Profiles of Volatile and Phenolic Compounds as Markers of Ripening Stage in Candonga Strawberries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents and Chemicals
2.3. Respiration Rate
2.4. Total Soluble Solids, Titratable Acidity and pH
2.5. Antioxidant Activity and Total Phenolic Content
2.6. Analysis of Polyphenols Compounds
2.6.1. Reversed Phase-High Performance Liquid Chromatographic-Diode Array Detector (RP-HPLC-DAD) Semi-Quantitative Determination of Polyphenols
2.6.2. Nanoflow HPLC-ESI MS/MS Analysis
2.7. Volatile Organic Compounds (VOCs) Analysis
2.7.1. Sample Preparation and HS SPME Procedure
2.7.2. Gas Chromatography-Quadrupole Mass Spectrometry Analysis (GC-qMS)
2.8. Statistical Data Analysis
3. Results and Discussion
3.1. Quality Traits in Half-Red and Red Strawberries
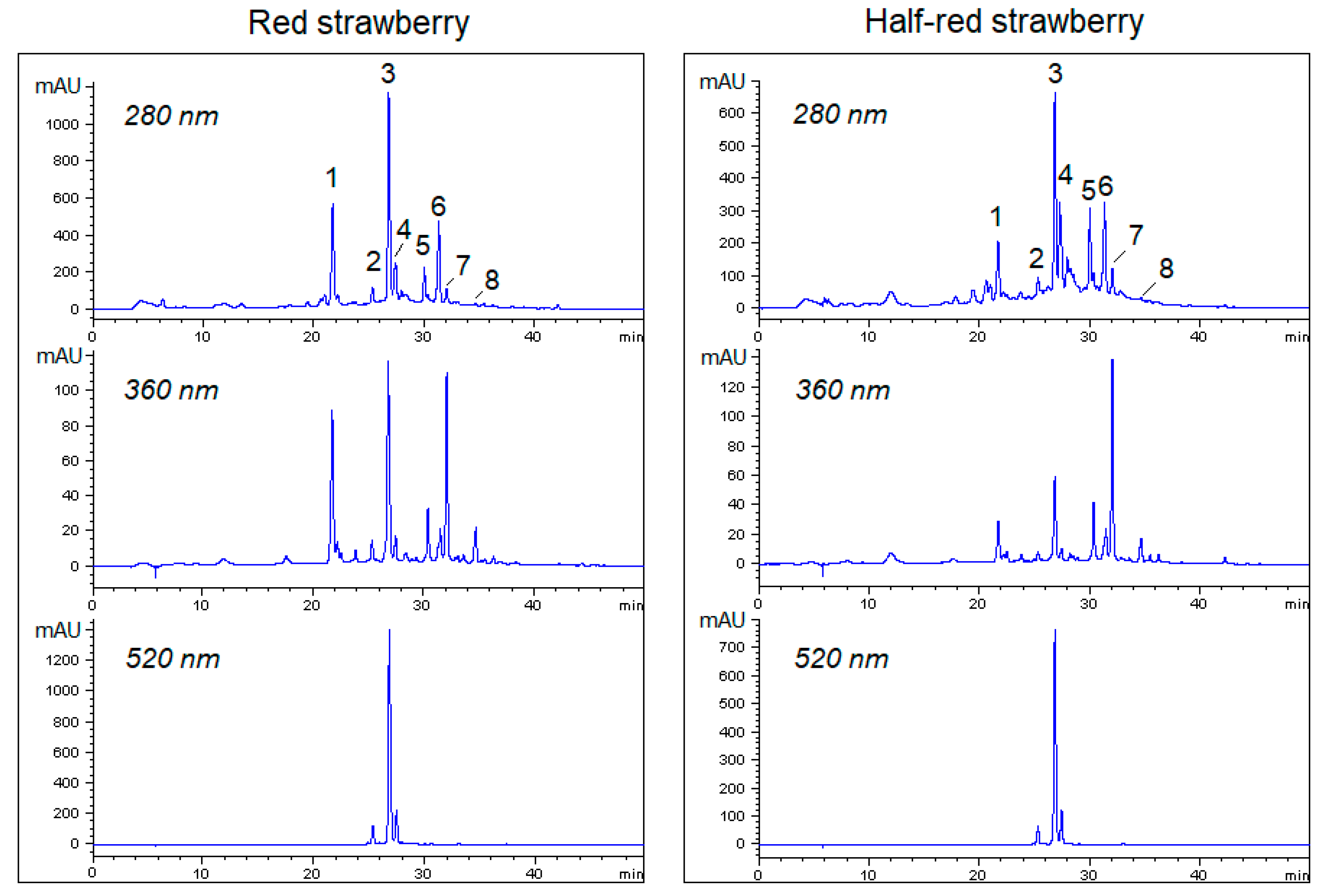
3.2. Phenolic Compounds in Half-Red and Red Strawberries
3.3. VOCs Compounds in Half-Red and Red Strawberries
3.3.1. Comparative Determination of VOCs in the “Candonga” Strawberry Samples at Two Different Ripening Stages
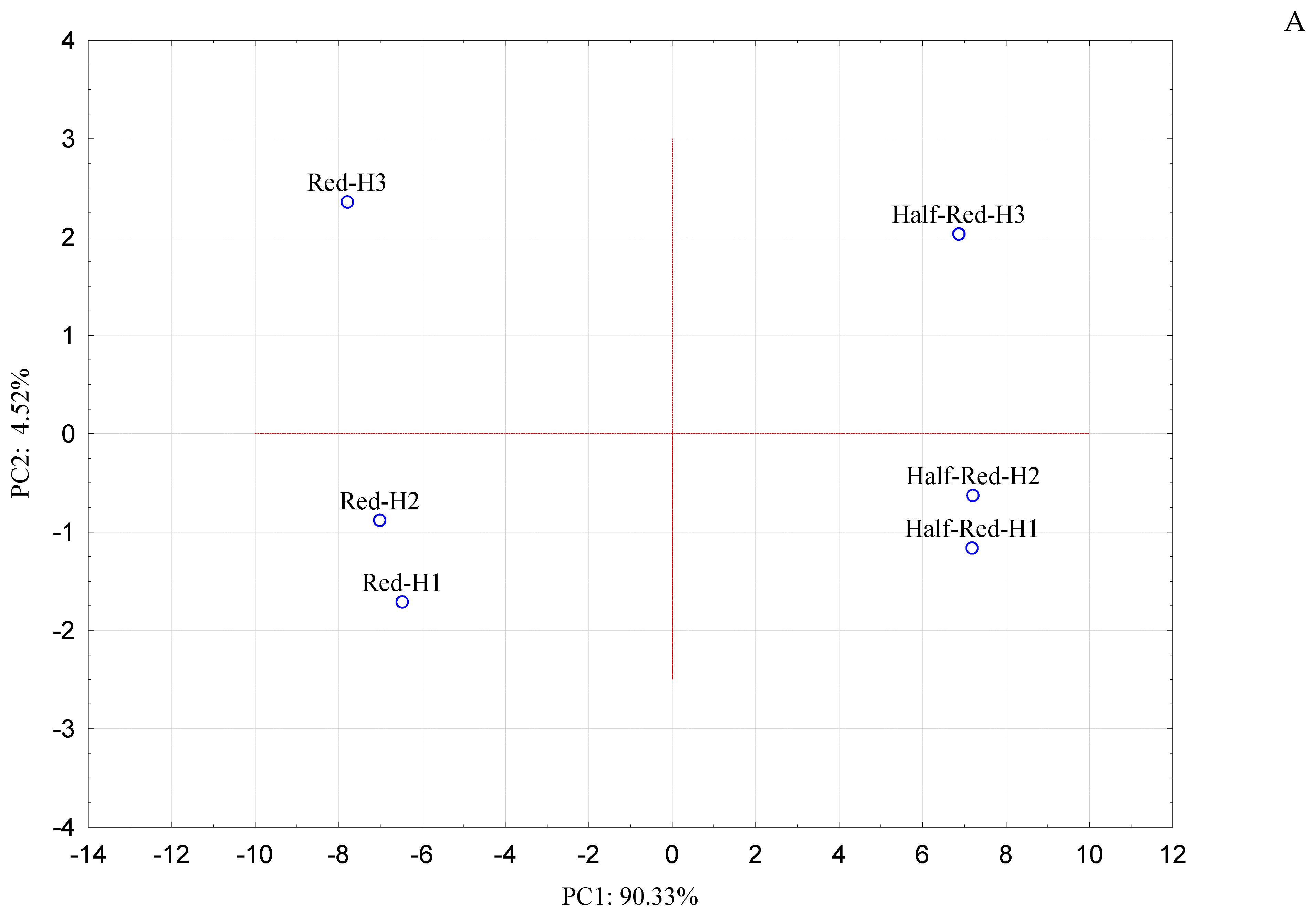
3.3.2. Selection of the VOCs Correlated to Ripening Stage of Strawberry by Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Liu, J.; Wang, F.; Wang, S.; Feng, H.; Xie, X.; Hao, F.; Zhang, L.; Fang, C. Volatile constituents and ellagic acid formation in strawberry fruits of selected cultivars. Food Res. Int. 2020, 138, 109767. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Jiménez, S.M.; Angoa-Pérez, M.V.; Mena-Violante, H.G.; Oyoque-Salcedo, G.; Montañez-Soto, J.L.; Oregel-Zamudio, E. Identification of Organic Volatile Markers Associated with Aroma during Maturation of Strawberry Fruits. Molecules 2021, 26, 504. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Ni, Y.; Wang, J.; Chen, Y.; Gao, H. Characteristic- aroma-component-based evaluation and classification of strawberry varieties by aroma type. Molecules 2021, 26, 6219. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Jiménez, S.M.; Angoa-Pérez, M.V.; Mena-Violante, H.G.; Oyoque-Salcedo, G.; Renteria-Ortega, M.; Oregel-Zamudio, E. Changes in the Aroma of Organic Blackberries (Rubus fruticosus) During Ripeness. Anal. Chem. Lett. 2019, 9, 64–73. [Google Scholar] [CrossRef]
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Li, H.; Brouwer, B.; Oud, N.; Verdonk, J.C.; Tikunov, Y.; Woltering, E.; Schouten, R.; Pereira da Silva, F. Sensory, GC-MS and PTR-ToF-MS profiling of strawberries varying in maturity at harvest with subsequent cold storage. Postharvest Biol. Technol. 2021, 182, 111719. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.A. Methods of gas mixing, sampling and analysis. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2002; pp. 145–148. [Google Scholar]
- Cefola, M.; Pace, B.; Sergio, L.; Baruzzi, F.; Gatto, M.A.; Carito, A.; Linsalata, V.; Cascarano, N.A.; Di Venere, D. Postharvest performance of fresh-cut ‘Big Top’ nectarine as affected by dipping in chemical preservatives and packaging in modified atmosphere. Int. J. Food Sci. Technol. 2014, 49, 1184–1195. [Google Scholar] [CrossRef]
- Fadda, A.; Pace, B.; Angioni, A.; Barberis, A.; Cefola, M. Suitability for ready-to-eat processing and preservation of six green and red baby leaves cultivars and evaluation of their antioxidant value during storage and after the expiration date. J. Food Process. Preserv. 2016, 40, 550–558. [Google Scholar] [CrossRef]
- Picariello, G.; Sciammaro, L.; Siano, F.; Volpe, M.G.; Puppo, M.C.; Mamone, G. Comparative analysis of C-glycosidic flavonoids from Prosopis spp. and Ceratonia siliqua seed germ flour. Food Res. Int. 2017, 99, 730–738. [Google Scholar] [CrossRef]
- Zorrilla-Fontanesi, Y.; Rambla, J.L.; Cabeza, A.; Medina, J.J.; Sánchez-Sevilla, J.F.; Valpuesta, V.; Botella, M.A.; Granell, A.; Amaya, I. Genetic analysis of strawberry fruit aroma and identification of O-methyltransferase FaOMT as the locus controlling natural variation in mesifurane content. Plant Physiol. 2012, 159, 851–870. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.A. Postharvest biology and technology: An overview. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2002; pp. 39–47. [Google Scholar]
- Janurianti, N.M.D.; Utama, I.M.S.; Gunam, I.B.W. Colour and quality of strawberry fruit (Fragaria x ananassa Duch.) at different levels of maturity. SEAS 2021, 5, 22–28. [Google Scholar] [CrossRef]
- Correia, P.J.; Pestana, M.; Martinez, F.; Ribeiro, E.; Gama, F.; Saavedra, T.; Palencia, P. Relationships between strawberry fruit quality attributes and crop load. Sci. Hortic. 2011, 130, 398–403. [Google Scholar] [CrossRef]
- Agüero, J.J.; Salazar, S.M.; Kirschbaum, D.S.; Jerez, E.F. Factors affecting fruit quality in strawberries grown in a subtropical environment. Int. J. Fruit Sci. 2015, 15, 223–234. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.J.; Shin, Y. Influence of ripening stage and cultivar on physicochemical properties, sugar and organic acid profiles, and antioxidant compositions of strawberries. Food Sci. Biotechnol. 2019, 28, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Shin, Y. Antioxidant profile, antioxidant activity, and physicochemical characteristics of strawberries from different cultivars and harvest locations. J. Appl. Biol. Chem. 2015, 58, 587–595. [Google Scholar] [CrossRef]
- Kandoliya, U.K.; Bajaniya, V.K.; Bhadja, N.K.; Bodar, N.P.; Golakiya, B.A. Antioxidant and nutritional components of eggplant (Solanum melongena L.) fruit grown in Saurastra region. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 806–813. [Google Scholar]
- Cefola, M.; Carbone, V.; Minasi, P.; Pace, B. Phenolic profiles and postharvest quality changes of fresh-cut radicchio (Cichorium intybus L.): Nutrient value in fresh vs. stored leaves. J. Food Comp. Anal. 2016, 51, 76–84. [Google Scholar] [CrossRef]
- Capotorto, I.; Innamorato, V.; Cefola, M.; Cervellieri, S.; Lippolis, V.; Longobardi, F.; Logrieco, A.F.; Pace, B. High CO2 short-term treatment to preserve quality and volatiles profile of fresh-cut artichokes during cold storage. Postharvest Biol. Technol. 2020, 160, 111056. [Google Scholar] [CrossRef]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of phenolic compounds in strawberry (Fragaria × ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007, 30, 4395. [Google Scholar] [CrossRef]
- La Barbera, G.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Zenezini Chiozzi, R.; Laganà, A. Comprehensive polyphenol profiling of a strawberry extract (Fragaria × ananassa) by ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 2127–2142. [Google Scholar] [CrossRef]
- Truchado, P.; Larrosa, M.; García-Conesa, M.T.; Cerdá, B.; Vidal-Guevara, M.L.; Tomás-Barberán, F.A.; Espín, J.C. Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. J. Agric. Food Chem. 2012, 60, 5749–5754. [Google Scholar] [CrossRef] [PubMed]
- Williner, M.R.; Pirovani, M.E.; Güemes, D.R. Ellagic acid content in strawberries of different cultivars and ripening stages. J. Sci. Food Agric. 2003, 83, 842–845. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Scheuller, H.S.; Heber, D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef] [Green Version]
- González-Domínguez, R.; Sayago, A.; Akhatou, I.; Fernández-Recamales, Á. Volatile profiling of strawberry fruits cultivated in a soilless system to investigate cultivar-dependent chemical descriptors. Foods 2020, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; San-Juan, F.; Concejero, B.; Callejón, R.M.; Troncoso, A.M.; Morales, M.L.; Ferreira, V.; Hernández-Orte, P. Glycosidically bound aroma compounds and impact odorants of four strawberry varieties. J. Agric. Food Chem. 2012, 60, 6095–6102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Q.; Li, J.; Luo, J.; Chen, W.; Li, X. Comparative study of volatile compounds in the fruit of two banana cultivars at different ripening stages. Molecules 2018, 23, 2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Forney, C.F. Flavour volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537550. [Google Scholar] [CrossRef]
- Ozcan, G.; Barringer, S. Effect of enzymes on strawberry volatiles during storage, at different ripeness level, in different cultivars and during eating. J. Food Sci. 2011, 71, C324–C333. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC–MS. Int. J. Food Prop. 2017, 20, S2926–S2938. [Google Scholar] [CrossRef] [Green Version]
- Arena, M.E.; Postemsky, P.; Curvetto, N.R. Accumulation patterns of phenolic compounds during fruit growth and ripening of Berberis buxifolia, a native Patagonian species. N. Z. J. Bot. 2012, 50, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Du, L.; Li, L.; Kalt, W.; Palmer, L.C.; Fillmore, S.; Zhang, Y.; Zhang, Z.; Li, X. Quantitative changes in proteins responsible for flavonoid and anthocyanin biosynthesis in strawberry fruit at different ripening stages: A targeted quantitative proteomic investigation employing multiple reaction monitoring. J. Proteom. 2015, 122, 1–10. [Google Scholar] [CrossRef]
- Yeh, S.Y.; Huang, F.C.; Hoffmann, T.; Mayershofer, M.; Schwab, W. FaPOD27 functions in the metabolism of polyphenols in strawberry fruit (Fragaria sp.). Front. Plant Sci. 2014, 5, 518. [Google Scholar] [CrossRef]
- Parra-Palma, C.; Úbeda, C.; Gil, M.; Ramos, P.; Castro, R.I.; Morales-Quintana, L. Comparative study of the volatile organic compounds of four strawberry cultivars and it relation to alcohol acyltransferase enzymatic activity. Sci. Hortic. 2019, 251, 65–72. [Google Scholar] [CrossRef]
- Ulrich, D.; Olbricht, K. A search for the ideal flavor of strawberry—Comparison of consumer acceptance and metabolite patterns in Fragaria × ananassa Duch. J. Appl. Bot. Food Qual. 2016, 89, 223–234. [Google Scholar]
- Fan, Z.; Plotto, A.; Bai, J.; Whitaker, V.M. Volatiles Influencing sensory attributes and bayesian modeling of the soluble solids–sweetness relationship in strawberry. Front. Plant Sci. 2021, 12, 640704. [Google Scholar] [CrossRef]
- Oh, Y.; Barbey, C.R.; Chandra, S.; Bai, J.; Fan, Z.; Plotto, A.; Pillet, J.; Folta, K.M.; Whitaker, V.M.; Lee, S. Genomic characterization of the fruity aroma gene, FaFAD1, reveals a gene dosage effect on γ-decalactone production in strawberry (Fragaria × ananassa). Front. Plant. Sci. 2021, 12, 639345. [Google Scholar] [CrossRef] [PubMed]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Hasing, T.; Johnson, T.S.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.R.; Whitaker, V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021, 8, 66. [Google Scholar] [CrossRef]

| Parameters | Harvest Time | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | |||||||||||||
| Ripening Stage | |||||||||||||||
| Red | Half-Red | p | Red | Half-Red | p | Red | Half-Red | p | |||||||
| Respiration rate (mL CO2 kg−1 h−1) | 16.74 | 14.70 | ns | 15.33 | 13.61 | ns | 16.50 | 14.00 | ns | ||||||
| Titratable acidity (% citric acid) | 0.78 | b | 0.99 | a | **** | 0.86 | b | 1.00 | a | **** | 0.76 | b | 0.97 | a | **** |
| pH | 3.56 | a | 3.31 | b | **** | 3.44 | a | 3.27 | b | *** | 3.54 | a | 3.46 | b | * |
| Total soluble solids (°Brix) | 9.61 | a | 8.37 | b | *** | 9.83 | a | 8.50 | b | **** | 10.17 | a | 9.73 | b | ** |
| Antioxidant activity (mg Trolox 100 g−1 fw) | 314.72 | a | 345.06 | b | *** | 308.12 | b | 339.11 | a | * | 287.5 | b | 347.25 | a | *** |
| Total phenols (mg GAE 100 g−1 fw ) | 175.91 | a | 207.29 | b | * | 191.46 | b | 218.05 | a | * | 183.42 | b | 207.84 | a | * |
| Polyphenols | Code | H1 | H2 | H3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Half-Red | Red | p | Half-Red | Red | p | Half-Red | Red | p | ||||||||
| p-coumaryl hexoside | P1 | 9.6 | b | 28.5 | a | **** | 11.4 | b | 25.9 | a | **** | 12.7 | b | 29.0 | a | **** |
| cyanidin-3-O-glucoside | P2 | 1.8 | b | 3.3 | a | **** | 2.0 | b | 3.8 | a | *** | 1.5 | b | 3.1 | a | *** |
| pelargonidin 3-O-glucoside | P3 | 25.1 | b | 48.7 | a | **** | 19.8 | b | 42.6 | a | **** | 22.4 | b | 43.6 | a | **** |
| pelargonidin 3-O-rutinoside | P4 | 3.1 | b | 5.6 | a | **** | 2.4 | b | 4.5 | a | *** | 3.5 | b | 5.1 | a | *** |
| quercetin-3-O-glucoside | P5 | 5.1 | a | 4.2 | b | **** | 4.1 | a | 3.6 | b | * | 3.3 | a | 3.2 | b | * |
| kaempferol-3-O-glucoside | P6 | 2.1 | b | 2.3 | a | * | 2.5 | a | 2.1 | b | *** | 2.1 | b | 2.3 | a | * |
| quercetin-3-O-glucuronide | P7 | 20.2 | a | 17.0 | b | *** | 18.6 | a | 15.8 | b | **** | 12.5 | b | 14.1 | a | *** |
| kaempferol-3-O-glucuronide | P8 | 2.9 | b | 3.1 | a | ** | 3.0 | b | 3.4 | a | ** | 2.5 | b | 2.8 | a | ** |
| Volatile Componds | Ripening Stage | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | ||||||||||||||
| Code | Red | Half-Red | p | Red | Half-Red | p | Red | Half-Red | p | |||||||
| Methyl propionate | E1 | 1.92 | a | 0.91 | b | * | 2.65 | a | 0.94 | b | **** | 2.57 | a | 0.90 | b | **** |
| Methyl butyrate | E2 | 682.95 | a | 246.38 | b | *** | 806.88 | a | 256.15 | b | **** | 812.99 | a | 266.61 | b | **** |
| Methyl isovalerate | E3 | 6.65 | a | 4.45 | b | ** | 7.70 | a | 3.97 | b | **** | 7.89 | a | 4.04 | b | **** |
| Ethyl butyrate | E4 | 46.52 | a | 4.77 | b | * | 63.08 | a | 4.24 | b | **** | 61.06 | a | 4.29 | b | **** |
| Isopropyl butyrate | E5 | 58.07 | a | 3.88 | b | **** | 73.39 | a | 3.62 | b | **** | 75.59 | a | 4.08 | b | **** |
| Butyl acetate | E6 | 4.18 | a | 0.00 | b | ** | 4.77 | a | 0.00 | b | *** | 5.99 | a | 0.00 | b | *** |
| Methyl pentanoate | E7 | 18.08 | a | 0.00 | b | **** | 28.53 | a | 0.00 | b | **** | 28.44 | a | 0.00 | b | **** |
| Ethyl pentanoate | E8 | 1.75 | a | 0.00 | b | ** | 4.51 | a | 0.00 | b | **** | 4.63 | a | 0.00 | b | **** |
| Methyl hexanoate | E9 | 472.43 | a | 188.74 | b | ** | 444.88 | a | 181.44 | b | **** | 465.00 | a | 182.94 | b | **** |
| Butyl butyrate | E10 | 48.35 | a | 0.00 | b | **** | 48.95 | a | 0.00 | b | **** | 49.57 | a | 0.00 | b | **** |
| Ethyl hexanoate | E11 | 219.48 | a | 0.00 | b | **** | 227.68 | a | 0.00 | b | **** | 278.43 | a | 0.00 | b | **** |
| Isoamyl butyrate | E12 | 3.84 | a | 0.00 | b | ** | 5.76 | a | 0.00 | b | **** | 5.07 | a | 0.00 | b | **** |
| Hexyl acetate | E13 | 473.52 | a | 35.15 | b | **** | 446.02 | a | 35.86 | b | **** | 441.31 | a | 35.77 | b | **** |
| Methyl 2-hexenoate | E14 | 21.51 | a | 3.86 | b | **** | 21.07 | a | 3.48 | b | **** | 24.98 | a | 11.61 | b | **** |
| cis-3-Hexen-1-ol acetate | E15 | 39.92 | a | 4.63 | b | **** | 39.40 | a | 3.97 | b | **** | 39.57 | a | 3.82 | b | **** |
| trans-2-Hexen-1-ol acetate | E16 | 2724.77 | a | 142.74 | b | **** | 2664.89 | a | 141.33 | b | **** | 2688.80 | a | 141.61 | b | **** |
| Methyl octanoate | E17 | 42.43 | a | 4.64 | b | **** | 41.15 | a | 4.73 | b | **** | 41.56 | a | 4.72 | b | **** |
| trans-2-Hexen-1-ol propionate | E18 | 93.04 | a | 4.49 | b | **** | 91.79 | a | 4.51 | b | **** | 91.15 | a | 4.72 | b | **** |
| n-Hexyl isobutyrate | E19 | 520.24 | a | 6.75 | b | **** | 515.75 | a | 6.89 | b | **** | 511.47 | a | 6.76 | b | **** |
| trans-2-Hexenyl butyrate | E20 | 1114.73 | a | 21.94 | b | **** | 1107.06 | a | 21.32 | b | **** | 1080.35 | a | 21.21 | b | **** |
| Methyl 3-(methylthio) propionate | E21 | 93.01 | a | 2.19 | b | **** | 91.28 | a | 2.33 | b | **** | 92.29 | a | 2.18 | b | **** |
| Hexyl hexanoate | E22 | 4.29 | a | 1.18 | b | **** | 4.10 | a | 1.29 | b | **** | 4.48 | a | 1.40 | b | **** |
| n-Octyl isobutyrate | E23 | 11.63 | a | 0.00 | b | **** | 11.31 | a | 0.00 | b | **** | 11.42 | a | 0.00 | b | **** |
| Octyl 2-methylbutyrate | E24 | 4.03 | a | 0.00 | b | **** | 4.00 | a | 0.00 | b | **** | 4.00 | a | 0.00 | b | **** |
| Methyl 3-hydroxyhexanoate | E25 | 4.62 | a | 1.67 | b | **** | 4.64 | a | 1.74 | b | **** | 4.07 | a | 1.73 | b | **** |
| Benzyl acetate | E26 | 15.53 | a | 7.49 | b | **** | 15.66 | a | 7.66 | b | **** | 15.93 | a | 7.65 | b | **** |
| Hexanal | Ald1 | 19.41 | a | 11.47 | b | * | 33.11 | a | 11.96 | b | **** | 33.88 | a | 12.69 | b | **** |
| 2-Hexenal | Ald2 | 532.42 | a | 234.98 | b | *** | 503.36 | a | 221.54 | b | **** | 523.03 | a | 219.89 | b | **** |
| Nonanal | Ald3 | 29.57 | a | 2.22 | b | **** | 26.25 | a | 2.46 | b | **** | 26.75 | a | 2.64 | b | **** |
| Benzaldehyde | Ald4 | 76.65 | a | 5.12 | b | **** | 77.97 | a | 5.22 | b | **** | 77.14 | a | 5.37 | b | **** |
| Dodecanal | Ald5 | 8.24 | a | 3.42 | b | **** | 8.08 | a | 3.66 | b | **** | 8.14 | a | 3.60 | b | **** |
| 1-Hexanol | Al1 | 209.84 | a | 27.21 | b | **** | 209.38 | a | 28.10 | b | **** | 215.20 | a | 27.69 | b | **** |
| trans-3-Hexen-1-ol | Al2 | 11.12 | a | 2.02 | b | **** | 11.08 | a | 2.11 | b | **** | 11.89 | a | 2.18 | b | **** |
| cis-3-Hexen-1-ol | Al3 | 11.69 | a | 4.92 | b | **** | 12.38 | a | 4.96 | b | **** | 12.79 | a | 4.95 | b | **** |
| trans-2-Hexen-1-ol | Al4 | 419.31 | a | 110.29 | b | **** | 404.16 | a | 111.24 | b | **** | 402.86 | a | 110.18 | b | **** |
| 1-Octanol | Al5 | 3.47 | a | 1.31 | b | **** | 3.03 | a | 1.31 | b | **** | 3.32 | a | 1.69 | b | **** |
| Propanoic acid | Ac1 | 37.29 | a | 1.56 | b | **** | 37.87 | a | 1.69 | b | **** | 37.72 | a | 1.78 | b | **** |
| 2-Methylpropionic acid | Ac2 | 70.11 | a | 1.30 | b | **** | 70.47 | a | 1.30 | b | **** | 71.67 | a | 1.33 | b | **** |
| Butyric acid | Ac3 | 24.85 | a | 8.13 | b | **** | 24.15 | a | 8.20 | b | **** | 24.27 | a | 8.31 | b | **** |
| 2-Methylbutanoic acid | Ac4 | 80.09 | a | 12.99 | b | **** | 88.70 | a | 12.82 | b | **** | 733.19 | a | 12.06 | b | **** |
| Hexanoic acid | Ac5 | 1423.08 | a | 332.86 | b | **** | 1424.30 | a | 331.78 | b | **** | 1426.72 | a | 332.20 | b | **** |
| Heptanoic acid | Ac6 | 33.53 | a | 4.26 | b | **** | 33.57 | a | 4.22 | b | **** | 33.89 | a | 4.32 | b | **** |
| Octanoic acid | Ac7 | 35.88 | a | 5.99 | b | **** | 35.50 | a | 6.05 | b | **** | 35.56 | a | 6.19 | b | **** |
| Nonanoic acid | Ac8 | 56.54 | a | 19.18 | b | **** | 56.05 | a | 19.35 | b | **** | 56.06 | a | 19.15 | b | **** |
| Decanoic acid | Ac9 | 28.59 | a | 2.52 | b | **** | 29.25 | a | 2.50 | b | **** | 29.71 | a | 2.54 | b | **** |
| Linalool | T1 | 111.83 | a | 62.76 | b | **** | 112.66 | a | 62.20 | b | **** | 112.18 | a | 62.92 | b | **** |
| β-Farnesene | T2 | 11.12 | a | 0.00 | b | **** | 8.88 | a | 0.00 | b | **** | 11.75 | a | 0.00 | b | **** |
| α-Terpineol | T3 | 98.36 | a | 10.26 | b | **** | 98.21 | a | 10.86 | b | **** | 98.37 | a | 10.57 | b | **** |
| β-Damascenone | T4 | 1.47 | a | 0.92 | b | **** | 1.47 | a | 0.92 | b | **** | 1.46 | a | 0.91 | b | **** |
| Nerolidol | T5 | 130.56 | a | 6.56 | b | **** | 130.15 | a | 6.21 | b | **** | 130.22 | a | 6.29 | b | **** |
| Mesifurane | F1 | 140.90 | a | 12.89 | b | **** | 133.47 | a | 12.81 | b | **** | 1837.09 | a | 12.65 | b | **** |
| Furaneol | F2 | 42.61 | a | 3.28 | b | **** | 41.99 | a | 3.26 | b | **** | 42.37 | a | 3.19 | b | **** |
| trans-γ-Jasmolactone | F3 | 46.65 | a | 0.00 | b | **** | 46.69 | a | 0.00 | b | **** | 46.48 | a | 0.00 | b | **** |
| γ-Octalactone | L1 | 4.31 | a | 0.00 | b | **** | 4.44 | a | 0.00 | b | **** | 4.57 | a | 0.00 | b | **** |
| γ-Decalactone | L2 | 2689.87 | a | 262.49 | b | **** | 2631.41 | a | 260.20 | b | **** | 2660.37 | a | 260.14 | b | **** |
| γ-Dodecalactone | L3 | 60.16 | a | 11.62 | b | **** | 61.88 | a | 11.00 | b | **** | 59.96 | a | 11.64 | b | **** |
| Acetophenone | O1 | 4.42 | a | 0.00 | b | **** | 4.47 | a | 0.00 | b | **** | 4.52 | a | 0.00 | b | **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzolino, R.; Pace, B.; Palumbo, M.; Laurino, C.; Picariello, G.; Siano, F.; De Giulio, B.; Pelosi, S.; Cefola, M. Profiles of Volatile and Phenolic Compounds as Markers of Ripening Stage in Candonga Strawberries. Foods 2021, 10, 3102. https://doi.org/10.3390/foods10123102
Cozzolino R, Pace B, Palumbo M, Laurino C, Picariello G, Siano F, De Giulio B, Pelosi S, Cefola M. Profiles of Volatile and Phenolic Compounds as Markers of Ripening Stage in Candonga Strawberries. Foods. 2021; 10(12):3102. https://doi.org/10.3390/foods10123102
Chicago/Turabian StyleCozzolino, Rosaria, Bernardo Pace, Michela Palumbo, Carmine Laurino, Gianluca Picariello, Francesco Siano, Beatrice De Giulio, Sergio Pelosi, and Maria Cefola. 2021. "Profiles of Volatile and Phenolic Compounds as Markers of Ripening Stage in Candonga Strawberries" Foods 10, no. 12: 3102. https://doi.org/10.3390/foods10123102
APA StyleCozzolino, R., Pace, B., Palumbo, M., Laurino, C., Picariello, G., Siano, F., De Giulio, B., Pelosi, S., & Cefola, M. (2021). Profiles of Volatile and Phenolic Compounds as Markers of Ripening Stage in Candonga Strawberries. Foods, 10(12), 3102. https://doi.org/10.3390/foods10123102










