Cognitive Keys in Psychophysical Estimation of Chemosensory Perception in University Students
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.3. Procedure
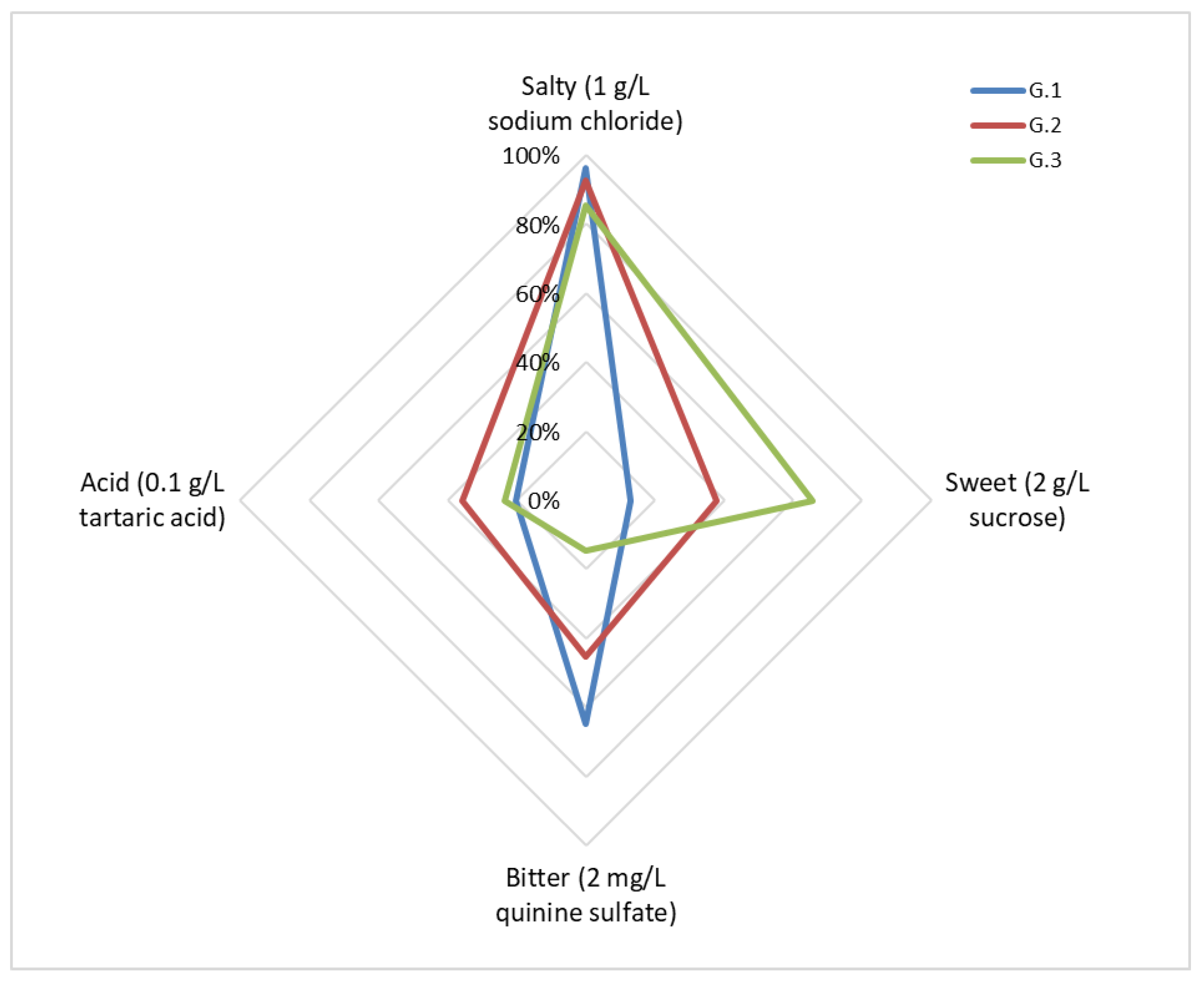
- G.1 (9:30 a.m. *): go to the classroom-laboratory at 9:00 a.m., after breakfast.Upon arriving at the place, participants had already distributed all the necessary material (informed consent, information sheets detection, paper mats to be numbered, glassware, bottles of water for mouth rinsing between tastings, dump bins, etc.) and, in the first half hour, a brief theoretical explanation of the lingual areas with the highest concentration of chemical receptors for each basic flavor was offered.During the experiment in exercise B, it was reported in this group that astringency was included in their tasting along with the four basic flavors.
- G.2 (4:00 p.m. *): go to the classroom-laboratory at 3:30 p.m., after lunch.Upon arriving at the place, participants had already distributed all the necessary material, and in the first half hour, they were offered an identical theoretical explanation as that given to G.1.During the experiment in exercise B, it was reported in this group that, along with the basic flavors, a “surprise” taste was included to identify among the five solutions.
- G.3 (12:00 p.m. *): do not consume any food or drink, except water, from 9:00 a.m. and go to the classroom-laboratory at 11:00 a.m.In the hour prior to the experiment, from 11 a.m. to 12 p.m., the necessary material was distributed, and they were offered an identical theoretical explanation as that given to G.1.During the experiment in exercise B, it was reported in this group that astringency was included for their tasting along with the four basic flavors.(*): Start time of the experiment.
2.4. Statistical Analyses
3. Results and Discussion
3.1. Detection Threshold (DT) for the Four Basic Tastes
3.2. Detection Thresholds (DT) in Conditions of Hunger and Food Satiety
3.3. Astringency Detection and Its Prior Knowledge
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pires, M.A.; Pastrana, L.M.; Fuciños, P.; Abreu, C.S.; Oliveira, S.M. Sensorial Perception of Astringency: Oral Mechanisms and Current Analysis Methods. Foods 2020, 9, 1124. [Google Scholar] [CrossRef] [PubMed]
- Bajec, M.; Pickering, G. Astringency: Mechanisms and perception. Crit. Rev. Food Sci. Nutr. 2008, 48, 858–875. [Google Scholar] [CrossRef]
- Boehm, M.; Yakubov, G.; Stokes, J.; Baier, S. The role of saliva in oral processing: Reconsidering the breakdown path paradigm. J. Texture Stud. 2020, 51, 67–77. [Google Scholar] [CrossRef] [Green Version]
- García-Estévez, I.; Ramos-Pineda, A.M.; Escribano-Bailón, M.T. Interactions between wine phenolic compounds and human saliva in astringency perception. Food Funct. 2018, 9, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- González-Royo, E.; Esteruelas, M.; Kontoudakis, N.; Fort, F.; Canals, J.M.; Zamora, F. The effect of supplementation with three commercial inactive dry yeasts on the colour, phenolic compounds, polysaccharides and astringency of a model wine solution and red wine. J. Sci. Food Agric. 2017, 97, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, N.N.; Matsunami, H. Astringency: A more stringent definition. Chem. Senses 2014, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Sun, B. Grape and wine polymeric polyphenols: Their importance in enology. Crit. Rev. Food Sci. Nutr. 2019, 59, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Okamoto, M.; Wada, Y.; Yamaguchi, Y.; Kimura, A.; Dan, H.; Masuda, T.; Singh, A.K.; Clowney, L.; Dan, I. Influences of Food-Name Labels on Perceived Tastes. Chem. Senses 2009, 34, 187–194. [Google Scholar] [CrossRef]
- Liang, P.; Jiang, J.; Ding, Q.; Tang, X.; Roy, S. Memory Load Influences Taste Sensitivities. Front. Psychol. 2018, 9, 2533. [Google Scholar] [CrossRef] [Green Version]
- Breslin, P.A.S. The Sense of Taste Encompasses Two Roles: Conscious Taste Perception and Subconscious Metabolic Responses. In Think Tank: Forty Neuroscientists Explore the Biological Roots of Human Experience; Linden, D.J., Ed.; Yale University Press: New Haven, CT, USA, 2018; pp. 110–118. ISBN 9780300235470. [Google Scholar]
- International Organisation of Vine and Wine: OIV. Review Document on Sensory Analysis of Wine. 2015, pp. 1–13. Available online: https://www.oiv.int/public/medias/3307/review-on-sensory-analysis-of-wine.pdf (accessed on 16 November 2021).
- International Organization for Standardization (ISO). ISO 3972. Sensory Analysis—Methodology—Method of Investigating Sensitivity of Taste, 2nd ed.; ISO: Geneva, Switzerland, 1991. [Google Scholar]
- Ato, M. Tipología de los diseños cuasiexperimentales. In Métodos de Investigación en Psicología; Anguera, M., Arnau, J., Ato, M., Martínez-Arias, R., Pascual, J., Vallejo, G., Eds.; Síntesis: Madrid, Spain, 1995; pp. 245–270. [Google Scholar]
- Ato, M.; López, J.J.; Benavente, A. Un sistema de clasificación de los diseños de investigación en psicología. An. Psicol. 2013, 29, 1038–1059. [Google Scholar] [CrossRef] [Green Version]
- IBM SPSS Statistics for MAC 2018. Document information for Software version: 20.0. Available online: https://www.ibm.com/support/pages/ibm-spss-statistics-200-release-notes (accessed on 16 November 2021).
- Mennella, J.A.; Beauchamp, G.K. Understanding the Origin of Flavor Preferences. Chem. Senses 2005, 30, i242–i243. [Google Scholar] [CrossRef]
- Mennella, J.; Pepino, N.; Reed, D. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 2005, 115. [Google Scholar] [CrossRef] [Green Version]
- Yeomans, M.R. Flavour–nutrient learning in humans: An elusive phenomenon? Physiol. Behav. 2012, 106, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louro, T.; Simões, C.; Castelo, P.M.; Capela e Silva, F.; Luis, H.; Moreira, P.; Lamy, E. How Individual Variations in the Perception of Basic Tastes and Astringency Relate with Dietary Intake and Preferences for Fruits and Vegetables. Foods 2021, 10, 1961. [Google Scholar] [CrossRef]
- Reed, D.; Knaapila, A. Genetics of taste and smell: Poisons and pleasures. Prog. Mol. Biol. Transl. Sci. 2010, 94, 213–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imoscopi, A.; Inelmen, E.M.; Sergi, G.; Miotto, F.; Manzato, E. Taste loss in the elderly: Epidemiology, causes and consequences. Aging Clin. Exp. Res. 2012, 24, 570–579. [Google Scholar] [CrossRef]
- Mojet, J.; Christ-Hazelhof, E.; Heidema, J. Taste perception with age: Generic or specific losses in threshold sensitivity to the five basic tastes? Chem. Senses 2001, 26, 845–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.; Kim, Y.; Min, S.; Song, M.; Son, S.; Lee, S. Taste Sensitivity of Elderly People Is Associated with Quality of Life and Inadequate Dietary Intake. Nutrients 2021, 13, 1693. [Google Scholar] [CrossRef]
- Martínez-García, C.; Martínez García, T.; Merchán Clavellino, A.; Salguero Alcañiz, M.; Parra Martínez, C.; Alameda Bailén, J. Cognitive implications in the psychophysical estimation of taste. Nutr. Hosp. 2018, 35, 1424–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, L.; Møller, P.; Flint, A.; Martens, M.; Raben, A. Effect of sensory perception of foods on appetite and food intake: A review of studies on humans. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1152–1166. [Google Scholar] [CrossRef] [Green Version]
- Hanci, D.; Altun, H. Hunger state affects both olfactory abilities and gustatory sensitivity. Eur. Arch. Otorhinolaryngol. 2016, 273, 1637–1641. [Google Scholar] [CrossRef]
- Haase, L.; Cerf-Ducastel, B.; Murphy, C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage 2009, 44, 1008–1021. [Google Scholar] [CrossRef] [Green Version]
- Zverev, Y.P. Effects of caloric deprivation and satiety on sensitivity of the gustatory system. BMC Neurosci. 2004, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Haase, L.; Green, E.; Murphy, C. Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite 2011, 57, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Silverthorn, D.U. Fisiología Humana: Un Enfoque Integrado, 8th ed.; Médica Panamericana: Buenos Aires, Argentina, 2009; ISBN 9786078546220. [Google Scholar]
- Prescott, J. Chemosensory learning and flavour: Perception, preference and intake. Physiol. Behav. 2012, 107, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Croijmans, I.; Speed, L.J.; Arshamian, A.; Majid, A. Expertise Shapes Multimodal Imagery for Wine. Cogn. Sci. 2020, 44, e12842. [Google Scholar] [CrossRef] [PubMed]

| Exercise A | Exercise B | Exercise C |
|---|---|---|
| (1). 2 g/L sodium chloride | (6). 0.75 g/L tartaric acid | (11). 0.1 g/L tartaric acid |
| (2). 1 g/L tartaric acid (acidic) | (7). 10 g/L sucrose | (12). 2 g/L sucrose |
| (3). 10 mg/L quinine sulphate (bitter) | (8). 5 mg/L quinine sulphate | (13). 5 g/L sucrose |
| (4). 20 g/L sucrose (sweet) | (9). 1 g/L sodium chloride | (14). 0.5 g/L tartaric acid |
| (5). 4 g/L sodium chloride (salty) | (10). 1 g/L tannic acid (astringent) | (15). 2 mg/L quinine sulphate |
| Student Group | ||||||
|---|---|---|---|---|---|---|
| Taste | Solution | Group 1 | Group 2 | Group 3 | Total | |
| Sweet threshold | 2 g/L sucrose | Head count | 7 | 20 | 36 | 63 |
| Within-group comparison (%) 1 | 13.0 | 37.7 | 65.5 | 38.9 | ||
| Between-group comparison (%) 2 | 11.1 | 31.7 | 57.1 | 100.0 | ||
| 5 g/L sucrose | Head count | 41 | 31 | 18 | 90 | |
| Within-group comparison (%) 1 | 75.9 | 58.5 | 32.7 | 55.6 | ||
| Between-group comparison (%) 2 | 45.6 | 34.4 | 20.0 | 100.0 | ||
| 10 g/L sucrose | Head count | 6 | 2 | 1 | 9 | |
| Within-group comparison (%) 1 | 11.1 | 3.8 | 1.8 | 5.6 | ||
| Between-group comparison (%) 2 | 66.7 | 22.2 | 11.1 | 100.0 | ||
| 20 g/L sucrose 3 | Head count | - | - | - | 162 | |
| Salty threshold | 1 g/L NaCl | Head count | 52 | 49 | 47 | 148 |
| Within-group comparison (%) 1 | 96.3 | 92.5 | 85.5 | 91.4 | ||
| Between-group comparison (%) 2 | 35.1 | 33.1 | 31.8 | 100.0 | ||
| 2 g/L NaCl | Head count | 1 | 3 | 4 | 8 | |
| Within-group comparison (%) 1 | 1.9 | 5.7 | 7.3 | 4.9 | ||
| Between-group comparison (%) 2 | 12.5 | 37.5 | 50.0 | 100.0 | ||
| 4 g/L NaCl | Head count | 1 | 0 | 4 | 5 | |
| Within-group comparison (%) 1 | 1.9 | 0.0 | 7.3 | 3.1 | ||
| Between-group comparison (%) 2 | 20.0 | 0.0 | 80.0 | 100.0 | ||
| Not detection | Head count | 0 | 1 | 0 | 1 | |
| Within-group comparison (%) 1 | 0.0 | 1.9 | 0.0 | 0.6 | ||
| Between-group comparison (%) 2 | 0.0 | 100.0 | 0.0 | 100.0 | ||
| Bitter threshold | 2 mg/L quinine sulphate | Head count | 35 | 24 | 8 | 67 |
| Within-group comparison (%) 1 | 64.8 | 45.3 | 14.5 | 41.4 | ||
| Between-group comparison (%) 2 | 52.2 | 35.8 | 11.9 | 100.0 | ||
| 5 mg/L quinine sulphate | Head count | 14 | 14 | 24 | 52 | |
| Within-group comparison (%) 1 | 25.9 | 26.4 | 43.6 | 32.1 | ||
| Between-group comparison (%) 2 | 26.9 | 26.9 | 46.2 | 100.0 | ||
| 10 mg/L quinine sulphate | Head count | 5 | 11 | 16 | 32 | |
| Within-group comparison (%) 1 | 9.3 | 20.8 | 29.1 | 19.8 | ||
| Between-group comparison (%) 2 | 15.6 | 34.4 | 50.0 | 100.0 | ||
| Not detection | Head count | 0 | 4 | 7 | 11 | |
| Within-group comparison (%) 1 | 0.0 | 7.5 | 12.7 | 6.8 | ||
| Between-group comparison (%) 2 | 0.0 | 36.4 | 63.6 | 100.0 | ||
| Acid threshold | 0.1 g/L tartaric acid | Head count | 11 | 19 | 13 | 43 |
| Within-group comparison (%) 1 | 20.4 | 35.8 | 23.6 | 26.5 | ||
| Between-group comparison (%) 2 | 25.6 | 44.2 | 30.2 | 100.0 | ||
| 0.5 g/L tartaric acid | Head count | 26 | 26 | 30 | 82 | |
| Within-group comparison (%) 1 | 48.1 | 49.1 | 54.5 | 50.6 | ||
| Between-group comparison (%) 2 | 31.7 | 31.7 | 36.6 | 100.0 | ||
| 0.75 g/L tartaric acid | Head count | 14 | 8 | 10 | 32 | |
| Within-group comparison (%) 1 | 25.9 | 15.1 | 18.2 | 19.8 | ||
| Between-group comparison (%) 2 | 43.8 | 25.0 | 31.2 | 100.0 | ||
| 1 g/L tartaric acid | Head count | 2 | 0 | 0 | 2 | |
| Within-group comparison (%) 1 | 3.7 | 0.0 | 0.0 | 1.2 | ||
| Between-group comparison (%) 2 | 100.0 | 0.0 | 0.0 | 100.0 | ||
| Not detection | Head count | 1 | 0 | 2 | 3 | |
| Within-group comparison (%) 1 | 1.9 | 0.0 | 3.6 | 1.9 | ||
| Between-group comparison (%) 2 | 33.3 | 0.0 | 66.7 | 100.0 | ||
| Student Group | |||||
|---|---|---|---|---|---|
| Astringency Detection | Group 1 | Group 2 | Group 3 | Total | |
| No detection | Head count | 7 | 0 | 9 | 16 |
| % of student group | 13.0 | 0.0 | 16.4 | 9.9 | |
| % of astringency detection | 43.8 | 0.0 | 56.2 | 100.0 | |
| Astringency detection | Head count | 42 | 11 | 45 | 98 |
| % of student group | 77.8 | 20.8 | 81.8 | 60.5 | |
| % of astringency detection | 42.9 | 11.2 | 45.9 | 100.0 | |
| Named bitter | Head count | 2 | 42 | 0 | 44 |
| % of student group | 3.7 | 79.2 | 0.0 | 27.2 | |
| % of astringency detection | 4.5 | 95.5 | 0.0 | 100.0 | |
| Named acid | Head count | 3 | 0 | 1 | 4 |
| % of student group | 5.6 | 0.0 | 1.8 | 2.5 | |
| % of astringency detection | 75.0 | 0.0 | 25.0 | 100.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sánchez, L.M.; Parra-Martínez, C.; Martínez-García, T.E.; Martínez-García, C. Cognitive Keys in Psychophysical Estimation of Chemosensory Perception in University Students. Foods 2021, 10, 3134. https://doi.org/10.3390/foods10123134
Martínez-Sánchez LM, Parra-Martínez C, Martínez-García TE, Martínez-García C. Cognitive Keys in Psychophysical Estimation of Chemosensory Perception in University Students. Foods. 2021; 10(12):3134. https://doi.org/10.3390/foods10123134
Chicago/Turabian StyleMartínez-Sánchez, Laura María, Cecilio Parra-Martínez, Tomás Eugenio Martínez-García, and Concha Martínez-García. 2021. "Cognitive Keys in Psychophysical Estimation of Chemosensory Perception in University Students" Foods 10, no. 12: 3134. https://doi.org/10.3390/foods10123134
APA StyleMartínez-Sánchez, L. M., Parra-Martínez, C., Martínez-García, T. E., & Martínez-García, C. (2021). Cognitive Keys in Psychophysical Estimation of Chemosensory Perception in University Students. Foods, 10(12), 3134. https://doi.org/10.3390/foods10123134






