Calcium Chelation by Phosphate Ions and Its Influence on Fouling Mechanisms of Whey Protein Solutions in a Plate Heat Exchanger
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Fouling Solution
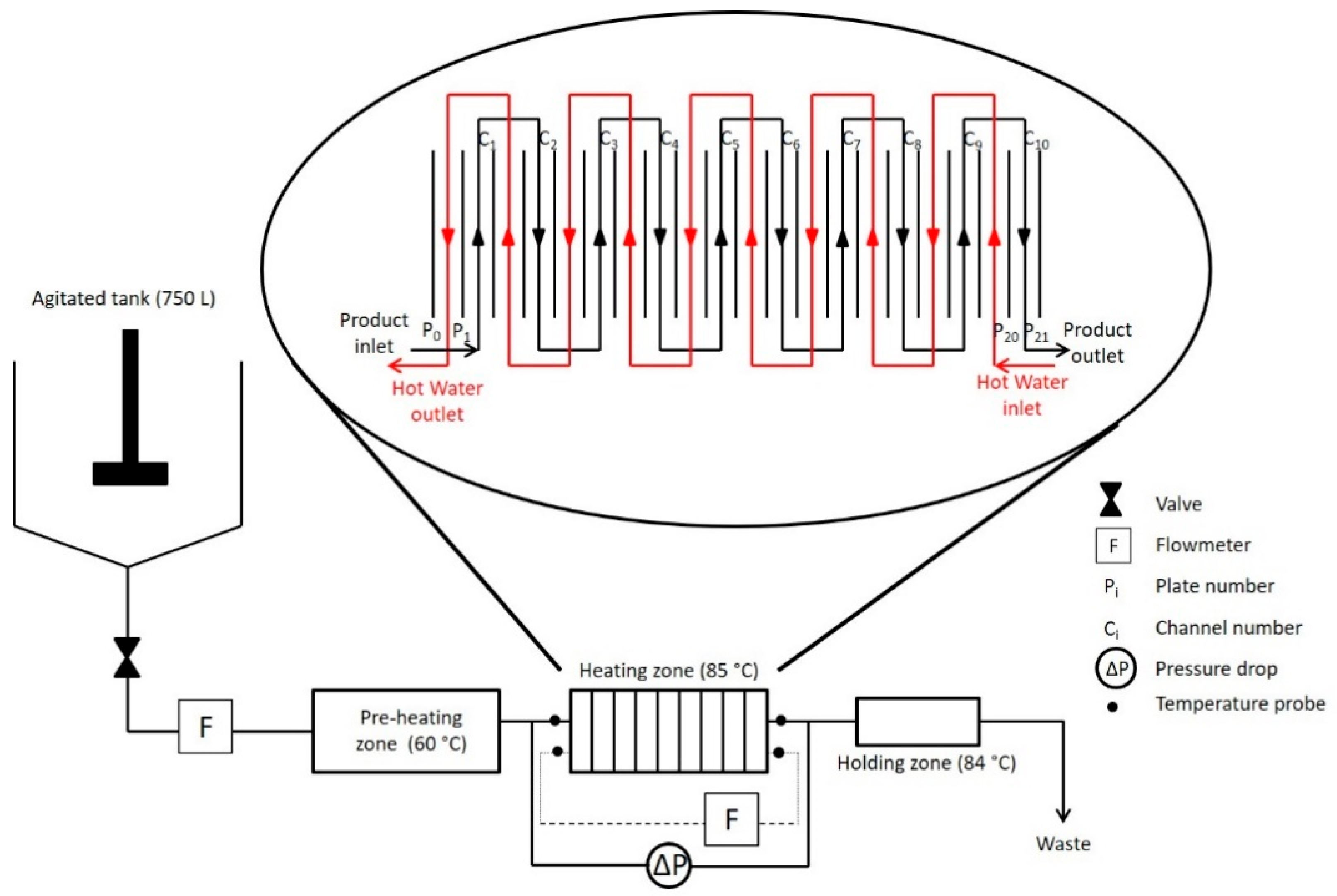
2.2. Experimental Fouling Installation
2.3. Fouling Mass
2.4. Monitoring of the Heat Transfer Coefficient and Pressure Drop in the Plate Heat Exchanger
2.5. β-Lg Denaturation Kinetics Determination
2.6. HPLC Analysis
2.7. Determination of β-Lg Kinetic Parameters
2.8. Electron-Probe Micro Analysis (EPMA)
3. Results and Discussions
3.1. Effect of Adding Phosphate on the Deposit Mass Distribution within the PHE and on Total Deposit Mass, and Highlight the Role of Free Calcium on Fouling Mechanism
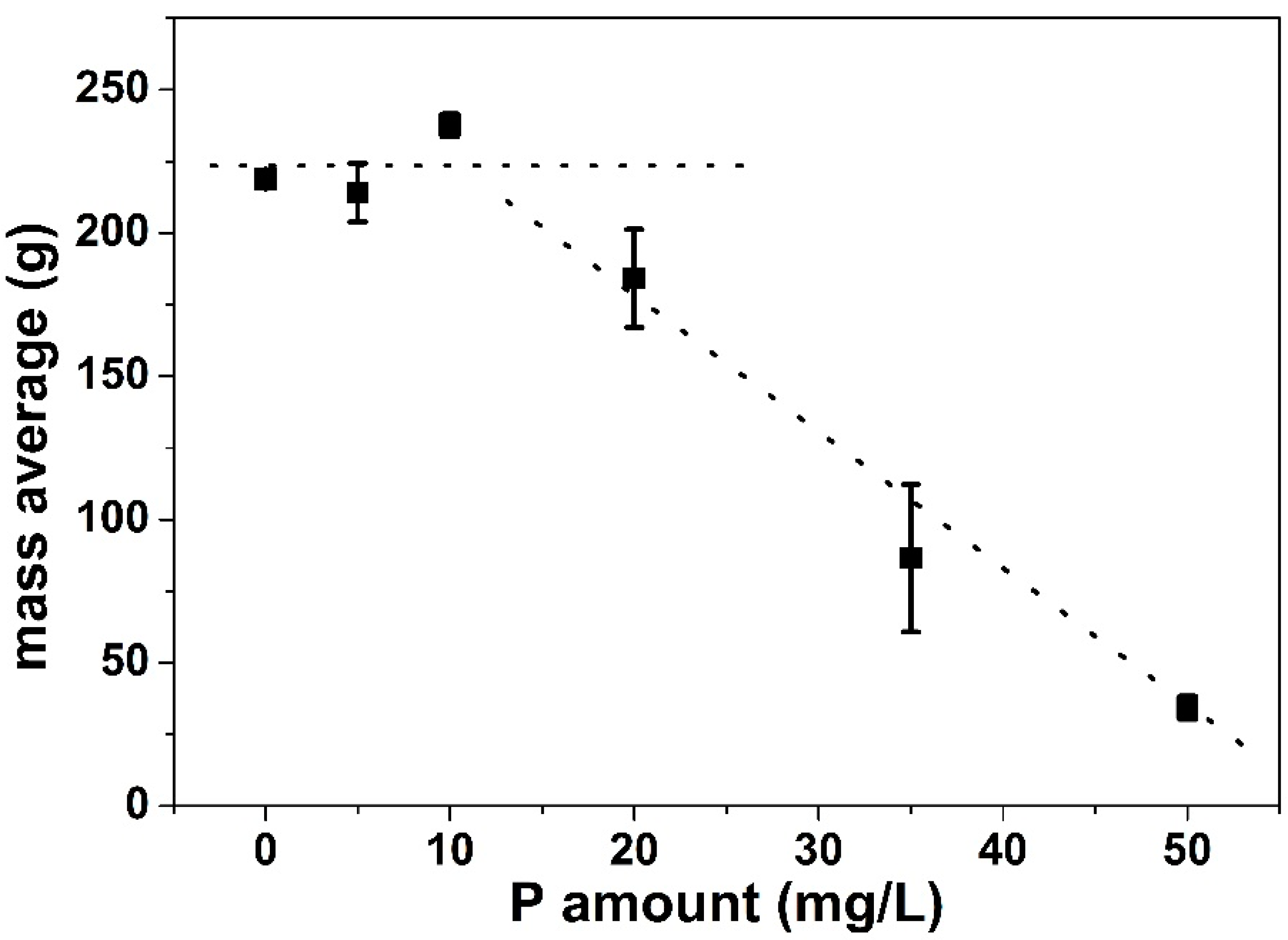
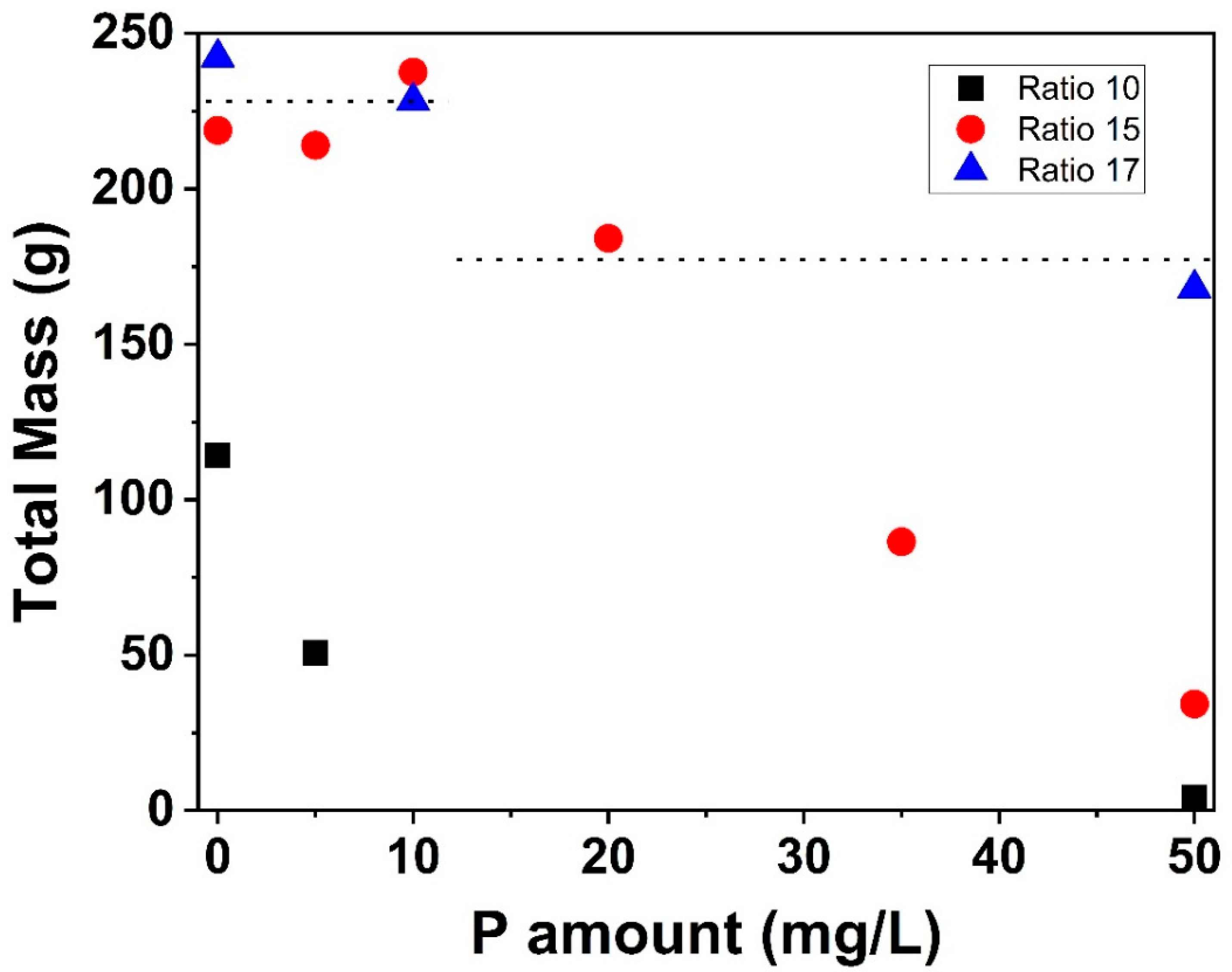
3.1.1. Total Deposit Mass
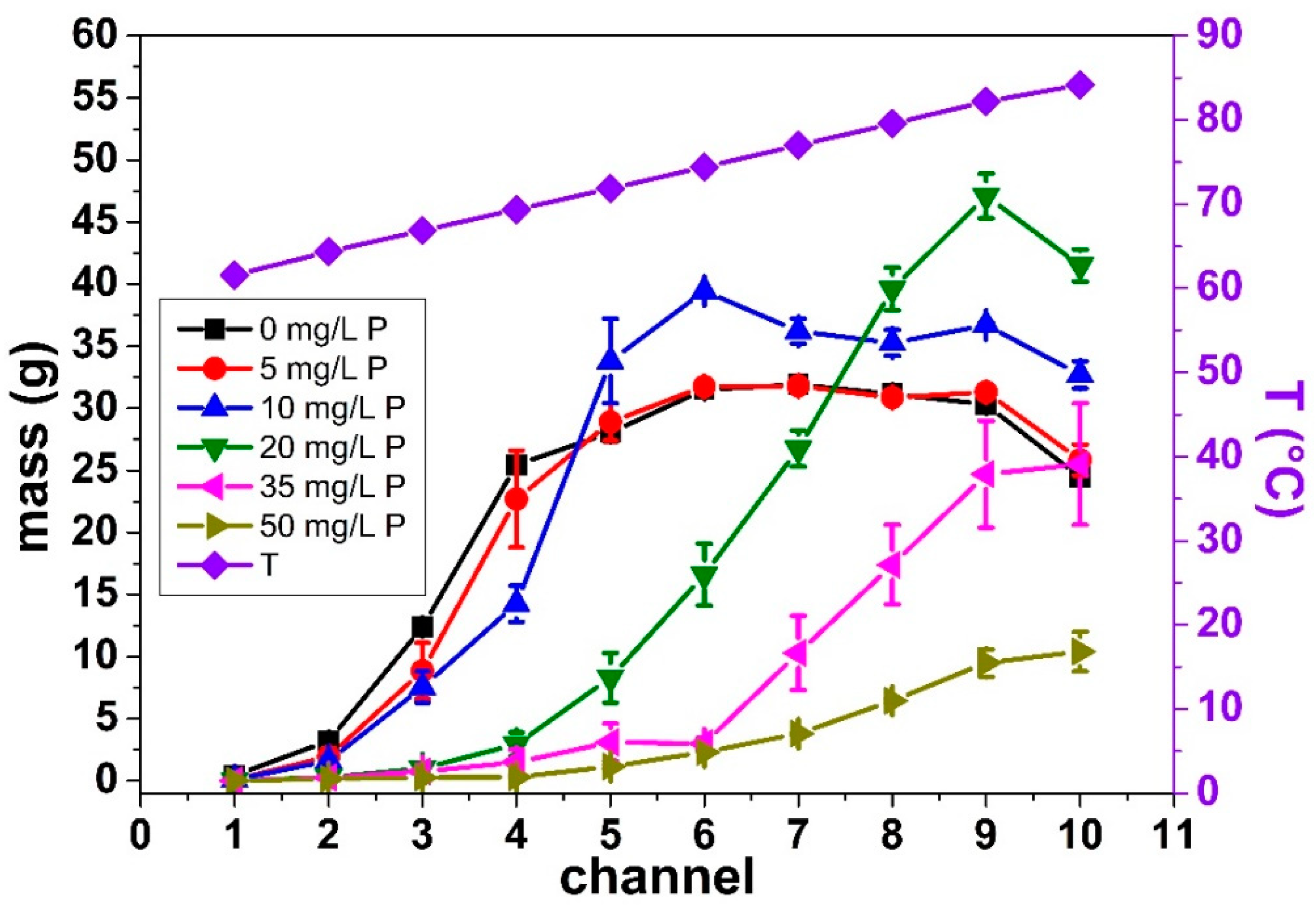
3.1.2. Deposit Mass Distribution
3.2. Effect of Adding Phosphate on the Pressure Drop, on the Heat Transfer, and on the Fouling Layer Structure, and Highlight on Fouling Build-Up in Function of Calcium Complexes
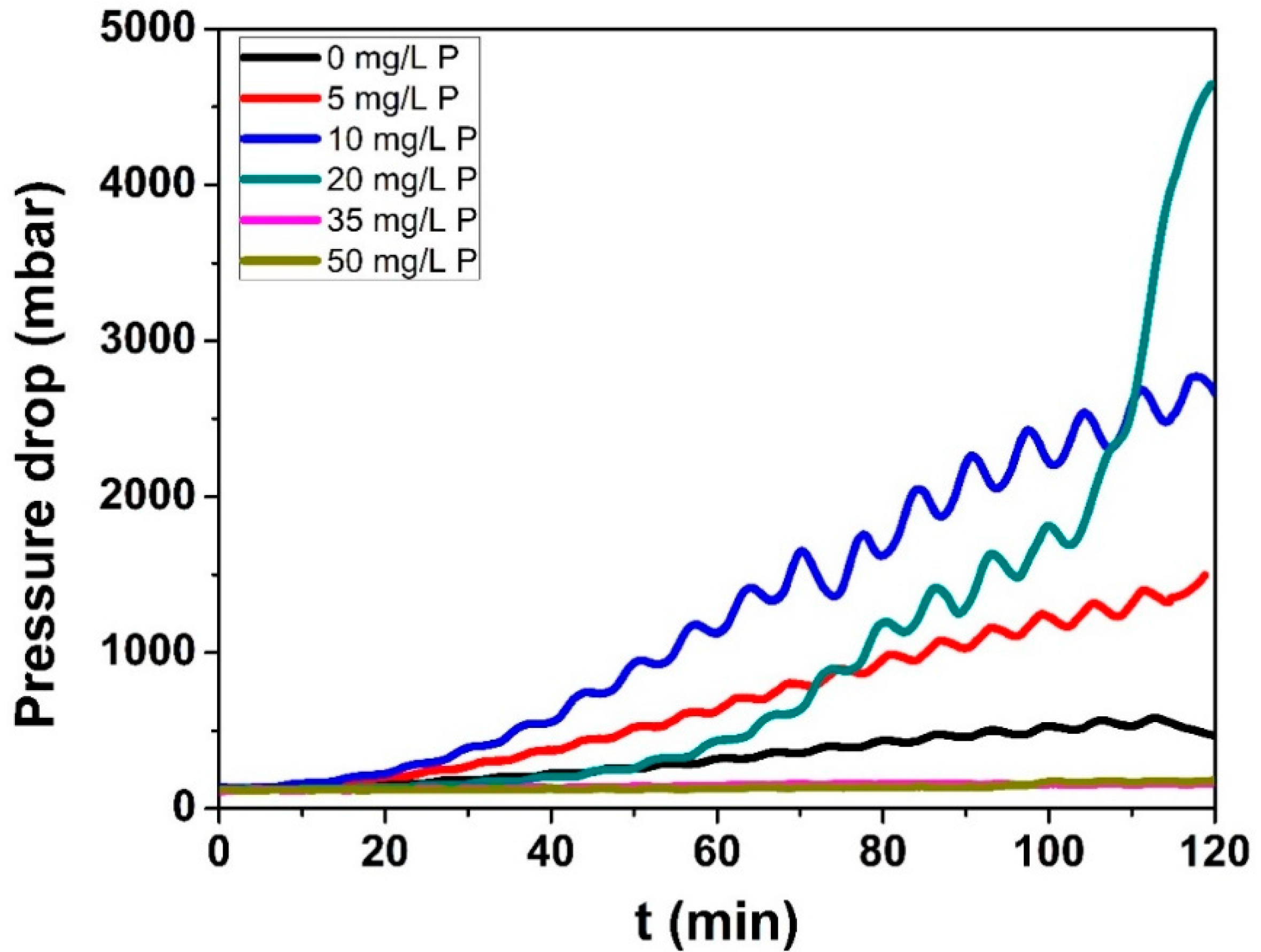
3.2.1. Pressure Drop
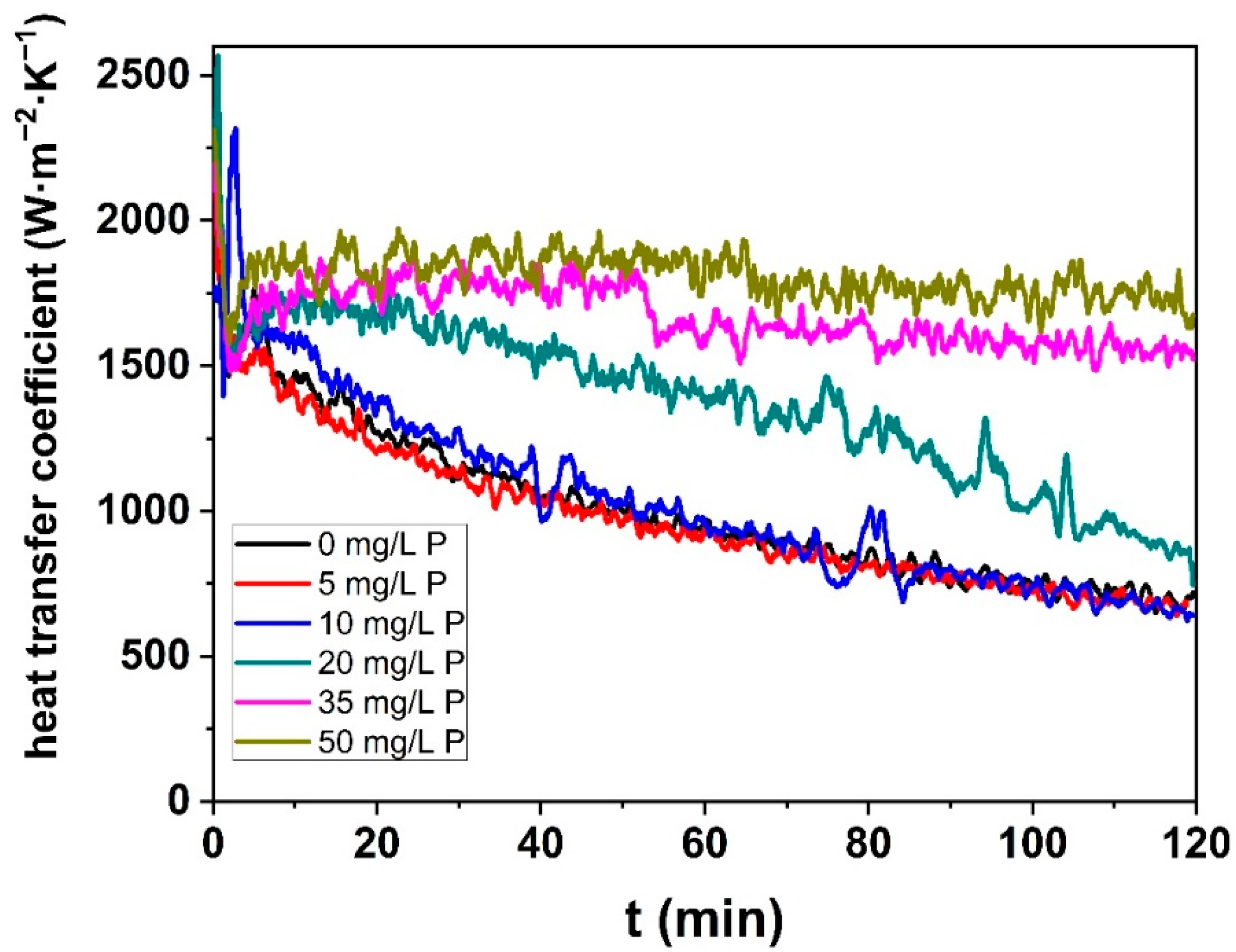
3.2.2. Heat Transfer
3.2.3. Fouling Layer Structure
3.3. Effect of Adding Phosphate on the β-Lg Denaturation Kinetics and Highlights on Fouling Build-Up in Function of Unfolding and Aggregation Reactions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bansal, B.; Chen, X.D. A Critical Review of Milk Fouling in Heat Exchangers. Compr. Rev. Food Sci. Food Saf. 2006, 5, 27–33. [Google Scholar] [CrossRef]
- Gillham, C.; Fryer, P.; Hasting, A.P.; Wilson, D. Enhanced cleaning of whey protein soils using pulsed flows. J. Food Eng. 2000, 46, 199–209. [Google Scholar] [CrossRef]
- Zouaghi, S.; Frémiot, J.; André, C.; Grunlan, M.A.; Gruescu, C.; Delaplace, G.; Duquesne, S.; Jimenez, M. Investigating the Effect of an Antifouling Surface Modification on the Environmental Impact of a Pasteurization Process: An LCA Study. ACS Sustain. Chem. Eng. 2019, 7, 9133–9142. [Google Scholar] [CrossRef]
- Burton, H.; Section, G. Deposits from whole milk in heat treatment plant—A review and discussion. J. Dairy Res. 1968, 35, 317–330. [Google Scholar] [CrossRef]
- Daufin, G.; Labbé, J.; Quemerais, A.; Brulé, G.; Michel, F.; Roignant, M.; Priol, M. Fouling of a heat exchange surface by whey, milk and model fluids. An analytical study. Lait 1987, 67, 339–364. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, M.; Delaplace, G.; Nuns, N.; Bellayer, S.; Deresmes, D.; Ronse, G.; Alogaili, G.; Collinet-Fressancourt, M.; Traisnel, M. Toward the understanding of the interfacial dairy fouling deposition and growth mechanisms at a stainless steel surface: A multiscale approach. J. Colloid Interface Sci. 2013, 404, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, X.D.; Jeantet, R.; André, C.; Bellayer, S.; Delaplace, G. Effect of casein/whey ratio on the thermal denaturation of whey proteins and subsequent fouling in a plate heat exchanger. J. Food Eng. 2021, 289, 110175. [Google Scholar] [CrossRef]
- Khaldi, M.; Croguennec, T.; André, C.; Ronse, G.; Jimenez, M.; Bellayer, S.; Blanpain-Avet, P.; Bouvier, L.; Six, T.; Bornaz, S.; et al. Effect of the calcium/protein molar ratio on β-lactoglobulin denaturation kinetics and fouling phenomena. Int. Dairy J. 2018, 78, 1–10. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Kazi, S.N.; Badarudin, A.; Zubair, M.N.M.; Dehkordi, B.L.; Oon, C.S. A review of milk fouling on heat exchanger surfaces. Rev. Chem. Eng. 2013, 29, 169–188. [Google Scholar] [CrossRef]
- Peixoto, P.D.S.; Trivelli, X.; André, C.; Moreau, A.; Delaplace, G. Formation of β-Lactoglobulin Aggregates from Quite, Unfolded Conformations upon Heat Activation. Langmuir 2019, 35, 446–452. [Google Scholar] [CrossRef]
- Xiong, Y.L. Influence of pH and ionic environment on thermal aggregation of whey proteins. J. Agric. Food Chem. 1992, 40, 380–384. [Google Scholar] [CrossRef]
- Joyce, A.M.; Kelly, A.L.; O’Mahony, J.A. Controlling denaturation and aggregation of whey proteins during thermal processing by modifying temperature and calcium concentration. Int. J. Dairy Technol. 2018, 71, 446–453. [Google Scholar] [CrossRef]
- Hebishy, E.; Joubran, Y.; Murphy, E.; O’Mahony, J.A. Influence of calcium-binding salts on heat stability and fouling of whey protein isolate dispersions. Int. Dairy J. 2019, 91, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Keowmaneechai, E.; McClements, D.J. Influence of EDTA and citrate on physicochemical properties of whey protein-stabilized oil-in-water emulsions containing CaCL2. J. Agric. Food Chem. 2002, 50, 7145–7153. [Google Scholar] [CrossRef] [PubMed]
- Keowmaneechai, E.; McClements, D.J. Influence of EDTA and citrate on thermal stability of whey protein stabilized oil-in-water emulsions containing calcium chloride. Food Res. Int. 2006, 39, 230–239. [Google Scholar] [CrossRef]
- Pathomrungsiyounggul, P.; Lewis, M.J.; Grandison, A.S. Effects of calcium-chelating agents and pasteurisation on certain properties of calcium-fortified soy milk. Food Chem. 2010, 118, 808–814. [Google Scholar] [CrossRef]
- de Kort, E.; Minor, M.; Snoeren, T.; van Hooijdonk, T.; van der Linden, E. Effect of calcium chelators on physical changes in casein micelles in concentrated micellar casein solutions. Int. Dairy J. 2011, 21, 907–913. [Google Scholar] [CrossRef]
- De Kort, E.; Minor, M.; Snoeren, T.; van Hooijdonk, T.; van der Linden, E. Effect of calcium chelators on heat coagulation and heat-induced changes of concentrated micellar casein solutions: The role of calcium-ion activity and micellar integrity. Int. Dairy J. 2012, 26, 112–119. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Power, O.; Wijayanti, H.B.; Kelly, P.M.; Mao, L.; Fenelon, M.A. Effects of calcium chelating agents on the solubility of milk protein concentrate. Int. J. Dairy Technol. 2017, 70, 415–423. [Google Scholar] [CrossRef]
- Renhe, I.R.T.; Indris, L.M.; Corredig, M. Effect of calcium chelators on heat stability and heat-induced changes of milk microfiltered concentrates. Int. Dairy J. 2018, 82, 4–10. [Google Scholar] [CrossRef]
- Christian, G.K.; Changani, S.D.; Fryer, P.J. The effect of adding minerals on fouling from whey protein concentrate: Development of a model fouling fluid for a plate heat exchanger. Food Bioprod. Process. Trans. Inst. Chem. Eng. Part C 2002, 80, 231–239. [Google Scholar] [CrossRef]
- Blanpain-Avet, P.; André, C.; Azevedo-Scudeller, L.; Croguennec, T.; Jimenez, M.; Bellayer, S.; Six, T.; Martins, G.A.S.; Delaplace, G. Effect of the phosphate/calcium molar ratio on fouling deposits generated by the processing of a whey protein isolate in a plate heat exchanger. Food Bioprod. Process. 2020, 121, 154–165. [Google Scholar] [CrossRef]
- CODEX STAN 192-1995. General standard for food additives. 1995.
- Lalande, M.; Rene, F. Fouling by Milk and Dairy Product and Cleaning of Heat Exchange Surfaces. In Fouling Science and Technology; Springer: Dordrecht, The Netherlands, 1988; pp. 557–573. [Google Scholar]
- Khaldi, M.; Blanpain-Avet, P.; Guérin, R.; Ronse, G.; Bouvier, L.; André, C.; Bornaz, S.; Croguennec, T.; Jeantet, R.; Delaplace, G. Effect of calcium content and flow regime on whey protein fouling and cleaning in a plate heat exchanger. J. Food Eng. 2015, 147, 68–78. [Google Scholar] [CrossRef]
- Blanpain-Avet, P.; André, C.; Khaldi, M.; Bouvier, L.; Petit, J.; Six, T.; Jeantet, R.; Croguennec, T. Predicting the distribution of a whey protein fouling in a plate heat exchanger using the kinetic parameters of the thermal denaturation reaction of β-lactoglobulin and the bulk temperature profiles. J. Dairy Sci. 2016, 99, 9611–9630. [Google Scholar] [CrossRef] [Green Version]
- Petit, J.; Six, T.; Moreau, A.; Ronse, G.; Delaplace, G. β-lactoglobulin denaturation, aggregation, and fouling in a plate heat exchanger: Pilot-scale experiments and dimensional analysis. Chem. Eng. Sci. 2013, 101, 432–450. [Google Scholar] [CrossRef]
- Leuliet, J.-C. Comportements Hydraulique et Thermique des Echangeurs à Plaques Traitant des Produits Non-Newtoniens. 1988. Available online: http://www.theses.fr/1988NAN10450 (accessed on 12 October 2020).
- Cooper, A. Protein Heat Capacity: An Anomaly that Maybe Never Was. J. Phys. Chem. Lett 2010, 1, 4. [Google Scholar] [CrossRef]
- Khaldi, M.; Ronse, G.; André, C.; Blanpain-Avet, P.; Bouvier, L.; Six, T.; Bornaz, S.; Croguennec, T.; Jeantet, R.; Delaplace, G. Denaturation Kinetics of Whey Protein Isolate Solutions and Fouling Mass Distribution in a Plate Heat Exchanger. Int. J. Chem. Eng. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Nielsen, B.T.; Singh, H.; Latham, J.M. Aggregation of bovine β-lactoglobulins A and B on heating at 75 °C. Int. Dairy J. 1996, 6, 519–527. [Google Scholar] [CrossRef]
- Lindmark-Månsson, H.; Timgren, A.; Aldén, G.; Paulsson, M. Two-dimensional gel electrophoresis of proteins and peptides in bovine milk. Int. Dairy J. 2005, 15, 111–121. [Google Scholar] [CrossRef]
- Oldfield, D.J.; Singh, H.; Taylor, M.W. Kinetics of heat-induced whey protein denaturation and aggregation in skim milks with adjusted whey protein concentration. J. Dairy Res. 2005, 72, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Anema, S.G.; McKenna, A.B. Reaction Kinetics of Thermal Denaturation of Whey Proteins in Heated Reconstituted Whole Milk. J. Agric. Food Chem. 1996, 44, 422–428. [Google Scholar] [CrossRef]
- Petit, J.; Herbig, A.-L.; Moreau, A.; Delaplace, G. Influence of calcium on β-lactoglobulin denaturation kinetics: Implications in unfolding and aggregation mechanisms. J. Dairy Sci. 2011, 94, 5794–5810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, J.W.F.A.; Kosters, H.A.; Visschers, R.W.; De Jongh, H.H.J. Role of calcium as trigger in thermal β-lactoglobulin aggregation. Arch. Biochem. Biophys. 2002, 406, 143–152. [Google Scholar] [CrossRef]
- Phan-Xuan, T.; Durand, D.; Nicolai, T.; Donato, L.; Schmitt, C.; Bovetto, L. Heat induced formation of beta-lactoglobulin microgels driven by addition of calcium ions. Food Hydrocoll. 2014, 34, 227–235. [Google Scholar] [CrossRef]
- Simmons, M.J.H.; Jayaraman, P.; Fryer, P.J. The effect of temperature and shear rate upon the aggregation of whey protein and its implications for milk fouling. J. Food Eng. 2007, 79, 517–528. [Google Scholar] [CrossRef]
- Burton, H. A comparison between a hot-wire laboratory apparatus and a plate heat exchanger for determining the sensitivity of milk to deposit formation. J. Dairy Res 1966, 33, 324. [Google Scholar] [CrossRef]
- Jeyarajah, S.; Allen, J.C. Calcium Binding and Salt-Induced Structural Changes of Native and Preheated β-Lactoglobulin. J. Agric. Food Chem. 1994, 42, 80–85. [Google Scholar] [CrossRef]
- Petit, J.; Moreau, A.; Ronse, G.; Debreyne, P.; Bouvier, L.; Blanpain-Avet, P.; Jeantet, R.; Delaplace, G. Role of Whey Components in the Kinetics and Thermodynamics of β-Lactoglobulin Unfolding and Aggregation. Food Bioprocess Technol. 2016, 9, 1367–1379. [Google Scholar] [CrossRef]
- Galani, D.; Apenten, R.K. Heat-induced denaturation and aggregation of beta-Lactoglobulin: Kinetics of formation of hydrophobic and disulphide-linked aggregates. Int. J. Food Sci. Technol. 1999, 34, 467–476. [Google Scholar] [CrossRef]
- Sava, N.; Van der Plancken, I.; Claeys, W.; Hendrickx, M. The Kinetics of Heat-Induced Structural Changes of β-Lactoglobulin. J. Dairy Sci. 2005, 88, 1646–1653. [Google Scholar] [CrossRef]
- Tolkach, A.; Kulozik, U. Reaction kinetic pathway of reversible and irreversible thermal denaturation of β-lactoglobulin. Lait 2007, 87, 301–315. [Google Scholar] [CrossRef] [Green Version]
- Dannenberg, F.; Kessler, H.-G. Reaction Kinetics of the Denaturation of Whey Proteins in Milk. J. Food Sci. 1988, 53, 258–263. [Google Scholar] [CrossRef]
- Blanpain-Avet, P.; Hédoux, A.; Guinet, Y.; Paccou, L.; Petit, J.; Six, T.; Delaplace, G. Analysis by Raman spectroscopy of the conformational structure of whey proteins constituting fouling deposits during the processing in a heat exchanger. J. Food Eng. 2012, 110, 86–94. [Google Scholar] [CrossRef]


| P Concentration (mg/L) | |||
|---|---|---|---|
| Denaturation Parameter | 0 | 10 | 50 |
| Unfolding | |||
| EAunf (kJ/mol) | 343.3 | 327.9 | 296.5 |
| ln(kounf) | 111.7 | 106.6 | 95.5 |
| Aggregation | |||
| EAagg (kJ/mol) | 119.2 | 140.1 | 52.6 |
| ln(koagg) | 37.0 | 43.5 | 13.8 |
| P Concentration (mg/L) | Non-Aggregated Protein Concentration (g/L) | Aggregation Level (%) | |
|---|---|---|---|
| Inlet (60 °C) | Outlet (85 °C) | ||
| 0 | 3.13 | 0.97 | 69 |
| 10 | 3.07 | 0.96 | 69 |
| 50 | 3.08 | 2.21 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scudeller, L.A.; Blanpain-Avet, P.; Six, T.; Bellayer, S.; Jimenez, M.; Croguennec, T.; André, C.; Delaplace, G. Calcium Chelation by Phosphate Ions and Its Influence on Fouling Mechanisms of Whey Protein Solutions in a Plate Heat Exchanger. Foods 2021, 10, 259. https://doi.org/10.3390/foods10020259
Scudeller LA, Blanpain-Avet P, Six T, Bellayer S, Jimenez M, Croguennec T, André C, Delaplace G. Calcium Chelation by Phosphate Ions and Its Influence on Fouling Mechanisms of Whey Protein Solutions in a Plate Heat Exchanger. Foods. 2021; 10(2):259. https://doi.org/10.3390/foods10020259
Chicago/Turabian StyleScudeller, Luisa A., Pascal Blanpain-Avet, Thierry Six, Séverine Bellayer, Maude Jimenez, Thomas Croguennec, Christophe André, and Guillaume Delaplace. 2021. "Calcium Chelation by Phosphate Ions and Its Influence on Fouling Mechanisms of Whey Protein Solutions in a Plate Heat Exchanger" Foods 10, no. 2: 259. https://doi.org/10.3390/foods10020259






