Bioactivity of Hydrolysates Obtained from Chicken Egg Ovalbumin Using Artichoke (Cynara scolymus L.) Proteases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining the Enzymatic Extract of Cynara scolymus L.
2.2. Obtaining the Hydrolysates
2.3. Peptide Concentration
2.4. Determination of the Hydrophobicity of Peptides
2.5. Angiotensin I-Converting Enzyme Inhibitory Activity
2.6. Antioxidant Activity
2.6.1. DPPH Radical Scavenging Activity
2.6.2. ABTS Radical Scavenging Activity
2.6.3. Iron (II) Chelating Activity
2.7. Antimicrobial Activity
2.8. Peptide Identification
2.9. Statistical Analysis
3. Results
3.1. Total Peptides and Hydrophilic and Hydrophobic Peptides of Ovalbumin Hydrolysates
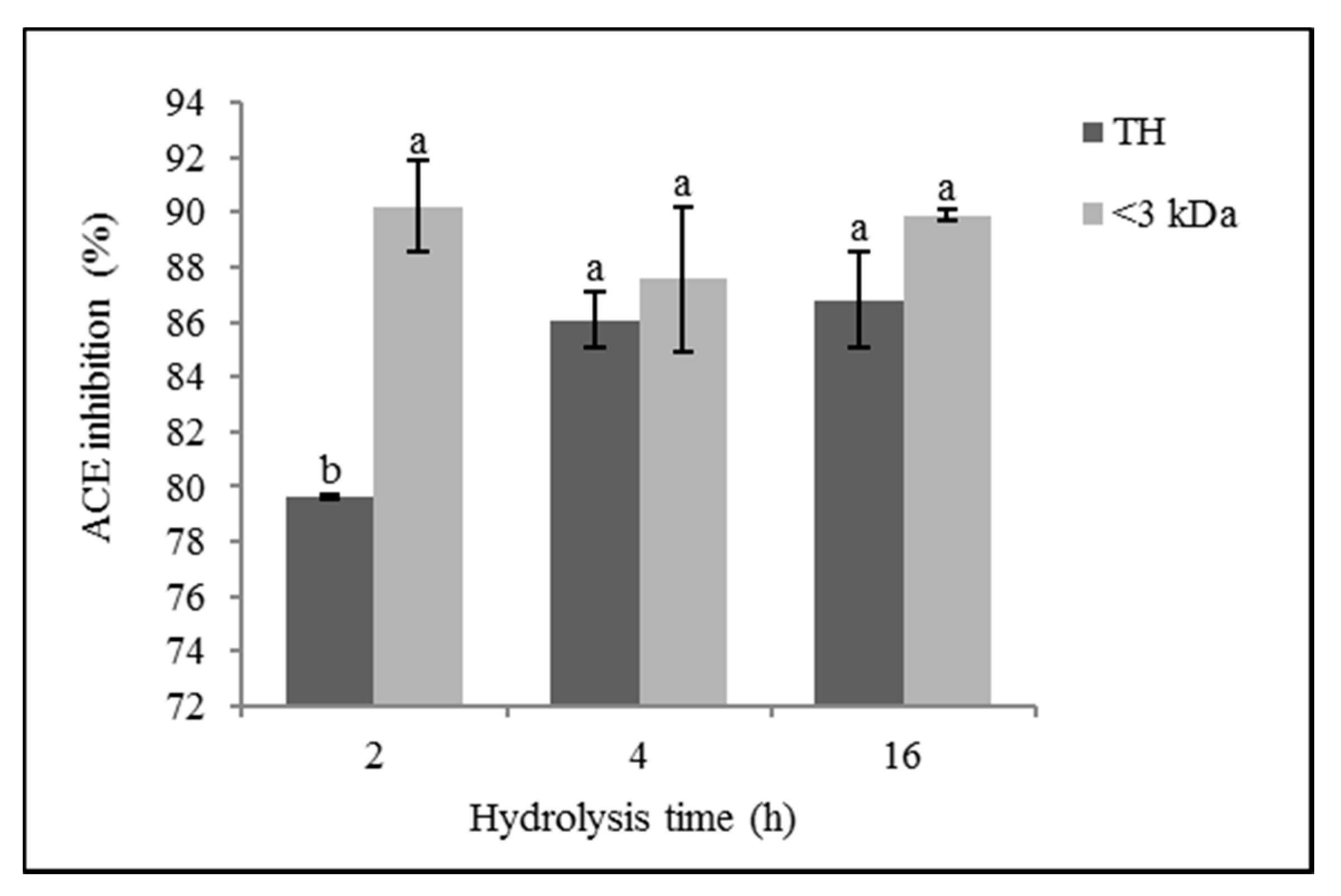
3.2. ACE Inhibitory Activity
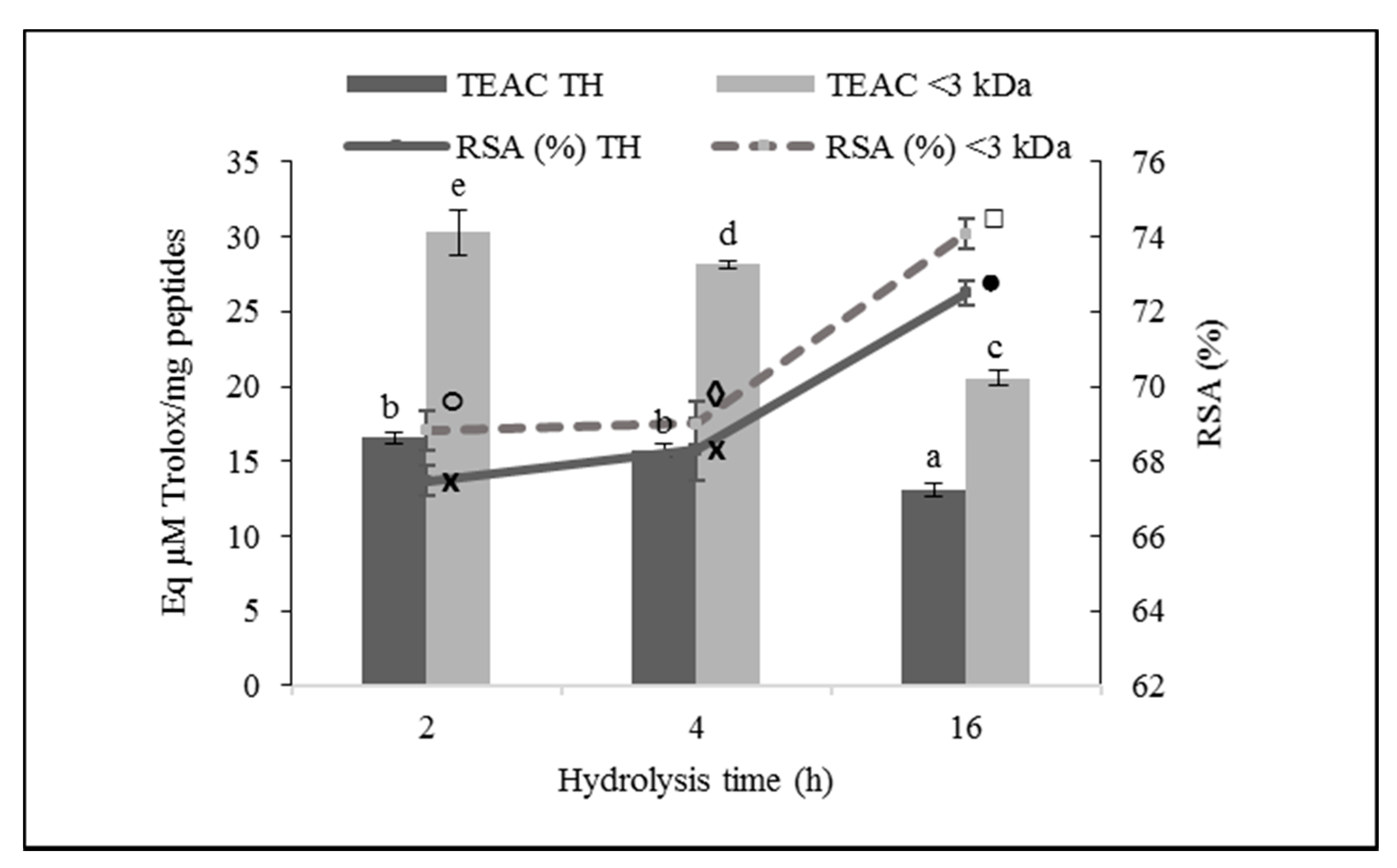
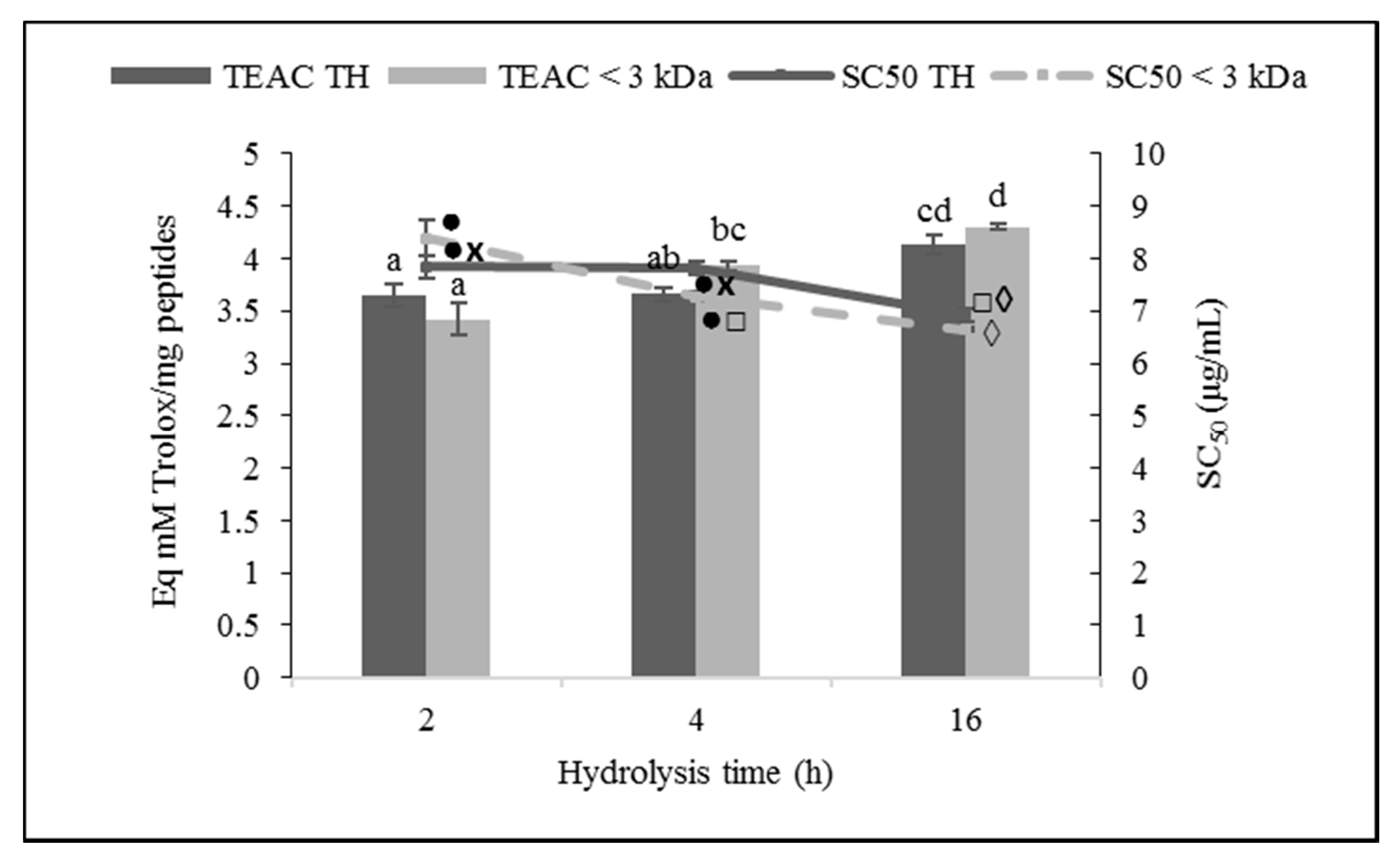
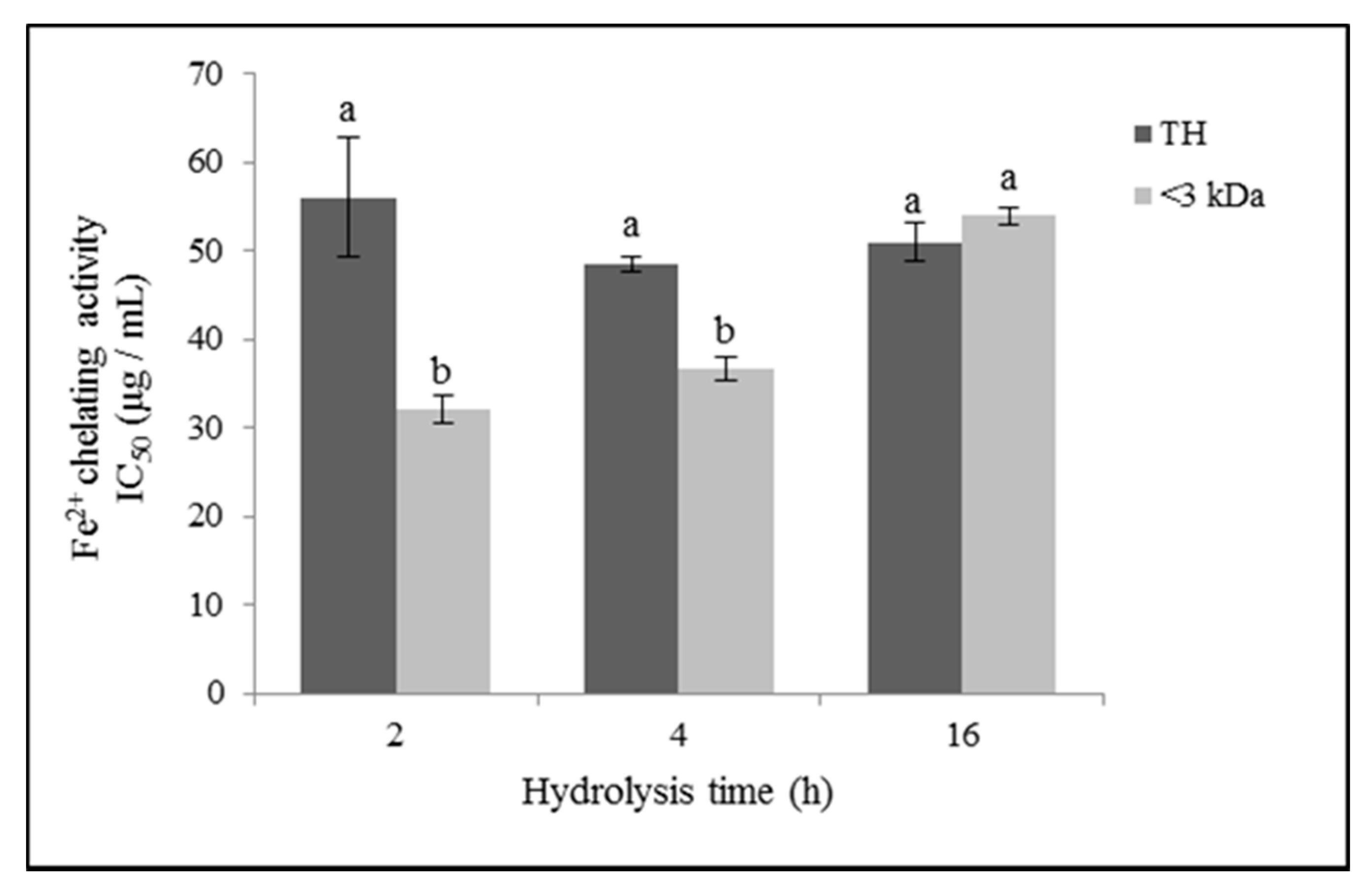
3.3. Antioxidant activity
3.3.1. DPPH Radical Scavenging Activity
3.3.2. ABTS Radical Scavenging Activity
3.3.3. Iron (II) Chelating Activity
3.4. Antimicrobial Activity
3.5. Identification of the Bioactive Peptides Present in the Oovalbumin Hydrolysates
4. Discussion
4.1. Total Peptides and Hydrophilic and Hydrophobic Peptides of Ovalbumin Hydrolysates
4.2. ACE Inhibitory Activity
4.3. Antioxidant Activity
4.4. Antimicrobial Activity
4.5. Peptide Identification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miranda, J.M.; Anton, X.; Redondo-Valbuena, C.; Roca-Saavedra, P.; Rodriguez, J.A.; Lamas, A.; Franco, C.M.; Cepeda, A. Egg and egg-derived foods: Effects on human health and use as functional foods. Nutrients 2015, 7, 706–729. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Peñas, E.; Frias, J. Chapter 2-Bioactive peptides in fermented foods: Production and evidence for health effects. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 23–47. ISBN 978-0-12-802309-9. [Google Scholar]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, A.; Eckert, E.; Pokora, M.; Bobak, Ł.; Dąbrowska, A.; Szołtysik, M.; Trziszka, T.; Chrzanowska, J. Antioxidant and antidiabetic activities of peptides isolated from a hydrolysate of an egg-yolk protein by-product prepared with a proteinase from Asian pumpkin (Cucurbita ficifolia). RSC Adv. 2015, 5, 10460–10467. [Google Scholar] [CrossRef]
- Lee, J.H.; Paik, H.-D. Anticancer and immunomodulatory activity of egg proteins and peptides: A review. Poult. Sci. 2019, 98, 6505–6516. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Jin, Y.; Liu, M.; Yang, Y.; Zhang, M.; Guo, Y.; Jones, G.; Liu, J.; Yin, Y. Research on the preparation of antioxidant peptides derived from egg white with assisting of high-intensity pulsed electric field. Food Chem. 2013, 139, 300–306. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.W. Proteomics in Food Biotechnology. In Omics Technologies: Tools for Food Science; Benkeblia, N., Ed.; Crc Press-Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 99–118. [Google Scholar]
- Yuan, J.; Zheng, Y.; Wu, Y.; Chen, H.; Tong, P.; Gao, J. Double enzyme hydrolysis for producing antioxidant peptide from egg white: Optimization, evaluation, and potential allergenicity. J. Food Biochem. 2020, 44, e13113. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Wang, Y.; Yu, Y.; Liu, J.; Liu, B.; Zhang, T. Identification of antioxidant peptides derived from egg-white protein and its protective effects on H2O2-induced cell damage. Int. J. Food Sci. 2019, 54, 2219–2227. [Google Scholar] [CrossRef]
- Benedé, S.; Molina, E. Chicken egg proteins and derived peptides with antioxidant properties. Foods 2020, 9, 735. [Google Scholar] [CrossRef]
- Llorente, B.E.; Brutti, C.B.; Caffini, N.O. Purification and characterization of a milk-clotting aspartic proteinase from globe artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2004, 52, 8182–8189. [Google Scholar] [CrossRef]
- Sidrach, L.; Garciacanovas, F.; Tudela, J.; Neptunorodriguezlopez, J. Purification of cynarases from artichoke (Cynara scolymus L.): Enzymatic properties of cynarase A. Phytochemistry 2005, 66, 41–49. [Google Scholar] [CrossRef]
- Tejada, L.; Abellán, A.; Cayuela, J.M.; Martínez-Cacha, A.; Fernández-Salguero, J. Proteolysis in goats’ milk cheese made with calf rennet and plant coagulant. Int. Dairy J. 2008, 18, 139–146. [Google Scholar] [CrossRef]
- Agboola, S.; Chen, S.; Zhao, J. Formation of bitter peptides during ripening of ovine milk cheese made with different coagulants. Int. J. Dairy Technol. 2004, 84, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Bueno-Gavilá, E. Utilización de Proteasas de Cynara Scolymus L. para la Obtención de Péptidos Bioactivos a partir de Ovoalbúmina, Caseína y leche; Universidad Católica de Murcia (UCAM): Murcia, Spain, 2017. [Google Scholar]
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008, 108, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Salguero, J.; Tejada, L.; Gómez, R. Use of powdered vegetable coagulant in the manufacture of ewe’s milk cheeses: Cheese-making with a powdered vegetable coagulant. J. Sci. Food Agric. 2002, 82, 464–468. [Google Scholar] [CrossRef]
- Association of Analytical Chemists. Official Methods of Analysis; Association of Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- González de Llano, D.; Polo, M.C.; Ramos, M. Study of proteolysis in artisanal cheeses: High performance liquid chromatography of peptides. J. Dairy Sci. 1994, 78, 1018–1024. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Miguel, M.; Recio, I.; Gómez-Ruiz, J.A.; Ramos, M.; López-Fandiño, R. Angiotensin I-converting enzyme inhibitory activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- De Gobba, C.; Espejo-Carpio, F.J.; Skibsted, L.H.; Otte, J. Antioxidant peptides from goat milk protein fractions hydrolysed by two commercial proteases. Int. Dairy J. 2014, 39, 28–40. [Google Scholar] [CrossRef]
- Wu, C.-R.; Huang, M.-Y.; Lin, Y.-T.; Ju, H.-Y.; Ching, H. Antioxidant properties of Cortex Fraxini and its simple coumarins. Food Chem. 2007, 104, 1464–1471. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT-Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Bueno-Gavilá, E.; Abellán, A.; Girón-Rodríguez, F.; Cayuela, J.M.; Salazar, E.; Gómez, R.; Tejada, L. Bioactivity of hydrolysates obtained from bovine casein using artichoke (Cynara scolymus L.) proteases. J. Dairy Sci. 2019, 102, 10711–10723. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chahal, B.; Majumder, K.; You, S.-J.; Wu, J. Identification of novel antioxidative peptides derived from a thermolytic hydrolysate of ovotransferrin by LC-MS/MS. J. Agric. Food Chem. 2010, 58, 7664–7672. [Google Scholar] [CrossRef]
- Konrad, B.; Anna, D.; Marek, S.; Marta, P.; Aleksandra, Z.; Józefa, C. The evaluation of dipeptidyl peptidase (DPP)-IV, α-glucosidase and angiotensin converting enzyme (ACE) inhibitory activities of whey proteins hydrolyzed with serine protease isolated from asian pumpkin (Cucurbita ficifolia). Int. J. Pept. Res. Ther. 2014, 20, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Wu, F.; Li, M.; Yang, N.; Wu, C.; Jin, Y.; Yang, J.; Jin, Z.; Xu, X. Naturally occurring angiotensin I-converting enzyme inhibitory peptide from a fertilized egg and its inhibitory mechanism. J. Agric. Food Chem. 2014, 62, 5500–5506. [Google Scholar] [CrossRef]
- Garcés-Rimón, M.; López-Expósito, I.; López-Fandiño, R.; Miguel, M. Egg white hydrolysates with in vitro biological multiactivities to control complications associated with the metabolic syndrome. Eur. Food Res. Technol. 2016, 242, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chi, Y.-J. Antioxidant, ACE inhibitory activities and functional properties of egg white protein hydrolysate. J. Food Biochem. 2011, 36, 383–394. [Google Scholar] [CrossRef]
- Huang, Q.; Li, S.; Teng, H.; Jin, Y.; Ma, M.; Song, H. Optimizing preparation conditions for angiotensin-I-converting enzyme inhibitory peptides derived from enzymatic hydrolysates of ovalbumin. Food Sci. Biotechnol. 2015, 24, 2193–2198. [Google Scholar] [CrossRef]
- Jakovetić, S.; Luković, N.; Jugović, B.; Gvozdenović, M.; Grbavčić, S.; Jovanović, J.; Knežević-Jugović, Z. Production of antioxidant egg white hydrolysates in a continuous stirred tank enzyme reactor coupled with membrane separation unit. Food Bioprocess Technol. 2015, 8, 287–300. [Google Scholar] [CrossRef]
- Chiang, W.-D.; Tsou, M.-J.; Weng, C.-H.; Tsai, T.-C. Production of angiotensin I-converting enzyme inhibitor derived from egg white protein hydrolysates using a membrane reactor. J. Food Drug Anal. 2008, 16, 54–60. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.-J.; Zhao, M.-Y.; Xu, W. Influence of degree of hydrolysis on functional properties, antioxidant and ACE inhibitory activities of egg white protein hydrolysate. Food Sci. Biotechnol. 2012, 21, 27–34. [Google Scholar] [CrossRef]
- Sun, S.; Niu, H.; Yang, T.; Lin, Q.; Luo, F.; Ma, M. Antioxidant and anti-fatigue activities of egg white peptides prepared by pepsin digestion: Antioxidant and anti-fatigue activities of egg white peptides. J. Sci. Food Agric. 2014, 94, 3195–3200. [Google Scholar] [CrossRef]
- You, S.-J.; Wu, J. Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activities of Egg Protein Hydrolysates Produced with Gastrointestinal and Nongastrointestinal Enzymes. J. Food Sci. 2011, 76, C801–C807. [Google Scholar] [CrossRef]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamauchi, T.; Katsuda, T.; Yamaji, H.; Katoh, S. Angiotensin-I converting enzyme (ACE) inhibitory mechanism of tripeptides containing aromatic residues. J. Biosci. Bioeng. 2008, 106, 310–312. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Quirós, A.; Chichón, R.; Recio, I.; López-Fandiño, R. The use of high hydrostatic pressure to promote the proteolysis and release of bioactive peptides from ovalbumin. Food Chem. 2007, 104, 1734–1739. [Google Scholar] [CrossRef]
- Tanzadehpanah, H.; Asoodeh, A.; Saberi, M.R.; Chamani, J. Identification of a novel angiotensin-I converting enzyme inhibitory peptide from ostrich egg white and studying its interactions with the enzyme. Innov. Food Sci. Emerg. Technol. 2013, 18, 212–219. [Google Scholar] [CrossRef]
- Pokora, M.; Zambrowicz, A.; Dąbrowska, A.; Eckert, E.; Setner, B.; Szołtysik, M.; Szewczuk, Z.; Zabłocka, A.; Polanowski, A.; Trziszka, T.; et al. An attractive way of egg white protein by-product use for producing of novel anti-hypertensive peptides. Food Chem. 2014, 151, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Jo, C.; Nam, K.C.; Ahn, D.U. Enzymatic hydrolysis of ovalbumin and the functional properties of the hydrolysates. Poult. Sci. 2014, 93, 2678–2686. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Timmer, M.; Eckert, E.; Trziszka, T. Evaluation of the ACE-Inhibitory Activity of Egg-White Proteins Degraded with Pepsin. Pol. J. Food Nutr. Sci. 2013, 63. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, A.; Saito, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Structural analysis of new antihypertensive peptides derived from cheese whey protein by proteinase K digestion. J. Dairy Sci. 1998, 81, 3131–3138. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Angiotensin-I-converting enzyme inhibitory activities of gastric and pancreatic proteinase digests of whey proteins. Int. Dairy J. 1997, 7, 299–303. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Identification of a novel angiotensin-I-converting enzyme inhibitory peptide corresponding to a tryptic fragment of bovine β-lactoglobulin. FEBS Lett. 1997, 402, 99–101. [Google Scholar] [CrossRef] [Green Version]
- Galán, E.; Prados, F.; Pino, A.; Tejada, L.; Fernández-Salguero, J. Influence of different amounts of vegetable coagulant from cardoon Cynara cardunculus and calf rennet on the proteolysis and sensory characteristics of cheeses made with sheep milk. Int. Dairy J. 2008, 18, 93–98. [Google Scholar] [CrossRef]
- Natesh, R.; Schwager, S.L.U.; Sturrock, E.D.; Acharya, K.R. Crystal structure of the human angiotensin-converting enzyme–lisinopril complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Bamdad, F.; Khey, K.; Sunwoo, H.H. Antioxidant and anti-inflammatory properties of chicken egg vitelline membrane hydrolysates. Poult. Sci. 2017, 96, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Regulation of exacerbated immune responses in human peripheral blood cells by hydrolysed egg white proteins. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.-J.; Zhao, M.-Y.; Lv, L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 2012, 43, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptides fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Homayouni-Tabrizi, M.; Asoodeh, A.; Abbaszadegan, M.-R.; Shahrokhabadi, K.; Nakhaie Moghaddam, M. An identified antioxidant peptide obtained from ostrich (Struthio camelus) egg white protein hydrolysate shows wound healing properties. Pharm. Biol. 2015, 53, 1155–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, D.O.; Suh, H.J. Preparation of egg white liquid hydrolysate (ELH) and its radical-scavenging activity. Prev. Nutr. Food Sci. 2015, 20, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baratzadeh, M.-H.; Asoodeh, A.; Chamani, J. Antioxidant peptides obtained from goose egg white proteins by enzymatic hydrolysis. Int. J. Food Sci. 2013, 48, 1603–1609. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, Radical Scavenging, and Chelation Properties of in Vitro Digests of Alcalase-Treated Zein Hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yamauchi, F.; Nokihara, K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 1996, 44, 2619–2623. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Zhao, W.; Chen, F.; Liu, J. Application and bioactive properties of proteins and peptides derived from hen eggs: Opportunities and challenges: Application of proteins and peptides from hen eggs. J. Sci. Food Agric. 2014, 94, 2839–2845. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Thammasirirak, S.; Pukcothanung, Y.; Preecharram, S.; Daduang, S.; Patramanon, R.; Fukamizo, T.; Araki, T. Antimicrobial peptides derived from goose egg white lysozyme. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Ma, F.; Lauriau, S. Antimicrobial peptides released by enzymatic hydrolysis of hen egg white lysozyme. J. Agric. Food Chem. 2004, 52, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, H.; Wang, L.; Qian, H. Antimicrobial peptide isolated from ovalbumin hydrolysate by immobilized liposome-binding extraction. Eur. Food Res. Technol. 2013, 237, 591–600. [Google Scholar] [CrossRef]
- Asoodeh, A.; Homayouni-Tabrizi, M.; Shabestarian, H.; Emtenani, S.; Emtenani, S. Biochemical characterization of a novel antioxidant and angiotensin I-converting enzyme inhibitory peptide from Struthio camelus egg white protein hydrolysis. J. Food Drug Anal. 2016, 24, 332–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Item 2 | Hydrolysis Time (H) | p-Value 3 | |||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 16 | H | MW | H × MW | ||
| Peptides | TH | 2.35 ± 0.07 c | 2.50 ± 0.04 d | 3.21 ± 0.10 e | ≤0.01 | ≤0.01 | >0.05 |
| (mg/mL) | <3 kDa | 1.31 ± 0.07 a | 1.41 ± 0.00 a | 2.08 ± 0.04 b | |||
| HI (%) | TH | 19.98 ± 0.45 b | 23.27 ± 0.17 c | 25.12 ± 4.18 c | ≤0.01 | ≤0.01 | >0.05 |
| <3 kDa | 27.17 ± 0.54 a | 44.32 ± 0.97 b | 50.71 ± 2.43 b | ||||
| HO (%) | TH | 80.02 ± 5.64 b | 76.73 ± 6.03 c | 74.88 ± 9.92 b | ≤0.05 | ≤0.01 | ≤0.05 |
| <3 kDa | 72.83 ± 1.91 a | 55.68 ± 0.97 a | 49.29 ± 8.75 a | ||||
| HO/HI | TH | 4.01 ± 0.25 d | 3.30 ± 0.23 bc | 2.98 ± 0.98 bc | ≤0.05 | ≤0.01 | >0.05 |
| <3 kDa | 2.68 ± 0.06 b | 1.26 ± 0.04 a | 0.97 ± 0.13 a | ||||
| MW 2 | Parameters | Control | Hydrolysis Time (h) | ||
|---|---|---|---|---|---|
| 2 | 4 | 16 | |||
| TH | Lag phase (min) | 719.78 ± 15.7 | 586.74 ± 8.2 ** | 598.00 ± 12.6 ** | 648.94 ± 12.7 |
| Growth rate (mU Abs/min) | 3.73 ± 0.2 | 2.96 ± 0.2 * | 3.15 ± 0.2 | 3.79 ± 0.2 | |
| Maximum growth (U Abs) | 0.566 ± 0.03 | 0.579 ± 0.01 | 0.581 ± 0.01 | 0.551 ± 0.01 | |
| <3 kDa | Lag phase (min) | 719.78 ± 15.7 | 554.50 ± 10.24 ** | 574.88 ± 14.0 ** | 614.04 ± 21.2 ** |
| Growth rate (mU Abs/min) | 3.73 ± 0.2 | 2.50 ± 0.1 ** | 2.65 ± 0.2 ** | 2.88 ± 0.2 ** | |
| Maximum growth (U Abs) | 0.566 ± 0.03 | 0.555 ± 0.00 | 0.557 ± 0.01 | 0.535 ± 0.01 | |
| Sequence (Origin Hydrolysate) | ID | Origin | MM | Activity |
|---|---|---|---|---|
| HLFGPPGKKDPV (4 h) | 8393 | Ovotransferrin | 1291.50 | ACE-inhibitor |
| IAAEVYEHTEGSTTSY (2 h, 4 h, 16 h) | 8235 | Ovotransferrin | 1757.81 | Antioxidative |
| PIAAEVYEHTEGSTTSY (2 h) | 8231 | Ovotransferrin | 1854.93 | Antioxidative |
| YAEERYPIL (2 h, 4 h, 16 h) | 8391 | Ovalbumin | 1153.28 | ACE-inhibitor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno-Gavilá, E.; Abellán, A.; Girón-Rodríguez, F.; Cayuela, J.M.; Tejada, L. Bioactivity of Hydrolysates Obtained from Chicken Egg Ovalbumin Using Artichoke (Cynara scolymus L.) Proteases. Foods 2021, 10, 246. https://doi.org/10.3390/foods10020246
Bueno-Gavilá E, Abellán A, Girón-Rodríguez F, Cayuela JM, Tejada L. Bioactivity of Hydrolysates Obtained from Chicken Egg Ovalbumin Using Artichoke (Cynara scolymus L.) Proteases. Foods. 2021; 10(2):246. https://doi.org/10.3390/foods10020246
Chicago/Turabian StyleBueno-Gavilá, Estefanía, Adela Abellán, Francisco Girón-Rodríguez, José María Cayuela, and Luis Tejada. 2021. "Bioactivity of Hydrolysates Obtained from Chicken Egg Ovalbumin Using Artichoke (Cynara scolymus L.) Proteases" Foods 10, no. 2: 246. https://doi.org/10.3390/foods10020246






