A Novel Method of a High Pressure Processing Pre-Treatment on the Juice Yield and Quality of Persimmon
Abstract
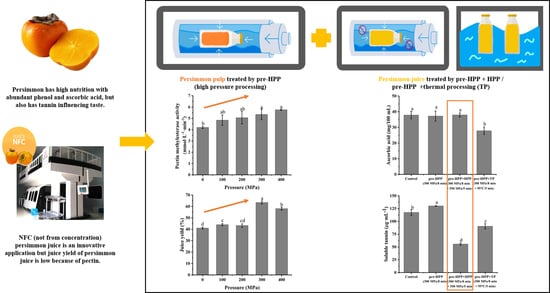
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Persimmon Pulp and Juice
2.3. HPP and TP Treatments of Persimmon Pulp and Juice
2.4. Determination of pH
2.5. Determination of Total Soluble Solids (TSS)
2.6. Determination of Juice Yield
2.7. Determination of Total Phenols
2.8. Determination of Ascorbic Acid (AA)
2.9. Sensory Evaluation
2.10. Determination of Pectin Methylesterase (PME) Activity
2.11. Determination of Polygalacturonase (PG) Activity
2.12. Microbiological Analysis
2.13. Determination of Water-Soluble Pectin
2.14. Determination of Soluble Tannin
2.15. Determination of Antioxidant Activity
2.15.1. DPPH Radical Scavenging Assay (DPPH)
2.15.2. Ferric Reducing/Antioxidant Power Assay (FRAP)
2.16. Determination of PPO and POD Activity
2.17. Color Assessment
2.18. Determination of Browning Degree (BD)
2.19. Determination of Clarity
2.20. Statistical Analysis
3. Results and Discussion
3.1. Differences on Physical and Chemical Indexes of Three Persimmon Cultivars
3.2. Effects of Different Pre-HPP Treatment Pressure and Time on Juice Yield, PME and PG Activity of Persimmon Pulp
3.3. Effects of HPP and TP on Microorganism, pH and TSS of Persimmon Juice
3.4. Effects of HPP and TP on PME, PG, and Water-Soluble Pectin in Persimmon Juice
3.5. Effects of HPP and TP on Total Phenols, Ascorbic Acid, Antioxidant Capacity and Soluble Tannin in Persimmon Juice
3.6. Effects of HPP and TP on the Polyphenol Oxidase Activity, Peroxidase Activity and Color of the Persimmon Juice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 7 May 2021).
- Anand, N.; Mukesh, K.P.; Sadhana, N. 8-Pectinases: Production and Applications for Fruit Juice Beverages. Process. Sustain. Beverages 2019, 2, 235–273. [Google Scholar]
- Sharma, H.P.; Patel, H. Sugandha, Enzymatic added extraction and clarification of fruit juices—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1215–1227. [Google Scholar] [CrossRef]
- Ortuno, C.; Trang, D.; Balaban, M.; Benedito, J. Combined high hydrostatic pressure and carbon dioxide inactivation of pectin methylesterase, polyphenol oxidase and peroxidase in feijoa puree. J. Supercrit. Fluids 2013, 82, 56–62. [Google Scholar] [CrossRef]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High pressure enhancement of enzymes: A review. Enzym. Microb. Technol. 2009, 45, 331–347. [Google Scholar] [CrossRef]
- Boulekou, S.S.; Katsaros, G.J.; Taoukis, P.S. Inactivation Kinetics of Peach Pulp Pectin Methylesterase as a Function of High Hydrostatic Pressure and Temperature Process Conditions. Food Bioprocess Technol. 2010, 3, 699–706. [Google Scholar] [CrossRef]
- Rodriguez, A.; Cap, M.; Ramos, N.; Godoy, F.; Mascheroni, R.H.; Vaudagna, S.R. High-pressure processing of persimmon purée: Stability during chilled storage. J. Food Process. Preserv. 2020, 44, e14306. [Google Scholar] [CrossRef]
- Kumari, A.; Farid, M. Optimization of high pressure processing for microbial load reduction in Diospyros kaki ‘Fuyu’ pulp using response surface methodology. J. Food Sci. Technol. 2020, 57, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- José Luis, V.-G.; Amparo, Q.; Erica, V.; Judith, A.J.; Isabel, H.; Nitin, N.; Diane, M.B. High hydrostatic pressure as a method to preserve fresh-cut Hachiya persimmons: A structural approach. Food Sci. Technol. Int. 2016, 22, 688–698. [Google Scholar]
- de Ancos, B.; Gonzalez, E.; Cano, M.P. Effect of High-Pressure Treatment on the Carotenoid Composition and the Radical Scavenging Activity of Persimmon Fruit Purees. J. Agric. Food Chem. 2000, 48, 3542–3548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucía, P.; Clara, C.; de Ancos, B.; Concepción, S.-M.; Cano, M.P. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.). Food Chem. 2012, 130, 591–597. [Google Scholar]
- Cano, M.P.; Gómez-Maqueo, A.; Welti-Chanes, J.; García-Cayuela, T. Characterization of Carotenoid and Carotenoid Esters of Astringent Persimmon Tissues (Diospyros kaki Thunb. var. Rojo Brillante). Effects of Thermal and High Pressure Non-Thermal Processing. Food Chem. 2018. [Google Scholar] [CrossRef]
- Vázquez-Gutiérrez, J.L.; Quiles, A.; Hernando, I.; Pérez-Munuera, I. Changes in the microstructure and location of some bioactive compounds in persimmons treated by high hydrostatic pressure. Postharvest Biol. Technol. 2011, 61, 137–144. [Google Scholar] [CrossRef]
- Hernández-Carrión, M.; Tárrega, A.; Hernando, I.; Fiszman, S.M.; Quiles, A. High hydrostatic pressure treatment provides persimmon good characteristics to formulate milk-based beverages with enhanced functionality. Food Funct. 2014, 5, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lounds-Singleton, A.J.; Talcott, S.T. Antioxidant phytochemical and quality changes associated with hot water immersion treatment of mangoes (Mangifera indica L.). Food Chem. 2009, 115, 989–993. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Da Pieve, S.; Butler, F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purees. Innov. Food Sci. Emerg. Technol. 2009, 10, 308–313. [Google Scholar] [CrossRef]
- Zhang, K. NY 82.2—1988. In Juice Determination Method-Sensory Inspection; Standards of the Ministry of Agriculture of the People’s Republic of China: Beijing, China, 1988. [Google Scholar]
- Bi, X.; Wu, J.; Zhang, Y.; Xu, Z.; Liao, X. High pressure carbon dioxide treatment for fresh-cut carrot slices. Innov. Food Sci. Emerg. Technol. 2011, 12, 298–304. [Google Scholar] [CrossRef]
- Amnuaysin, N.; Jones, M.L.; Seraypheap, K. Changes in activities and gene expression of enzymes associated with cell wall modification in peels of hot water treated bananas. Sci. Hortic. 2012, 142, 98–104. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Bi, X.; Guo, X.; Fu, S.; Liao, X. Comparison of Microbial Inactivation and Rheological Characteristics of Mango Pulp after High Hydrostatic Pressure Treatment and High Temperature Short Time Treatment. Food Bioprocess Technol. 2013, 6, 2675–2684. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Hadfield, K.A.; Labavitch, J.M.; Bennett, A.B. Temporal sequence of cell wall disassembly in rapidly ripening melon fruit. Plant Physiol. 1998, 117, 345–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juang, L.J.; Sheu, S.J.; Lin, T.C. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J. Sep. Sci. 2004, 27, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, I.; Duru, M.E.; Ozturk, M.; Mercan, N.; Harmandar, M.; Topcu, G. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chem. 2009, 116, 470–479. [Google Scholar] [CrossRef]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Zhao, F.; Sun, Z.; Liao, X. Quality comparison of carrot juices processed by high-pressure processing and high-temperature short-time processing. Innov. Food Sci. Emerg. Technol. 2016, 33, 135–144. [Google Scholar] [CrossRef]
- Meydav, S.; Saguy, I.; Kopelman, I.J. Browning determination in citrus products. J. Agric. Food Chem. 1977, 25, 602–604. [Google Scholar] [CrossRef]
- Rai, P.; Majumdar, G.C.; Dasgupta, S.; De, S. Optimizing pectinase usage in pretreatment of mosambi juice for clarification by response surface methodology. J. Food Eng. 2004, 64, 397–403. [Google Scholar] [CrossRef]
- Hsu, K.-C. Evaluation of processing qualities of tomato juice induced by thermal and pressure processing. Lwt-Food Sci. Technol. 2008, 41, 450–459. [Google Scholar] [CrossRef]
- dos Santos Aguilar, J.G.; Cristianini, M.; Sato, H.H. Modification of enzymes by use of high-pressure homogenization. Food Res. Int. 2018, 109, 120–125. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Kermani, Z.J.; Moelants, K.R.N.; Ngouemazong, E.D.; Van Loey, A.; Hendrickx, M.E.G. Process-Structure-Function Relations of Pectin in Food. Crit. Rev. Food Sci. Nutr. 2016, 56, 1021–1042. [Google Scholar] [CrossRef] [PubMed]
- Crelier, S.; Robert, M.C.; Claude, J.; Juillerat, M.A. Tomato (Lycopersicon esculentum) pectin methylesterase and polygalacturonase behaviors regarding heat- and pressure-induced inactivation. J. Agric. Food Chem. 2001, 49, 5566–5575. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Chaula, D.; Van Loey, A.M.; Hendrickx, M.E. Unravelling process-induced pectin changes in the tomato cell wall: An integrated approach. Food Chem. 2012, 132, 1534–1543. [Google Scholar] [CrossRef]
- Jiang, X.P.; Chen, P.; Yin, M.L.; Yang, Q. Constitutive expression, purification and characterisation of pectin methylesterase from Aspergillus niger in Pichia pastoris for potential application in the fruit juice industry. J. Sci. Food Agric. 2013, 93, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Gutierrez, J.L.; Hernandez-Carrion, M.; Quiles, A.; Hernando, I.; Perez-Munuera, I. Impact of high hydrostatic pressures on the structure, diffusion of soluble compounds and textural properties of persimmon ‘Rojo Brillante’. Food Res. Int. 2012, 47, 218–222. [Google Scholar] [CrossRef]
- Ribas-Agusti, A.; Van Buggenhout, S.; Palmero, P.; Hendrickx, M.; Van Loey, A. Investigating the role of pectin in carrot cell wall changes during thermal processing: A microscopic approach. Innov. Food Sci. Emerg. Technol. 2014, 24, 113–120. [Google Scholar] [CrossRef]
- Vazquez, L.H.; Palazon, J.; Navarro-Ocana, A. The Pentacyclic Triterpenes alpha, beta-amyrins: A Review of Sources and Biological Activities. In Phytochemicals-a Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2012; pp. 487–502. [Google Scholar]
- Vazquez-Gutierrez, J.L.; Hernando, I.; Quiles, A. Changes in tannin solubility and microstructure of high hydrostatic pressure-treated persimmon cubes during storage at 4 A degrees C. Eur. Food Res. Technol. 2013, 237, 9–17. [Google Scholar] [CrossRef]
- Hernandez-Carrion, M.; Vazquez-Gutierrez, J.L.; Hernando, I.; Quiles, A. Impact of High Hydrostatic Pressure and Pasteurization on the Structure and the Extractability of Bioactive Compounds of Persimmon “Rojo Brillante”. J. Food Sci. 2014, 79, C32–C38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Wang, Y.; Li, R.; Bi, X.; Liao, X. Effects of high hydrostatic pressure and high temperature short time on antioxidant activity, antioxidant compounds and color of mango nectars. Innov. Food Sci. Emerg. Technol. 2014, 21, 35–43. [Google Scholar] [CrossRef]
- Fevrier, H.; Le Quere, J.M.; Le Bail, G.; Guyot, S. Polyphenol profile, PPO activity and pH variation in relation to colour changes in a series of red-fleshed apple juices. Lwt-Food Sci. Technol. 2017, 85, 353–362. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, R.F.; Zhao, X.Y.; Ma, Y.; Wang, D.; Zhang, B.B. Effect of high hydrostatic pressure processing on qualities of strawberry juice. Adv. Mater. Res. 2014, 894, 305–310. [Google Scholar] [CrossRef]
- Sulaiman, A.; Soo, M.J.; Yoon, M.M.L.; Farid, M.; Silva, F.V.M. Modeling the polyphenoloxidase inactivation kinetics in pear, apple and strawberry purees after High Pressure Processing. J. Food Eng. 2015, 147, 89–94. [Google Scholar] [CrossRef]
- Serra, S.; Anthony, B.; Boscolo Sesillo, F.; Masia, A.; Musacchi, S. Determination of Post-Harvest Biochemical Composition, Enzymatic Activities, and Oxidative Browning in 14 Apple Cultivars. Foods 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, E.; Mateos, M.; Perez-Gago, M.B. Effect of maturity stage at processing and antioxidant treatments on the physico-chemical, sensory and nutritional quality of fresh-cut ‘Rojo Brillante’ persimmon. Postharvest Biol. Technol. 2015, 105, 34–44. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Protein/Polyphenol Interactions: Past and Present Contributions. Mechanisms of Astringency Perception. Curr. Org. Chem. 2012, 16, 724–746. [Google Scholar] [CrossRef] [Green Version]








| Gongcheng (Guangxi) | Mopan (Beijing) | Yangfeng (Shanxi) | |
|---|---|---|---|
| Juice yield (%) | 41.03 ± 0.75 a | 21.84 ± 2.41 b | 10.27 ± 0.47 c |
| pH | 5.83 ± 0.01 c | 6.00 ± 0.01 b | 6.28 ± 0.02 a |
| TSS (oBrix) | 14.60 ± 0.10 c | 15.00 ± 0.10 b | 17.30 ± 0.10 a |
| Total phenols (mg GAE/100 g FW) | 6.78 ± 0.33 a | 2.78 ± 0.28 b | 1.93 ± 0.17 c |
| Ascorbic acid (mg/100 g FW) | 28.32 ± 1.42 b | 18.25 ± 2.79 c | 97.53 ± 3.40 a |
| Sensory evaluation | 16.70 | 15.60 | 14.50 |
| Treatments | Control | Pre-HPP (300 MPa/8 Min) | Pre-HPP + HPP (300 MPa/8 min + 550 MPa/5 Min) | Pre-HPP + TP (300 MPa/8 min + 95 °C/5 Min) |
|---|---|---|---|---|
| TAB (lg CFU/mL) | 5.61 ± 0.17 a | 5.25 ± 0.03 b | 1.29 ± 0.08 c | ND |
| Y&M (lg CFU/mL) | ND | ND | ND | ND |
| pH | 5.96 ± 0.10 a | 5.94 ± 0.09 a | 5.63 ± 0.03 b | 4.87 ± 0.08 c |
| TSS (oBrix) | 15.2 ± 0.3 a | 14.7 ± 0.3 ab | 14.4 ± 0.3 b | 14.6 ± 0.4 ab |
| Juice yield (%) | 48.9 ± 0.1 | 60.1 ± 1.4 | 60.1 ± 1.4 | 60.1 ± 1.4 |
| Treatments | Control | Pre-HPP (300 MPa/8 Min) | Pre-HPP + HPP (300 MPa/8 min + 550 MPa/5 Min) | Pre-HPP + TP (300 MPa/8 min + 95 °C/5 Min) |
|---|---|---|---|---|
| L* | 35.90 ± 0.29 b | 35.88 ± 0.24 b | 37.39 ± 1.91 b | 46.91 ± 4.39 a |
| a* | 1.10 ± 0.04 a | 0.92 ± 0.48 a | 0.83 ± 0.16 a | 2.18 ± 1.66 a |
| b* | 4.66 ± 0.25 b | 4.37 ± 0.32 b | 2.45 ± 1.16 b | 15.35 ± 5.87 a |
| ΔE | 0 | 0.35 | 2.68 | 15.38 |
| BD | 0.585 ± 0.011 a | 0.203 ± 0.028 b | 0.189 ± 0.022 b | 0.123 ± 0.010 c |
| Clarity (%) | 18.2 ± 0.3 b | 25.7 ± 3.3 a | 18.6 ± 0.5 b | 0.20 ± 0.1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wang, Y.; Zhang, X.; Zhao, Z.; Yang, Y.; Yang, X.; Wang, Y.; Liao, X.; Zhao, L. A Novel Method of a High Pressure Processing Pre-Treatment on the Juice Yield and Quality of Persimmon. Foods 2021, 10, 3069. https://doi.org/10.3390/foods10123069
Xu J, Wang Y, Zhang X, Zhao Z, Yang Y, Yang X, Wang Y, Liao X, Zhao L. A Novel Method of a High Pressure Processing Pre-Treatment on the Juice Yield and Quality of Persimmon. Foods. 2021; 10(12):3069. https://doi.org/10.3390/foods10123069
Chicago/Turabian StyleXu, Jiayue, Yilun Wang, Xinyue Zhang, Zhen Zhao, Yao Yang, Xin Yang, Yongtao Wang, Xiaojun Liao, and Liang Zhao. 2021. "A Novel Method of a High Pressure Processing Pre-Treatment on the Juice Yield and Quality of Persimmon" Foods 10, no. 12: 3069. https://doi.org/10.3390/foods10123069