition. | [63] |
| A randomized controlled trial. The aim of the study was to compare the effect of a standard calorie- and fat-restricted diet vs. a lacto-ovo-vegetarian diet on total and high-molecular-weight and on total adiponectin levels. The participants were overweight/obese adults. | 143 | 6 months | Standard diet (n= 79)/vegetarian diet (n= 64). | A significant weight loss was reported in both groups (no significant differences between the groups). | [64] |
| A randomized pilot study in which participants were individuals with type 2 diabetes and painful diabetic neuropathy. The participants were randomly assigned to 2 groups. The participants’ mean age was 57 years. The mean BMI was 36 kg/m2. | 34 | 20 weeks | A low-fat, plant-based diet (n = 17)/omnivorous diet (n = 17) | The body weight was reduced by a mean of 7.0 kg over 20 weeks in the intervention group. In the control group, the mean weight loss was 0.6 kg. | [66] |
| Number of the Participants in Group I | Number of the Participants in Group II | Study Duration | Mean Weight Loss in Group I/Reduction in BMI | Mean Weight Loss in Group II/Reduction in BMI | Ref. |
|---|---|---|---|---|---|
| 49 | 50 (ADA) | 22 weeks | Body weight decreased by 6.5 kg. | Body weight decreased by 3.1 kg. | [23] |
| 49 | 50 (ADA) | 74 weeks | The mean weight loss was 22%. | The mean weight loss was 20%. | [43,44] |
| 30 | 32 (Mediterranean diet) | 16 weeks | The mean weight loss was 7.9 kg. | The mean weight loss was 1.5 kg. | [28] |
| 46 | 47 (a diet by Korean Diabetes Association) | 12 weeks | Reduction in BMI with −0.5 ± 0.9. | Reduction in BMI with −0.1 ± 0.6. | [32] |
| 28 | 34 (NCEP diet) | 2 years | −4.9 kg at the first year and −3.1 kg at the second year. | −1.8 kg at the first year and −0.8 kg at the second year. | [39] |
| 50 | 50 (American-Heart- Association-recommended diet) | 8 weeks | BMI index was reduced from 30.5 to 29.0 kg/m2. | BMI index was reduced from 30.9 to 29.5 kg/m2. | [42] |
| 9 | 9 (a low-cal. omnivorous diet) | 3 mounts | 1.8% weight loss in the first 3 mounts. | 0.0% weight loss in the first 3 mounts. | [51] |
| 29 | 30 (National Cholesterol Education Program Step II diet) | 14 weeks | BMI index reduced from 33.6 ± 5.2 to 31.5 ± 5.2 kg/m2. | BMI index reduced from 32.6 ± 3.3 to 31.2 ± 3.5 kg/m2. | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, S.; Delattre, C.; Karcheva-Bahchevanska, D.; Benbasat, N.; Nalbantova, V.; Ivanov, K. Plant-Based Diet as a Strategy for Weight Control. Foods 2021, 10, 3052. https://doi.org/10.3390/foods10123052
Ivanova S, Delattre C, Karcheva-Bahchevanska D, Benbasat N, Nalbantova V, Ivanov K. Plant-Based Diet as a Strategy for Weight Control. Foods. 2021; 10(12):3052. https://doi.org/10.3390/foods10123052
Chicago/Turabian StyleIvanova, Stanislava, Cédric Delattre, Diana Karcheva-Bahchevanska, Niko Benbasat, Vanya Nalbantova, and Kalin Ivanov. 2021. "Plant-Based Diet as a Strategy for Weight Control" Foods 10, no. 12: 3052. https://doi.org/10.3390/foods10123052
APA StyleIvanova, S., Delattre, C., Karcheva-Bahchevanska, D., Benbasat, N., Nalbantova, V., & Ivanov, K. (2021). Plant-Based Diet as a Strategy for Weight Control. Foods, 10(12), 3052. https://doi.org/10.3390/foods10123052








