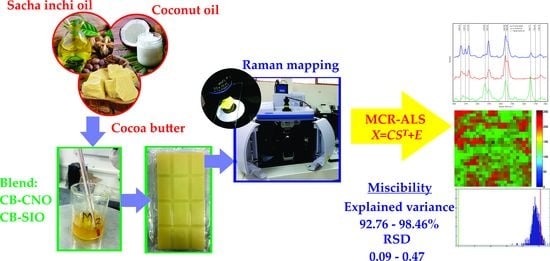
Evaluation of the Miscibility of Novel Cocoa Butter Equivalents by Raman Mapping and Multivariate Curve Resolution–Alternating Least Squares
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Raman Mapping
2.4. Data Analysis of Chemical Maps
Preprocessing and Constraints
2.5. Miscibility
3. Results
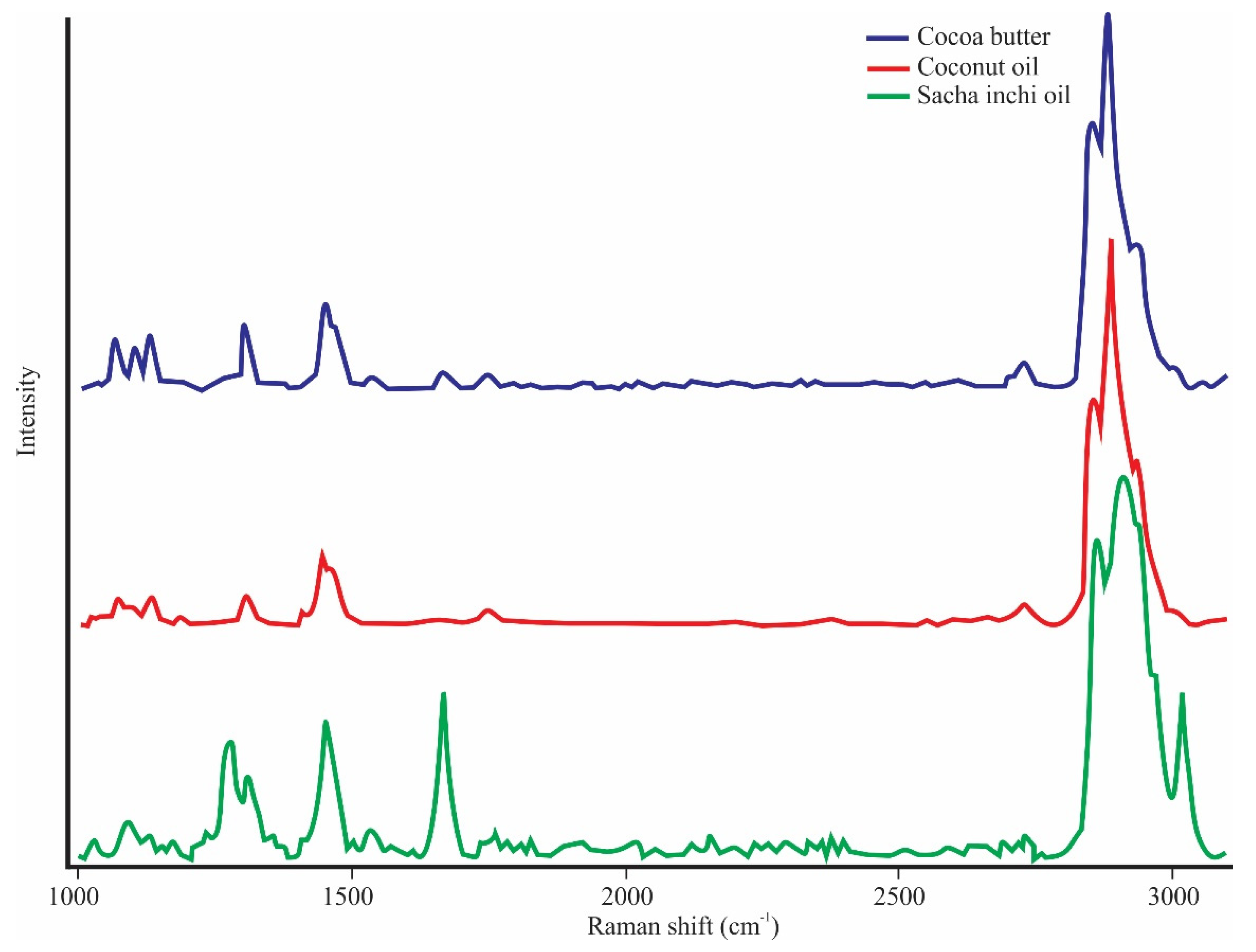
3.1. Characterization of the Spectra of Cocoa Butter and Vegetable Oils
3.2. Miscibility of Cocoa Butter and Vegetable Oils
4. Discussion
4.1. Characterization of the Spectra of Cocoa Butter and Vegetable Oils
4.2. Miscibility of Cocoa Butter and Vegetable Oils
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ewens, H.; Metilli, L.; Simone, E. Analysis of the effect of recent reformulation strategies on the crystallization behaviour of cocoa butter and the structural properties of chocolate. Curr. Res. Food Sci. 2021, 4, 105–114. [Google Scholar] [CrossRef]
- Norazlina, M.; Jahurul, M.; Hasmadi, M.; Mansoor, A.; Norliza, J.; Patricia, M.; George, M.R.; Noorakmar, A.; Lee, J.; Fan, H. Trends in blending vegetable fats and oils for cocoa butter alternative application: A review. Trends Food Sci. Technol. 2021, 116, 102–114. [Google Scholar] [CrossRef]
- Watanabe, S.; Yoshikawa, S.; Sato, K. Formation and properties of dark chocolate prepared using fat mixtures of cocoa butter and symmetric/asymmetric stearic-oleic mixed-acid triacylglycerols: Impact of molecular compound crystals. Food Chem. 2021, 339, 127808. [Google Scholar] [CrossRef] [PubMed]
- Toro-Vazquez, J.F.; Charó-Alonso, M.A.; Morales-Rueda, J.A.; Pérez-Martínez, J.D. Molecular Interactions of Triacylglycerides in Blends of Cocoa Butter with trans-free Vegetable Oils. In Cocoa Butter and Related Compounds; AOCS Press: Urbana, IL, USA, 2012; pp. 393–416. [Google Scholar]
- Bootello, M.A.; Hartel, R.W.; Garcés, R.; Martínez-Force, E.; Salas, J.J. Evaluation of high oleic-high stearic sunflower hard stearins for cocoa butter equivalent formulation. Food Chem. 2012, 134, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Bahari, A.; Akoh, C.C. Texture, rheology and fat bloom study of ‘chocolates’ made from cocoa butter equivalent synthesized from illipe butter and palm mid-fraction. LWT—Food Sci. Technol. 2018, 97, 349–354. [Google Scholar] [CrossRef]
- Jahurul, M.; Zaidul, I.; Norulaini, N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K.; Omar, A.M. Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J. Food Eng. 2013, 117, 467–476. [Google Scholar] [CrossRef]
- Segman, O.; Wiesman, Z.; Yarmolinsky, L. Methods Ant Technologies Related to Shea Butter Chemophysical Properties and to the Delivery of Bioactives in Chocolate and Related Products. In Cocoa Butter and Related Compounds; AOCS Press: Urbana, IL, USA, 2012; pp. 417–441. [Google Scholar]
- Kang, K.K.; Jeon, H.; Kim, I.-H.; Kim, B.H. Cocoa butter equivalents prepared by blending fractionated palm stearin and shea stearin. Food Sci. Biotechnol. 2013, 22, 347–352. [Google Scholar] [CrossRef]
- Talbot, G. Chocolate and Cocoa Butter—Structure and Composition. In Cocoa Butter and Related Compounds; AOCS Press: Urbana, IL, USA, 2012; pp. 1–34. ISBN 978-0-9830791-2-5. [Google Scholar]
- Beckett, T.S. Industrial Chocolate Manufacture and Use, 4th ed.; Wiley-Blackwell: Chichester, UK, 2009; ISBN 978-1-4051-3949-6. [Google Scholar]
- Rodriguez-Negrette, A.C.; Huck-Iriart, C.; Herrera, M.L. Physical Chemical Properties of Shea/Cocoa Butter Blends and their Potential for Chocolate Manufacture. J. Am. Oil Chem. Soc. 2019, 96, 239–248. [Google Scholar] [CrossRef]
- EUR-Lex Directive 2000/36/EC of the European Parliament and of the Council of 23 June 2000 Relating to Cocoa and Chocolate Products Intended for Human Consumption. Available online: https://eur-lex.europa.eu/eli/dir/2000/36/2013-11-18 (accessed on 2 December 2021).
- CFR Code of Federal Regulations. Title 21-Part 163: Cacao Products. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-163 (accessed on 2 December 2021).
- Medina-Mendoza, M.; Rodriguez-Pérez, R.J.; Rojas-Ocampo, E.; Torrejón-Valqui, L.; Fernández-Jeri, A.B.; Idrogo-Vásquez, G.; Cayo-Colca, I.S.; Castro-Alayo, E.M. Rheological, bioactive properties and sensory preferences of dark chocolates with partial incorporation of Sacha Inchi (Plukenetia volubilis L.) oil. Heliyon 2021, 7, e06154. [Google Scholar] [CrossRef]
- Chirinos, R.; Zuloeta, G.; Pedreschi, R.; Mignolet, E.; Larondelle, Y.; Campos, D. Sacha inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chem. 2013, 141, 1732–1739. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Morales, M.T.; Escudero, M.R.; Muñoz, A.M.; Chavez, K.C.; Asuero, A.G. Assessment of phenolic and volatile compounds of commercial Sacha inchi oils and sensory evaluation. Food Res. Int. 2021, 140, 110022. [Google Scholar] [CrossRef]
- Neves, M.D.G.; Poppi, R.J. Monitoring of Adulteration and Purity in Coconut Oil Using Raman Spectroscopy and Multivariate Curve Resolution. Food Anal. Methods 2017, 11, 1897–1905. [Google Scholar] [CrossRef]
- Jayawardena, R.; Swarnamali, H.; Ranasinghe, P.; Misra, A. Health effects of coconut oil: Summary of evidence from systematic reviews and meta-analysis of interventional studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 549–555. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.H.R.; Ribeiro, L.N.; Mitsutake, H.; Guilherme, V.A.; DE Castro, S.; Poppi, R.; Breitkreitz, M.C.; de Paula, E. Optimised NLC: A nanotechnological approach to improve the anaesthetic effect of bupivacaine. Int. J. Pharm. 2017, 529, 253–263. [Google Scholar] [CrossRef]
- Scoutaris, N.; Vithani, K.; Slipper, I.; Chowdhry, B.; Douroumis, D. SEM/EDX and confocal Raman microscopy as complementary tools for the characterization of pharmaceutical tablets. Int. J. Pharm. 2014, 470, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Mitsutake, H.; Ribeiro, L.N.; da Silva, G.H.R.; Castro, S.R.; de Paula, E.; Poppi, R.; Breitkreitz, M.C. Evaluation of miscibility and polymorphism of synthetic and natural lipids for nanostructured lipid carrier (NLC) formulations by Raman mapping and multivariate curve resolution (MCR). Eur. J. Pharm. Sci. 2019, 135, 51–59. [Google Scholar] [CrossRef]
- Mitsutake, H.; DE Castro, S.; de Paula, E.; Poppi, R.; Rutledge, D.N.; Breitkreitz, M.C. Comparison of different chemometric methods to extract chemical and physical information from Raman images of homogeneous and heterogeneous semi-solid pharmaceutical formulations. Int. J. Pharm. 2018, 552, 119–129. [Google Scholar] [CrossRef]
- Breitkreitz, M.C.; Sabin, G.P.; Polla, G.; Poppi, R. Characterization of semi-solid Self-Emulsifying Drug Delivery Systems (SEDDS) of atorvastatin calcium by Raman image spectroscopy and chemometrics. J. Pharm. Biomed. Anal. 2013, 73, 3–12. [Google Scholar] [CrossRef]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman Spectroscopic Methods in Food Safety: A Review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef]
- Yaseen, T.; Sun, D.-W.; Cheng, J.-H. Raman imaging for food quality and safety evaluation: Fundamentals and applications. Trends Food Sci. Technol. 2017, 62, 177–189. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N.; Yu, L.; Zhou, S.; Shanks, R.; Zheng, J. Imaging the phase of starch–gelatin blends by confocal Raman microscopy. Food Hydrocoll. 2016, 60, 7–10. [Google Scholar] [CrossRef]
- Neves, A.C.d.O.; Zougagh, M.; Ríos, Á.; Tauler, R.; Wakamatsu, K.; Galván, I. Pheomelanin Subunit Non-Destructive Quantification by Raman Spectroscopy and Multivariate Curve Resolution-Alternating Least Squares (MCR-ALS). Chemom. Intell. Lab. Syst. 2021, 217, 104406. [Google Scholar] [CrossRef]
- Vajna, B.; Pataki, H.; Nagy, Z.; Farkas, I.; Marosi, G. Characterization of melt extruded and conventional Isoptin formulations using Raman chemical imaging and chemometrics. Int. J. Pharm. 2011, 419, 107–113. [Google Scholar] [CrossRef] [PubMed]
- de Juan, A.; Jaumot, J.; Tauler, R. Multivariate Curve Resolution (MCR). Solving the mixture analysis problem. Anal. Methods 2014, 6, 4964–4976. [Google Scholar] [CrossRef]
- Osorio, J.G.; Stuessy, G.; Kemeny, G.J.; Muzzio, F.J. Characterization of pharmaceutical powder blends using in situ near-infrared chemical imaging. Chem. Eng. Sci. 2014, 108, 244–257. [Google Scholar] [CrossRef]
- Amigo, J.M. Practical issues of hyperspectral imaging analysis of solid dosage forms. Anal. Bioanal. Chem. 2010, 398, 93–109. [Google Scholar] [CrossRef]
- Gendrin, C.; Roggo, Y.; Collet, C. Pharmaceutical applications of vibrational chemical imaging and chemometrics: A review. J. Pharm. Biomed. Anal. 2008, 48, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Bresson, S.; Rousseau, D.; Ghosh, S.; El Marssi, M.; Faivre, V. Raman spectroscopy of the polymorphic forms and liquid state of cocoa butter. Eur. J. Lipid Sci. Technol. 2011, 113, 992–1004. [Google Scholar] [CrossRef]
- Jiménez-Sanchidrián, C.; Ruiz, J.R. Use of Raman spectroscopy for analyzing edible vegetable oils. Appl. Spectrosc. Rev. 2016, 51, 417–430. [Google Scholar] [CrossRef]
- Carmona, M.Á.; Lafont, F.; Jiménez–Sanchidrián, C.; Ruiz, J.R. Raman spectroscopy study of edible oils and determination of the oxidative stability at frying temperatures: Raman Spectroscopy Study of Edible Oils. Eur. J. Lipid Sci. Technol. 2014, 116, 1451–1456. [Google Scholar] [CrossRef]
- Wang, H.; Xin, Y.; Ma, H.; Fang, P.; Li, C.; Wan, X.; He, Z.; Jia, J.; Ling, Z. Rapid detection of Chinese-specific peony seed oil by using confocal Raman spectroscopy and chemometrics. Food Chem. 2021, 362, 130041. [Google Scholar] [CrossRef] [PubMed]
- Bresson, S.; Lecuelle, A.; Bougrioua, F.; El Hadri, M.; Baeten, V.; Courty, M.; Pilard, S.; Rigaud, S.; Faivre, V. Comparative structural and vibrational investigations between cocoa butter (CB) and cocoa butter equivalent (CBE) by ESI/MALDI-HRMS, XRD, DSC, MIR and Raman spectroscopy. Food Chem. 2021, 363, 130319. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.C.; Ribeiro, D.S.; Santos, J.L.; Páscoa, R.N. Comparison of near infrared spectroscopy and Raman spectroscopy for the identification and quantification through MCR-ALS and PLS of peanut oil adulterants. Talanta 2021, 230, 122373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; de Juan, A.; Tauler, R. Multivariate Curve Resolution Applied to Hyperspectral Imaging Analysis of Chocolate Samples. Appl. Spectrosc. 2015, 69, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- de Juan, A.; Tauler, R. Multivariate Curve Resolution: 50 years addressing the mixture analysis problem—A review. Anal. Chim. Acta 2021, 1145, 59–78. [Google Scholar] [CrossRef]
- Gupta, S.; Román-Ospino, A.D.; Baranwal, Y.; Hausner, D.; Ramachandran, R.; Muzzio, F.J. Performance assessment of linear iterative optimization technology (IOT) for Raman chemical mapping of pharmaceutical tablets. J. Pharm. Biomed. Anal. 2021, 205, 114305. [Google Scholar] [CrossRef]
- MCR Constraints—Eigenvector Research Documentation Wiki. Available online: https://www.wiki.eigenvector.com/index.php?title=MCR_Constraints (accessed on 30 October 2021).
- Firmani, P.; Hugelier, S.; Marini, F.; Ruckebusch, C. MCR-ALS of hyperspectral images with spatio-spectral fuzzy clustering constraint. Chemom. Intell. Lab. Syst. 2018, 179, 85–91. [Google Scholar] [CrossRef]
- Gendrin, C. Monitoring galenical process development by near infrared chemical imaging: One case study. Eur. J. Pharm. Biopharm. 2008, 68, 828–837. [Google Scholar] [CrossRef]
- Lyon, R.C.; Lester, D.S.; Lewis, E.N.; Lee, E.; Yu, L.X.; Jefferson, E.H.; Hussain, A.S. Near-infrared spectral imaging for quality assurance of pharmaceutical products: Analysis of tablets to assess powder blend homogeneity. AAPS PharmSciTech 2002, 3, E17. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Z.; Li, J.; Lin, H. Raman spectroscopic techniques for nondestructive analysis of agri-foods: A state-of-the-art review. Trends Food Sci. Technol. 2021, 118, 490–504. [Google Scholar] [CrossRef]
- Metilli, L.; Francis, M.; Povey, M.; Lazidis, A.; Marty-Terrade, S.; Ray, J.; Simone, E. Latest advances in imaging techniques for characterizing soft, multiphasic food materials. Adv. Colloid Interface Sci. 2020, 279, 102154. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Tran, C.; O’Callaghan, T.; Hogan, S.A. Use of confocal Raman imaging to understand the microstructure of anhydrous milk fat-based oleogels. Food Struct. 2021, 30, 100228. [Google Scholar] [CrossRef]
- Long, Y.; Huang, W.; Wang, Q.; Fan, S.; Tian, X. Integration of textural and spectral features of Raman hyperspectral imaging for quantitative determination of a single maize kernel mildew coupled with chemometrics. Food Chem. 2021, 372, 131246. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, Z.; Peng, W.; Chen, M.; Yu, L.; Zhu, F. Chemical mapping analysis of compatibility in gelatin and hydroxypropyl methylcellulose blend films. Food Hydrocoll. 2020, 104, 105734. [Google Scholar] [CrossRef]
- Mitsutake, H.; da Silva, G.; Ribeiro, L.; de Paula, E.; Poppi, R.; Rutledge, D.; Breitkreitz, M. Raman Imaging and Chemometrics Evaluation of Natural and Synthetic Beeswaxes as Matrices for Nanostructured Lipid Carriers Development. Braz. J. Anal. Chem. 2021, 8, 116–130. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, Z.; Xie, D.; Cao, Z.; Chen, L.; Wang, H.; Jiang, L.; Shen, Q. Rhizomucor miehei lipase-catalysed synthesis of cocoa butter equivalent from palm mid-fraction and stearic acid: Characteristics and feasibility as cocoa butter alternative. Food Chem. 2021, 343, 128407. [Google Scholar] [CrossRef]





| Sample | Cocoa Butter (%) | Coconut Oil (%) | Sacha Inchi Oil (%) |
|---|---|---|---|
| CB55-CNO45 | 55 | 45 | --- |
| CB65-CNO35 | 65 | 35 | --- |
| CB75-CNO25 | 75 | 25 | --- |
| CB85-CNO15 | 85 | 15 | --- |
| CB95-CNO05 | 95 | 05 | --- |
| CB55-SIO45 | 55 | --- | 45 |
| CB65-SIO35 | 65 | --- | 35 |
| CB75-SIO25 | 75 | --- | 25 |
| CB85-SIO15 | 85 | --- | 15 |
| CB95-SIO05 | 95 | --- | 05 |
| Assignments 1 | Cocoa Butter (cm−1) | Coconut Oil (cm−1) | Sacha Inchi Oil (cm−1) |
|---|---|---|---|
| νas(C–C)T | 1066.2 | 1069.3 | Nd |
| ν(C–C)G | 1102.9 | 1092.4 | Nd |
| νs(C–C)T | 1132.3 | 1132.3 | 1125.7 |
| τ(CH2) | Nd | 1268.8 | Nd |
| τ(CH2) | Nd | Nd | 1276.7 |
| τ(CH2) | 1301.3 | 1303.4 | 1308.8 |
| δ(CH2) | 1445.9 | 1445.1 | 1449.9 |
| δa(CH3) | 1462.9 | Nd | Nd |
| νs(C=C) | 1662.3 | 1662.2 | 1662.7 |
| ν(C=O) | 1733.8 | Nd | 1734.8 |
| ν(C=O) | 1745.4 | 1747.9 | 1746.8 |
| ν(CH3–CH2) | 2728.7 | 2730.8 | 2733.9 |
| νs(CH2) | 2855.7 | 2856.7 | 2863.4 |
| νas(CH2) | 2886.1 | 2886.1 | 2907.6 |
| νs(CH3) | 2936.5 | 2932.3 | Nd |
| (=CH)2 | Nd | Nd | 3020.2 |
| Component | 1733.84 cm−1 | 1745.43 cm−1 | Area Ratio | ||
|---|---|---|---|---|---|
| Area | FWHM | Area | FWHM | A1733.84/A1745.43 | |
| Sacha inchi oil | 288.19 | 3.99 | 2751.58 | 9.04 | 0.11 |
| Coconut oil | Nd | Nd | 8112.33 | 27.52 | Nd |
| Cocoa butter | 2448.96 | 9.85 | 5366.85 | 17.38 | 0.46 |
| Sample | Numbers of Factor | Explained Variance (%) | MCR-ALS Component | Cocoa Butter | Vegetable Oil |
|---|---|---|---|---|---|
| CB55-CNO45 | 2 | 96.74 | Comp 1 | 0.9999 | 0.9396 |
| Comp 2 | 0.9384 | 0.9999 | |||
| CB65-CNO35 | 2 | 96.60 | Comp 1 | 0.9997 | 0.9546 |
| Comp 2 | 0.9397 | 0.9996 | |||
| CB75-CNO25 | 2 | 98.14 | Comp 1 | 0.9998 | 0.9378 |
| Comp 2 | 0.9401 | 0.9999 | |||
| CB85-CNO15 | 2 | 98.46 | Comp 1 | 0.9996 | 0.9377 |
| Comp 2 | 0.9408 | 0.9999 | |||
| CB95-CNO05 | 2 | 92.76 | Comp 1 | 0.9999 | 0.9386 |
| Comp 2 | 0.9404 | 0.9998 | |||
| CB55-SIO45 | 2 | 94.59 | Comp 1 | 0.9998 | 0.5944 |
| Comp 2 | 0.6117 | 0.9993 | |||
| CB65-SIO35 | 2 | 97.46 | Comp 1 | 0.9999 | 0.5976 |
| Comp 2 | 0.6099 | 0.9995 | |||
| CB75-SIO25 | 2 | 97.99 | Comp 1 | 0.9998 | 0.9995 |
| Comp 2 | 0.6121 | 0.5976 | |||
| CB85-SIO15 | 2 | 98.01 | Comp 1 | 0.9999 | 0.5924 |
| Comp 2 | 0.6147 | 0.9993 | |||
| CB95-SIO05 | 2 | 97.39 | Comp 1 | 0.9997 | 0.5914 |
| Comp 2 | 0.6140 | 0.9995 |
| Sample | Cocoa Butter RSD 1 | Vegetable Oil RSD 1 |
|---|---|---|
| CB55-CNO45 | 0.12 ± 0.01 ab | 0.09 ± 0.02 b |
| CB65-CNO35 | 0.17 ± 0.03 ab | 0.21 ± 0.09 ab |
| CB75-CNO25 | 0.23 ± 0.06 a | 0.29 ± 0.09 ab |
| CB85-CNO15 | 0.21 ± 0.12 a | 0.47 ± 0.13 a |
| CB95-CNO05 | 0.18 ± 0.04 ab | 0.44 ± 0.23 a |
| CB55-SIO45 | 0.12 ± 0.02 ab | 0.25 ± 0.03 ab |
| CB65-SIO35 | 0.10 ± 0.01 ab | 0.15 ± 0.04 b |
| CB75-SIO25 | 0.10 ± 0.04 ab | 0.18 ± 0.04 ab |
| CB85-SIO15 | 0.07 ± 0.01 b | 0.24 ± 0.03 ab |
| CB95-SIO05 | 0.07 ± 0.02 b | 0.19 ± 0.03 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Alayo, E.M.; Torrejón-Valqui, L.; Cayo-Colca, I.S.; Cárdenas-Toro, F.P. Evaluation of the Miscibility of Novel Cocoa Butter Equivalents by Raman Mapping and Multivariate Curve Resolution–Alternating Least Squares. Foods 2021, 10, 3101. https://doi.org/10.3390/foods10123101
Castro-Alayo EM, Torrejón-Valqui L, Cayo-Colca IS, Cárdenas-Toro FP. Evaluation of the Miscibility of Novel Cocoa Butter Equivalents by Raman Mapping and Multivariate Curve Resolution–Alternating Least Squares. Foods. 2021; 10(12):3101. https://doi.org/10.3390/foods10123101
Chicago/Turabian StyleCastro-Alayo, Efraín M., Llisela Torrejón-Valqui, Ilse S. Cayo-Colca, and Fiorella P. Cárdenas-Toro. 2021. "Evaluation of the Miscibility of Novel Cocoa Butter Equivalents by Raman Mapping and Multivariate Curve Resolution–Alternating Least Squares" Foods 10, no. 12: 3101. https://doi.org/10.3390/foods10123101
APA StyleCastro-Alayo, E. M., Torrejón-Valqui, L., Cayo-Colca, I. S., & Cárdenas-Toro, F. P. (2021). Evaluation of the Miscibility of Novel Cocoa Butter Equivalents by Raman Mapping and Multivariate Curve Resolution–Alternating Least Squares. Foods, 10(12), 3101. https://doi.org/10.3390/foods10123101