Safety Assessment of Vitamin D and Its Photo-Isomers in UV-Irradiated Baker’s Yeast
Abstract
:1. Introduction
1.1. General Background
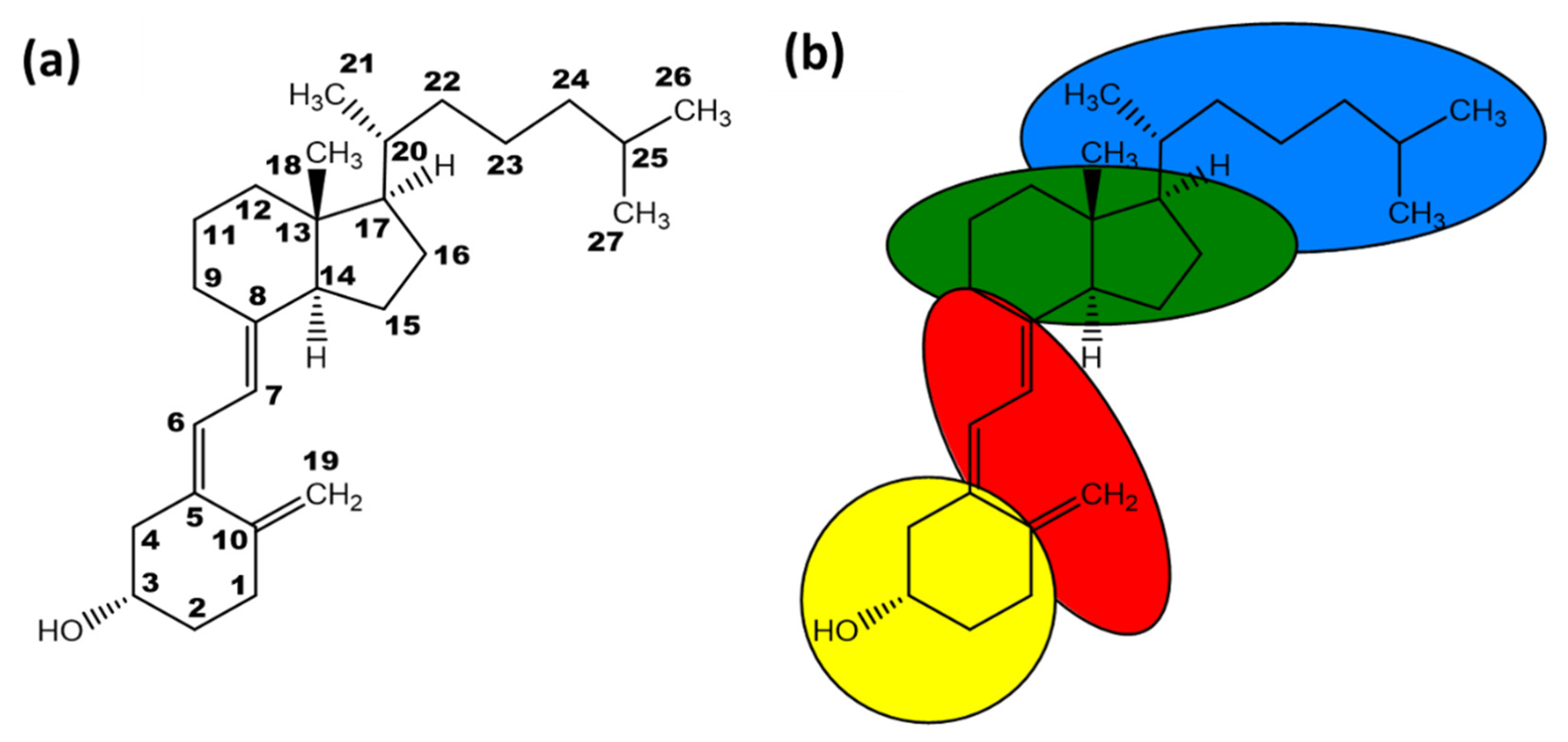
1.2. Vitamin D Synthesis and Activity
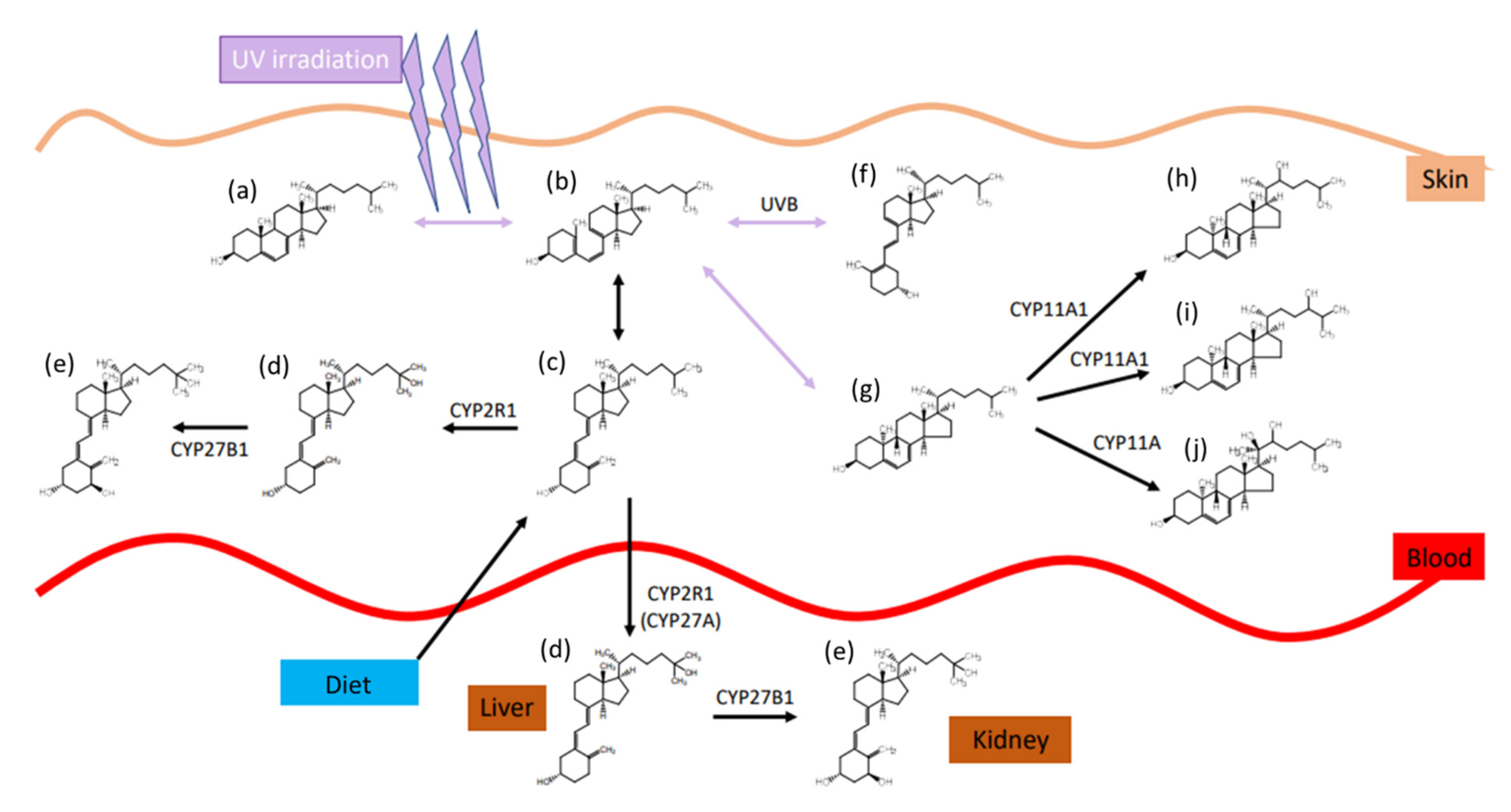
1.3. Vitamin D Transport, Metabolism, and Elimination
1.4. Vitamin D Toxicity
- Through an increased intake of vitamin D, the plasma 1,25(OH)2D concentrations, and consequently the cellular 1,25(OH)2D concentrations increase.
- Vitamin D intake raises plasma 25(OH)D to concentrations that exceed the DBP binding capacity and “free 25(OH)D” enters the cell, where it has direct effects on gene expression by binding to VDR, although it has a lower binding affinity compared with 1,25(OH)2D.
- Vitamin D intake raises the concentrations of many vitamin D metabolites, especially vitamin D itself and 25(OH)D. These compounds at high concentrations compete for the DBP binding capacity and cause the release of free 1,25(OH)2D, which enters the target cells and binds to VDR.
2. Other Isoforms of Vitamin D
3. Products and Site Products in Mushrooms and Yeast after UV Irradiation to Induce Vitamin D2 Formation
3.1. Baker’s Yeast (Saccharomyces cerevisiae)
3.2. Yield of Vitamin D2 after UV Irradiation of Mushrooms and Yeast
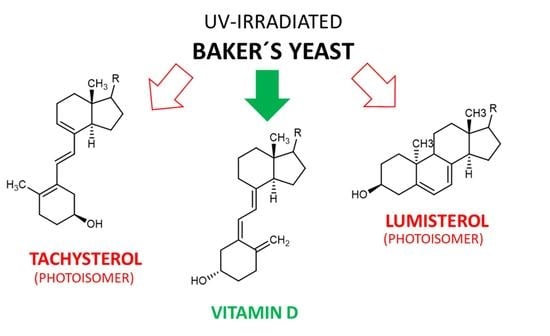
3.3. Tachysterol and Lumisterol as Major Photoproducts Generated by UV Irradiation during Vitamin D Synthesis
3.4. Other Photoproducts Generated by UV Irradiation during Vitamin D Synthesis
4. Bioavailability of Vitamin D2 from Fungal Nutritional Sources
5. Safety Assessment of UV-Irradiated Saccharomyces cerevisiae
5.1. The European Food Safety Authority Assessment of 2014
5.2. Update of the Safety Assessment of UV-Irradiated Baker’s Yeast
6. Conclusions
7. Literature Search Strategy
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maretzke, F.; Bechthold, A.; Egert, S.; Ernst, J.B.; van Melo Lent, D.; Pilz, S.; Reichrath, J.; Stangl, G.I.; Stehle, P.; Volkert, D.; et al. Role of Vitamin D in Preventing and Treating Selected Extraskeletal Diseases-An Umbrella Review. Nutrients 2020, 12, 969. [Google Scholar] [CrossRef] [Green Version]
- Amrein, K. Vitamin-D-Mangel—Aktuelle Diagnostik und Prophylaxe in Fallbeispielen; UNI-MED: Bremen, Germany, 2015; ISBN 9783837414530. [Google Scholar]
- Fraser, D. Vitamin D. Lancet 1995, 345, 104–107. [Google Scholar] [CrossRef]
- Stumpf, W.E. Vitamin D sites and mechanisms of action: A histochemical perspective. Reflections on the utility of autoradiography and cytopharmacology for drug targeting. Histochem. Cell Biol. 1995, 104, 417–427. [Google Scholar] [CrossRef]
- Reichrath, J. (Ed.) Sunlight, Vitamin D and Skin Cancer, 3rd ed.; Springer Nature: Cham, Switzerland, 2014; ISBN 978-3-030-462226-0. [Google Scholar]
- Wolf, G. The discovery of vitamin D: The contribution of Adolf Windaus. J. Nutr. 2004, 134, 1299–1302. [Google Scholar] [CrossRef]
- Windaus, A.; Thiele, W. Über die Konstitution des Vitamins D2. Ann. Chem. 1936, 521, 160–175. [Google Scholar] [CrossRef]
- Holick, M.F.; MacLaughlin, J.A.; Clark, M.B.; Holick, S.A.; Potts, J.T.; Anderson, R.R.; Blank, I.H.; Parrish, J.A.; Elias, P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 1980, 210, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Havinga, E. Vitamin D, example and challenge. Experientia 1973, 29, 1181–1193. [Google Scholar] [CrossRef]
- Abillon, E.; Mermet-Bouvier, R. Effect of wavelength on production of previtamin D2. J. Pharm. Sci. 1973, 62, 1688–1691. [Google Scholar] [CrossRef]
- Chen, T.C.; Chimeh, F.; Lu, Z.; Mathieu, J.; Person, K.S.; Zhang, A.; Kohn, N.; Martinello, S.; Berkowitz, R.; Holick, M.F. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch. Biochem. Biophys. 2007, 460, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Vieth, R. The mechanisms of vitamin D toxicity. Bone Miner. 1990, 11, 267–272. [Google Scholar] [CrossRef]
- Bikle, D. Endotext: Vitamin D: Production, Metabolism, and Mechanisms of Action. In Endotext [Internet]; Bikle, D., Ed.; MDText.com, Inc.: South Dartmouth, MA, USA, 2017. [Google Scholar]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieth, R.; McCarten, K.; Norwich, K.H. Role of 25-hydroxyvitamin D3 dose in determining rat 1,25-dihydroxyvitamin D3 production. Am. J. Physiol. 1990, 258, E780–E789. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Metabolism of vitamin D: Current status. Am. J. Clin. Nutr. 1976, 29, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agoston, E.S.; Hatcher, M.A.; Kensler, T.W.; Posner, G.H. Vitamin D Analogs as Anti-Carcinogenic Agents. Anti-Cancer Agents Med. Chem. 2005, 6, 53–71. [Google Scholar] [CrossRef]
- Vieth, R. Vitamin D toxicity, policy, and science. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V64–V68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Maestro, M.A.; Molnár, F.; Carlberg, C. Vitamin D and Its Synthetic Analogs. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, P.P.; Norman, A.W. 1,25(OH)2-vitamin D3 receptors: Gene regulation and genetic circuitry. FASEB J. 1988, 2, 3043–3053. [Google Scholar] [CrossRef]
- Walters, M.R. Newly identified actions of the vitamin D endocrine system. Endocr. Rev. 1992, 13, 719–764. [Google Scholar] [CrossRef]
- Bronner, F. Mechanisms of intestinal calcium absorption. J. Cell. Biochem. 2003, 88, 387–393. [Google Scholar] [CrossRef]
- Barrett, K.E.; Ganong, W.F. Ganong’s Review of Medical Physiology, 23rd ed.; McGraw-Hill Medical; McGraw-Hill [Distributor]: New York, NY, USA; London, UK, 2010; ISBN 9780071605670. [Google Scholar]
- Wu, S.; Chun, R.; Gacad, M.A.; Ren, S.; Chen, H.; Adams, J.S. Regulation of 1,25-dihydroxyvitamin d synthesis by intracellular vitamin d binding protein-1. Endocrinology 2002, 143, 4135. [Google Scholar] [CrossRef] [Green Version]
- Kiourtzidis, M.; Kühn, J.; Schutkowski, A.; Baur, A.C.; Hirche, F.; Stangl, G.I. Inhibition of Niemann-Pick C1-like protein 1 by ezetimibe reduces uptake of deuterium-labeled vitamin D in mice. J. Steroid Biochem. Mol. Biol. 2020, 197, 105504. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.-F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Hsu, J.J.; Tintut, Y. Steroid Hormone Vitamin D: Implications for Cardiovascular Disease. Circ. Res. 2018, 122, 1576–1585. [Google Scholar] [CrossRef]
- Silva, M.C.; Furlanetto, T.W. Intestinal absorption of vitamin D: A systematic review. Nutr. Rev. 2018, 76, 60–76. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef]
- Shepard, R.M.; DeLuca, H.F. Plasma concentrations of vitamin D3 and its metabolites in the rat as influenced by vitamin D3 or 25-hydroxyvitamin D3 intakes. Arch. Biochem. Biophys. 1980, 202, 43–53. [Google Scholar] [CrossRef]
- Bikle, D.D.; Gee, E. Free, and not total, 1,25-dihydroxyvitamin D regulates 25-hydroxyvitamin D metabolism by keratinocytes. Endocrinology 1989, 124, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Davies, J.S. A review of the growing risk of vitamin D toxicity from inappropriate practice. Br. J. Clin. Pharmacol. 2018, 84, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thacher, T.D.; Clarke, B.L. Vitamin D insufficiency. Mayo Clin. Proc. 2011, 86, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity—A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trang, H.M.; Cole, D.E.; Rubin, L.A.; Pierratos, A.; Siu, S.; Vieth, R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am. J. Clin. Nutr. 1998, 68, 854–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Biancuzzo, R.M.; Chen, T.C.; Klein, E.K.; Young, A.; Bibuld, D.; Reitz, R.; Salameh, W.; Ameri, A.; Tannenbaum, A.D. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J. Clin. Endocrinol. Metab. 2008, 93, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.W.; Peiris, A.N. The lack of vitamin D toxicity with megadose of daily ergocalciferol (D2) therapy: A case report and literature review. South Med. J. 2009, 102, 765–768. [Google Scholar] [CrossRef]
- Lehmann, U.; Hirche, F.; Stangl, G.I.; Hinz, K.; Westphal, S.; Dierkes, J. Bioavailability of vitamin D2 and D3 in healthy volunteers, a randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 4339–4345. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Vitamin D4 in mushrooms. PLoS ONE 2012, 7, e40702. [Google Scholar] [CrossRef]
- Urbain, P.; Valverde, J.; Jakobsen, J. Impact on Vitamin D2, Vitamin D4 and Agaritine in Agaricus bisporus Mushrooms after Artificial and Natural Solar UV Light Exposure. Plant Foods Hum. Nutr. 2016, 71, 314–321. [Google Scholar] [CrossRef]
- Wittig, M.; Krings, U.; Berger, R.G. Single-run analysis of vitamin D photoproducts in oyster mushroom (Pleurotus ostreatus) after UV-B treatment. J. Food Compos. Anal. 2013, 31, 266–274. [Google Scholar] [CrossRef]
- DeLuca, H.F.; Weller, M.; Blunt, J.W.; Neville, P.F. Synthesis, biological activity, and metabolism of 22, 23−3H-vitamin D4. Arch. Biochem. Biophys. 1968, 124, 122–128. [Google Scholar] [CrossRef]
- Napoli, J.L.; Fivizzani, M.A.; Schnoes, H.K.; Deluca, H.F. Synthesis of vitamin D5: Its biological activity relative to vitamins D3 and D2. Arch. Biochem. Biophys. 1979, 197, 119–125. [Google Scholar] [CrossRef]
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evolution 2012, 66, 2961–2968. [Google Scholar] [CrossRef]
- Shobayashi, M.; Mitsueda, S.; Ago, M.; Fujii, T.; Iwashita, K.; Iefuji, H. Effects of culture conditions on ergosterol biosynthesis by Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2005, 69, 2381–2388. [Google Scholar] [CrossRef] [Green Version]
- Mattila, P.H.; Piironen, V.I.; Uusi-Rauva, E.J.; Koivistoinen, P.E. Vitamin D Contents in Edible Mushrooms. J. Agric. Food Chem. 1994, 42, 2449–2453. [Google Scholar] [CrossRef]
- Kiribuchi, T. Contents of Vitamin D2 in Fungi Irradiated by Ultraviolet Light under Various Conditions and Changes in the Contents during Storage after Irradiation. J. Home Econ. Jpn. 1992, 43, 649–654. [Google Scholar] [CrossRef]
- Mau, J.-L.; Chen, P.-R.; Yang, J.-H. Ultraviolet Irradiation Increased Vitamin D2 Content in Edible Mushrooms. J. Agric. Food Chem. 1998, 46, 5269–5272. [Google Scholar] [CrossRef]
- Göring, H. Vitamin D in Nature: A Product of Synthesis and/or Degradation of Cell Membrane Components. Biochem. Mosc. 2018, 83, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Williams, R. Increasing Vitamin D Content of Mushrooms with UV Light. U.S. Patent 12/716,936, 3 March 2010. [Google Scholar]
- Takamura, K.; Hoshino, H.; Toyomasu, T.; Tanaka, T.; Nakazawa, T. Treatment of Mushroom. Patent JP10334869A, 26 November 1998. [Google Scholar]
- Kidder, J.W.; Romig, W.R.; Lobato, A.; Lodder, S.C. Ready-to-Use Mushrooms with Enhanced Vitamin D Content and Improved Shelf Life. U.S. Patent 12/437,394, 7 May 2009. [Google Scholar]
- Mattila, P.; Lampi, A.-M.; Ronkainen, R.; Toivo, J.; Piironen, V. Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chem. 2002, 76, 293–298. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, J.; Yan, R.; Shao, S.; Zhou, T.; Lei, J.; Wang, Z. Effects of UV-C treatment and cold storage on ergosterol and vitamin D2 contents in different parts of white and brown mushroom (Agaricus bisporus). Food Chem. 2016, 210, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Krings, U.; Berger, R.G. Dynamics of sterols and fatty acids during UV-B treatment of oyster mushroom. Food Chem. 2014, 149, 10–14. [Google Scholar] [CrossRef]
- Hu, D.; Chen, W.; Li, X.; Yue, T.; Zhang, Z.; Feng, Z.; Li, C.; Bu, X.; Li, Q.X.; Hu, C.Y.; et al. Ultraviolet Irradiation Increased the Concentration of Vitamin D2 and Decreased the Concentration of Ergosterol in Shiitake Mushroom (Lentinus edodes) and Oyster Mushroom (Pleurotus ostreatus) Powder in Ethanol Suspension. ACS Omega 2020, 5, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jägerstad, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. LWT—Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Ko, J.A.; Lee, B.H.; Lee, J.S.; Park, H.J. Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J. Agric. Food Chem. 2008, 56, 3671–3674. [Google Scholar] [CrossRef]
- Kristensen, H.L.; Rosenqvist, E.; Jakobsen, J. Increase of vitamin D2 by UV-B exposure during the growth phase of white button mushroom (Agaricus bisporus). Food Nutr. Res. 2012, 56, 7114. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.S.; Teichert, A.; McHugh, T.H. Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J. Agric. Food Chem. 2008, 56, 4541–4544. [Google Scholar] [CrossRef]
- Szabó, A. The Effect of UV Radiation on the Vitamin D Level, Bioactive Matter Content and Organileptic Properties of Cultivated Button and Oyster Mushrooms. Ph.D. Thesis, Corvinus University of Budapesti, Budapest, Hungary, 2015. [Google Scholar]
- Simon, R.R.; Phillips, K.M.; Horst, R.L.; Munro, I.C. Vitamin D mushrooms: Comparison of the composition of button mushrooms (Agaricus bisporus) treated postharvest with UVB light or sunlight. J. Agric. Food Chem. 2011, 59, 8724–8732. [Google Scholar] [CrossRef]
- Koyyalamudi, S.R.; Jeong, S.-C.; Song, C.-H.; Cho, K.Y.; Pang, G. Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. J. Agric. Food Chem. 2009, 57, 3351–3355. [Google Scholar] [CrossRef]
- Urbain, P.; Singler, F.; Ihorst, G.; Biesalski, H.-K.; Bertz, H. Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: A randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Jasinghe, V.J.; Perera, C.O.; Barlow, P.J. Vitamin D2 from irradiated mushrooms significantly increases femur bone mineral density in rats. J. Toxicol. Environ. Health A 2006, 69, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Kalaras, M.D.; Beelman, R.B.; Holick, M.F.; Elias, R.J. Generation of potentially bioactive ergosterol-derived products following pulsed ultraviolet light exposure of mushrooms (Agaricus bisporus). Food Chem. 2012, 135, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Aan, B.-Y. Optimization of ergosterol to vitamin D2 synthesis in Agaricus bisporus powder using ultraviolet-B radiation. Food Sci. Biotechnol. 2016, 25, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Jasinghe, V.J.; Perera, C.O.; Sablani, S.S. Kinetics of the conversion of ergosterol in edible mushrooms. J. Food Eng. 2007, 79, 864–869. [Google Scholar] [CrossRef]
- Chen, J.; Slominski, A.T.; Miller, D.D.; Li, W. Effects of sidechain length and composition on the kinetic conversion and product distribution of vitamin D analogs determined by real-time NMR. Dermato-Endocrinol. 2013, 5, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Havinga, E.; de Kock, R.J.; Rappoldt, M.P. The photochemical interconversions of provitamin D, lumisterol, previtamin D and tachysterol. Tetrahedron 1960, 11, 276–284. [Google Scholar] [CrossRef]
- Mermet-Bouvier, R.; Abillon, E. Ergosterol photoisomerization reaction scheme. J. Pharm. Sci. 1973, 62, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, C.; Thompson, T.; Baluyot, N.; Smith, A.C.; Tapavicza, E. The role of tachysterol in vitamin D photosynthesis—A non-adiabatic molecular dynamics study. Phys. Chem. Chem. Phys. 2017, 19, 5763–5777. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; MacLaughlin, J.A.; Doppelt, S.H. Regulation of cutaneous previtamin D3 photosynthesis in man: Skin pigment is not an essential regulator. Science 1981, 211, 590–593. [Google Scholar] [CrossRef]
- Kotwan, J.; Kühn, J.; Baur, A.C.; Stangl, G.I. Oral Intake of Lumisterol Affects the Metabolism of Vitamin D. Mol. Nutr. Food Res. 2021, 65, e2001165. [Google Scholar] [CrossRef]
- Braun, M.; Fuß, W.; Kompa, K.L.; Wolfrum, J. Improved photosynthesis of previtamin D by wavelengths of 280–300 nm. J. Photochem. Photobiol. A Chem. 1991, 61, 15–26. [Google Scholar] [CrossRef]
- Boomsma, F.; Jacobs, H.J.C.; Havinga, E.; van der Gen, A. The “overirradiation products” of previtamin D and tachysterol: Toxisterols. Recl. Trav. Chim. Pays-Bas 1977, 96, 104–112. [Google Scholar] [CrossRef]
- Jacobs, H.J.C.; Boomsma, F.; Havinga, E.; van der Gen, A. The photochemistry of previtamin D and tachysterol. Recl. Trav. Chim. Pays-Bas 1977, 96, 113–117. [Google Scholar] [CrossRef]
- Mattila, P.; Ronkainen, R.; Lehikoinen, K.; Piironen, V. Effect of Household Cooking on the Vitamin D content in Fish, Eggs, and Wild Mushrooms. J. Food Compos. Anal. 1999, 12, 153–160. [Google Scholar] [CrossRef]
- Simon, R.R.; Borzelleca, J.F.; DeLuca, H.F.; Weaver, C.M. Safety assessment of the post-harvest treatment of button mushrooms (Agaricus bisporus) using ultraviolet light. Food Chem. Toxicol. 2013, 56, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Outila, T.A.; Mattila, P.H.; Piironen, V.I.; Lamberg-Allardt, C.J. Bioavailability of vitamin D from wild edible mushrooms (Cantharellus tubaeformis) as measured with a human bioassay. Am. J. Clin. Nutr. 1999, 69, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, A.; Calvo, M.S.; Beelman, R.B.; Levy, E.; Siuty, J.; Kalaras, M.D.; Uribarri, J. Bioavailability of vitamin D2 from enriched mushrooms in prediabetic adults: A randomized controlled trial. Eur. J. Clin. Nutr. 2014, 68, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; O’Mahony, L.; O’Sullivan, A.; Collier, J.; Fraser, W.D.; Gibney, M.J.; Nugent, A.P.; Brennan, L. Effect of supplementation with vitamin D2-enhanced mushrooms on vitamin D status in healthy adults. J. Nutr. Sci. 2013, 2, e29. [Google Scholar] [CrossRef] [Green Version]
- Stephensen, C.B.; Zerofsky, M.; Burnett, D.J.; Lin, Y.-P.; Hammock, B.D.; Hall, L.M.; McHugh, T. Ergocalciferol from mushrooms or supplements consumed with a standard meal increases 25-hydroxyergocalciferol but decreases 25-hydroxycholecalciferol in the serum of healthy adults. J. Nutr. 2012, 142, 1246–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasinghe, V.J.; Perera, C.O.; Barlow, P.J. Bioavailability of vitamin D2 from irradiated mushrooms: An in vivo study. Br. J. Nutr. 2005, 93, 951–955. [Google Scholar] [CrossRef] [Green Version]
- Hohman, E.E.; Martin, B.R.; Lachcik, P.J.; Gordon, D.T.; Fleet, J.C.; Weaver, C.M. Bioavailability and efficacy of vitamin D2 from UV-irradiated yeast in growing, vitamin D-deficient rats. J. Agric. Food Chem. 2011, 59, 2341–2346. [Google Scholar] [CrossRef] [Green Version]
- Fleet, J.C.; Gliniak, C.; Zhang, Z.; Xue, Y.; Smith, K.B.; McCreedy, R.; Adedokun, S.A. Serum Metabolite Profiles and Target Tissue Gene Expression Define the Effect of Cholecalciferol Intake on Calcium Metabolism in Rats and Mice. J. Nutr. 2008, 138, 1114–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itkonen, S.T.; Skaffari, E.; Saaristo, P.; Saarnio, E.M.; Erkkola, M.; Jakobsen, J.; Cashman, K.D.; Lamberg-Allardt, C. Effects of vitamin D2-fortified bread v. supplementation with vitamin D2 or D3 on serum 25-hydroxyvitamin D metabolites: An 8-week randomised-controlled trial in young adult Finnish women. Br. J. Nutr. 2016, 115, 1232–1239. [Google Scholar] [CrossRef] [Green Version]
- Calvo, M.S.; Babu, U.S.; Garthoff, L.H.; Woods, T.O.; Dreher, M.; Hill, G.; Nagaraja, S. Vitamin D2 from light-exposed edible mushrooms is safe, bioavailable and effectively supports bone growth in rats. Osteoporos. Int. 2013, 24, 197–207. [Google Scholar] [CrossRef]
- Delaney, M.A.; Treuting, P.M.; Rothenburger, J.L. Rodentia. Pathol. Wildl. Zoo Anim. 2018, 499–515. [Google Scholar] [CrossRef]
- Lipkie, T.E.; Ferruzzi, M.G.; Weaver, C.M. Low bioaccessibility of vitamin D2 from yeast-fortified bread compared to crystalline D2 bread and D3 from fluid milks. Food Funct. 2016, 7, 4589–4596. [Google Scholar] [CrossRef]
- Itkonen, S.T.; Pajula, E.T.; Dowling, K.G.; Hull, G.L.; Cashman, K.D.; Lamberg-Allardt, C.J. Poor bioavailability of vitamin D2 from ultraviolet-irradiated D2-rich yeast in rats. Nutr. Res. 2018, 59, 36–43. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, H. Bioavailability of vitamin D. Eur. J. Clin. Nutr. 1997, 51 (Suppl. 1), S76–S79. [Google Scholar]
- European Food Safety Authority. Tolerable Upper Intake Levels for Vitamins and Minerals; European Food Safety Authority: Parma, Italy, 2006; ISBN 92-9199-014-0.
- European Food Safety Authority. Scientific Opinion on the safety of vitamin D-enriched UV-treated baker’s yeast. EFSA J. 2014, 12, 3520. [Google Scholar] [CrossRef] [Green Version]
- FoodEx2: Level 2. Available online: https://www.efsa.europa.eu/en/microstrategy/foodex2-level-2 (accessed on 4 June 2021).
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the Tolerable Upper Intake Level of vitamin D. EFSA J. 2012, 10, 2813. [Google Scholar]
- Food and Drug Administration. Food Additives Permitted for Direct Addition to Food for Human Consumption; Vitamin D2. Fed. Regist. 2012, 77. [Google Scholar]
- Health Canada Department of Health, Food and Drugs Regulation. Canada Gazette Part I (Ottawa ON K1A 0S5); Health Canada Department of Health, Food and Drugs Regulation: Ottawa, ON, Canada, 2011; pp. 439–440.
- Wittsiepe, J.; Fürst, P.; Wilhelm, M. The 2005 World Health Organization re-evaluation of TEFs for dioxins and dioxin-like compounds—What are the consequences for German human background levels? Int. J. Hyg. Environ. Health 2007, 210, 335–339. [Google Scholar] [CrossRef]
- Chen, T.C.; Persons, K.S.; Lu, Z.; Mathieu, J.S.; Holick, M.F. An evaluation of the biologic activity and vitamin D receptor binding affinity of the photoisomers of vitamin D3 and previtamin D3. J. Nutr. Biochem. 2000, 11, 267–272. [Google Scholar] [CrossRef]
- Verlinden, L.; Verstuyf, A.; Verboven, C.; Eelen, G.; de Ranter, C.; Gao, L.-J.; Chen, Y.-J.; Murad, I.; Choi, M.; Yamamoto, K.; et al. Previtamin D3 with a trans-fused decalin CD-ring has pronounced genomic activity. J. Biol. Chem. 2003, 278, 35476–35482. [Google Scholar] [CrossRef] [Green Version]
- Bouillon, R.; Sarandeses, L.A.; Allewaert, K.; Zhao, J.; Mascareñas, J.L.; Mouriño, A.; Vrielynck, S.; de Clercq, P.; Vandewalle, M. Biologic activity of dihydroxylated 19-nor-(pre)vitamin D3. J. Bone Miner. Res. 1993, 8, 1009–1015. [Google Scholar] [CrossRef]
- Tian, X.Q.; Chen, T.C.; Matsuoka, L.Y.; Wortsman, J.; Holick, M.F. Kinetic and thermodynamic studies of the conversion of previtamin D3 to vitamin D3 in human skin. J. Biol. Chem. 1993, 268, 14888–14892. [Google Scholar] [CrossRef]
- Tian, X.Q.; Holick, M.F. A liposomal model that mimics the cutaneous production of vitamin D3. Studies of the mechanism of the membrane-enhanced thermal isomerization of previtamin D3 to vitamin D3. J. Biol. Chem. 1999, 274, 4174–4179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steuerle, H. Bestimmung von vitamin D3 und seinen fotoisomeren in D3-harzen sowie in mischungen mit vitamin A-acetat. J. Chromatogr. A 1975, 115, 447–453. [Google Scholar] [CrossRef]
- Mahmoodani, F.; Perera, C.O.; Fedrizzi, B.; Abernethy, G.; Chen, H. Degradation studies of cholecalciferol (vitamin D3) using HPLC-DAD, UHPLC-MS/MS and chemical derivatization. Food Chem. 2017, 219, 373–381. [Google Scholar] [CrossRef]
- Houghton, L.A.; Vieth, R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am. J. Clin. Nutr. 2006, 84, 694–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavelli, V.; D’Incecco, P.; Pellegrino, L. Vitamin D Incorporation in Foods: Formulation Strategies, Stability, and Bioaccessibility as Affected by the Food Matrix. Foods 2021, 10, 1989. [Google Scholar] [CrossRef] [PubMed]







| Category | nmol/L | µg/L |
|---|---|---|
| Deficiency | <30 | <12 |
| Insufficiency | 30–50 | 12–20 |
| Sufficient | >50 | >20 |
| Excess | >250 | >100 |
| Intoxication | >375 | >150 |
| Mushroom | Latin Name | Form | UV Wavelength | Irradiation Dose, J/cm2 | Yield, µg/g Dryweight | Time of Exposure, min | Source |
|---|---|---|---|---|---|---|---|
| Shiitake | Lentinus edodes | powder in ethanol | UV-C | 24 | 629 | 120 | [59] |
| Oyster | Pleurotus ostreatus | powder in ethanol | UV-C | 24 | 275 | 120 | [59] |
| Funnel chanterelle | Cantharellus tubaeformis | lyophilized | 254 nm | 379 | 14 | 120 | [60] |
| White Button Mushroom | Agaricus bisporus/white | fresh | 254 nm | 379 | 10 | 120 | [60] |
| White Button Mushroom | Agaricus bisporus/white | whole pileus | UV-B | 2 | 13.03 | not provided | [61] |
| White Button Mushroom | Agaricus bisporus/white | whole gill | UV-B | 2 | 16.7 | not provided | [61] |
| Shiitake | Lentinus edodes | pileus layer | UV-B | 2.5 | 37 | not provided | [61] |
| Shiitake | Lentinus edodes | middle layer | UV-B | 2.5 | 69 | not provided | [61] |
| Shiitake | Lentinus edodes | gill layer | UV-B | 2.5 | 106 | not provided | [61] |
| White Button Mushroom | Agaricus bisporus/white | fresh, growing | UV-B | 0.25 | 1.64 | 4320 | [62] |
| White Button Mushroom | Agaricus bisporus/white | fresh | 305 nm | 1.5 | 7.43 | 25 | [63] |
| White Button Mushroom | Agaricus bisporus/white | fresh, growing | UV-B | 3.24 | 0.73 | 3 × 60 | [64] |
| Brown Button Mushroom | Agaricus bisporus/brown | fresh, growing | UV-B | 3.24 | 0.88 | 3 × 45 | [64] |
| Oyster | Pleurotus ostreatus | fresh, growing | UV-B | 3.24 | 2.28 | 3 × 90 | [64] |
| White Button Mushroom | Agaricus bisporus/white | fresh | UV-B | 1.08 | 4.1 | not provided | [65] |
| White Button Mushroom | Agaricus bisporus/white | fresh | UV-C | 0.25 | 23 | 60 | [66] |
| White Button Mushroom | Agaricus bisporus/white | sliced, dried | UV-B | 0.53 | 67.1 | not provided | [67] |
| Shiitake | Lentinus edodes | fresh | UV-B | 3.53 | 6.05 | 120 | [68] |
| Oyster | Pleurotus ostreatus | fresh | UV-B | 3.53 | 4.4 | 120 | [68] |
| White Button Mushroom | Agaricus bisporus/white | fresh | UV-B | 3.53 | 7.8 | 120 | [68] |
| Enoki | Flamulina velutipes | fresh | UV-B | 3.53 | 0.68 | 120 | [68] |
| Woddy mushroom | Auricularia auricula | fresh | UV-B | 3.53 | 0.68 | 120 | [68] |
| White Ear mushroom | Tremella fuciformis | fresh | UV-B | 3.53 | 0.32 | 120 | [69] |
| Portabella mushroom | Agaricus bisporus/white | fresh | UV-B | 3.53 | 6.76 | 120 | [68] |
| brown beech mushrom | Hypsizugus tessulatus | fresh | UV-B | 3.53 | 3.07 | 120 | [68] |
| Abalone mushroom | Pleurotus ostreatus | fresh | UV-B | 3.53 | 4.35 | 120 | [68] |
| White Button Mushroom | Agaricus bisporus/white | cap | UV-C | 0.2 | 1340 | 3 | [57] |
| White Button Mushroom | Agaricus bisporus/white | stem | UV-C | 0.2 | 1170 | 3 | [57] |
| Brown Button Mushroom | Agaricus bisporus/brown | cap | UV-C | 0.2 | 950 | 3 | [57] |
| Brown Button Mushroom | Agaricus bisporus/brown | stem | UV-C | 0.2 | 1050 | 3 | [57] |
| White Button Mushroom | Agaricus bisporus/white | fresh | UV-B | 1.47 | 12.48 | 120 | [51] |
| Agaricus bitorquis | fresh | UV-C | 1.47 | 5.32 | 120 | [51] | |
| Shiitake | Lentinus edodes | fresh | UV-B | 1.47 | 6.58 | 120 | [51] |
| Straw mushrooms | Volvariella volvacea | fresh | UV-B | 1.47 | 7.58 | 120 | [51] |
| Oyster | Pleurotus ostreatus | fresh, from bottom | UV-B | 4.14 | 160 | 60 | [58] |
| Compound | Hot Alkaline Hydrolysis, µg/g, µg/g [44] | Percentage, Vitamin D2 Set to 100 | Hypothetical Amount, µg/100 g Bread | Biological Activity, % | Equivalence Amount, µg/100 g Bread | Percentage, Normalised to Vitamin D2, % |
|---|---|---|---|---|---|---|
| previtamin D2 | 32.07 | 22.69 | 1.13 | 40 | 0.45 | 9.08 |
| tachysterol2 | 30.36 | 21.48 | 1.07 | 0 | 0.00 | 0.00 |
| vitamin D2 | 141.32 | 100.00 | 5.00 | 100 | 5.00 | 100.00 |
| lumisterol2 | 50.67 | 35.85 | 1.79 | 0 | 0.00 | 0.00 |
| previtamin D4 | 5.23 | 3.70 | 0.19 | 24 | 0.04 | 0.89 |
| tachysterol4 | 5.11 | 3.62 | 0.18 | 0 | 0.00 | 0.00 |
| vitamin D4 | 22.72 | 16.08 | 0.80 | 60 | 0.48 | 9.65 |
| lumisterol4 | 8.59 | 6.08 | 0.30 | 0 | 0.00 | 0.00 |
| Sum | 296.07 | 10.48 | 5.98 | 119.61 |
| Compound | Cold Alkaline Hydrolysis, µg/g [44] | Percentage, Vitamin D2 Set to 100 | Hypothetical Amount, µg/100 g Bread | Biological Activity, % | Equivalence Amount, µg/100 G Bread | Percentage, Normalised to Vitamin D2, % |
|---|---|---|---|---|---|---|
| previtamin D2 | 68.40 | 86.81 | 4.34 | 40 | 1.74 | 34.73 |
| tachysterol2 | 24.07 | 30.55 | 1.53 | 0 | 0.00 | 0.00 |
| vitamin D2 | 78.79 | 100.00 | 5.00 | 100 | 5.00 | 100.00 |
| lumisterol2 | 41.12 | 52.19 | 2.61 | 0 | 0.00 | 0.00 |
| previtamin D4 | 11.35 | 14.41 | 0.72 | 24 | 0.17 | 3.46 |
| tachysterol4 | 4.30 | 5.46 | 0.27 | 0 | 0.00 | 0.00 |
| vitamin D4 | 12.08 | 15.33 | 0.77 | 60 | 0.46 | 9.20 |
| lumisterol4 | 7.58 | 9.62 | 0.48 | 0 | 0.00 | 0.00 |
| Sum | 247.69 | 15.72 | 7.37 | 147.38 |
| Compound | Ultrasound Assisted Extraction, µg/g [44] | Percentage, Vitamin D2 Set to 100 | Hypothetical Amount, µg/100 g Bread | Biological Activity, % | Equivalence Amount, µg/100 g Bread | Percentage, Normalised to Vitamin D2, % |
|---|---|---|---|---|---|---|
| previtamin D2 | 59.02 | 84.06 | 4.20 | 40 | 1.68 | 33.62 |
| tachysterol2 | 16.65 | 23.71 | 1.19 | 0 | 0.00 | 0.00 |
| vitamin D2 | 70.21 | 100.00 | 5.00 | 100 | 5.00 | 100.00 |
| lumisterol2 | 37.39 | 53.25 | 2.66 | 0 | 0.00 | 0.00 |
| previtamin D4 | 7.73 | 11.01 | 0.55 | 24 | 0.13 | 2.64 |
| tachysterol4 | 2.72 | 3.87 | 0.19 | 0 | 0.00 | 0.00 |
| vitamin D4 | 9.45 | 13.46 | 0.67 | 60 | 0.40 | 8.08 |
| lumisterol4 | 5.58 | 7.95 | 0.40 | 0 | 0.00 | 0.00 |
| Sum | 208.75 | 14.87 | 7.22 | 144.34 |
| Search Term | Hits | Used |
|---|---|---|
| mushroom vitamin D UV | 51 | 19 |
| cerevisiae vitamin D UV-light | 10 | 2 |
| cerevisiae vitamin D enrichment | 1 | 0 |
| tachysterol | 38 | 7 |
| tachysterol toxic | 2 | 0 |
| mushroom tachysterol | 1 | 1 |
| tachysterol food | 8 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schümmer, T.; Stangl, G.I.; Wätjen, W. Safety Assessment of Vitamin D and Its Photo-Isomers in UV-Irradiated Baker’s Yeast. Foods 2021, 10, 3142. https://doi.org/10.3390/foods10123142
Schümmer T, Stangl GI, Wätjen W. Safety Assessment of Vitamin D and Its Photo-Isomers in UV-Irradiated Baker’s Yeast. Foods. 2021; 10(12):3142. https://doi.org/10.3390/foods10123142
Chicago/Turabian StyleSchümmer, Tobias, Gabriele I. Stangl, and Wim Wätjen. 2021. "Safety Assessment of Vitamin D and Its Photo-Isomers in UV-Irradiated Baker’s Yeast" Foods 10, no. 12: 3142. https://doi.org/10.3390/foods10123142
APA StyleSchümmer, T., Stangl, G. I., & Wätjen, W. (2021). Safety Assessment of Vitamin D and Its Photo-Isomers in UV-Irradiated Baker’s Yeast. Foods, 10(12), 3142. https://doi.org/10.3390/foods10123142