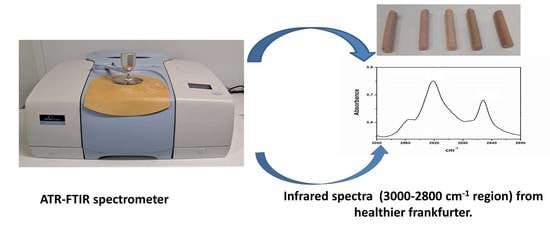
Development of Meat Products with Healthier Lipid Content: Vibrational Spectroscopy
Abstract
:1. Introduction
2. Meat and Meat Products with a Healthier Lipid Content
Lipid Materials Based on Structured Lipid: Emulsion Gels and Oil Bulking Agents
3. Basic Concepts of Vibrational Spectroscopic Techniques: Infrared and Raman Spectroscopy
3.1. Analysis of Infrared and Raman Spectra Data
3.1.1. Infrared Spectra
3.1.2. Raman Spectra
4. Application of Vibrational Spectroscopy in Meat Products with Healthier Lipid Content
4.1. Infrared and Raman Spectroscopy to Study Lipid Material Based on Structured Lipids
4.2. Infrared and Raman Spectroscopy to Examine Meat Products with Healthier Lipid Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiménez-Colmenero, F. Healthier lipid formulation approaches in meat-based functional foods. Technological options for replacement of meat fats by non-meat fats. Trends Food Sci. Technol. 2007, 18, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Herrero, A.M.; Ruiz-Capillas, C. Novel lipid materials based on gelling procedures as fat analogues in the development of healthier meat products. Curr. Opin. Food Sci. 2021, 39, 1–6. [Google Scholar] [CrossRef]
- Pintado, T.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Carmona, P.; Herrero, A.M. Oil-in-water emulsion gels stabilized with chia (Salvia hispanica L.) and cold gelling agents: Technological and infrared spectroscopic characterization. Food Chem. 2015, 185, 470–478. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Polysaccharide gels as oil bulking agents: Technological and structural properties. Food Hydrocoll. 2014, 36, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Herrero, A.M.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Carmona, P. Raman spectroscopic study of structural changes upon chilling storage of frankfurters containing olive oil bulking agents as fat replacers. J. Agric. Food Chem. 2014, 62, 5963–5971. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Herrero, A.M.; Ruiz-Capillas, C.; Triki, M.; Carmona, P.; Jiménez-Colmenero, F. Effects of emulsion gels containing bioactive compounds on sensorial, technological and structural properties of frankfurters. Food Sci. Technol. Int. 2016, 22, 132–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poyato, C.; Ansorena, D.; Astiasarán, I. Linseed oil gelled emulsion: A successful fat replacer in dry fermented sausages. Meat Sci. 2016, 121, 107–113. [Google Scholar]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Pasqualin Cavalheiro, C.; Ruiz-Capillas, C. Chia and oat emulsion gels as new animal fat replacers and healthy bioactive sources in fresh sausage formulation. Meat Sci. 2018, 135, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Ansorena, D.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I. Influence of a gel emulsion containing microalgal oil and a blackthorn (Prunus spinosa L.) branch extract on the antioxidant capacity and acceptability of reduced-fat beef patties. Meat Sci. 2019, 148, 219–222. [Google Scholar]
- Domínguez, R.; Munekata, P.E.S.; Pateiro, M.; López-Fernández, O.; Lorenzo, J.M. Immobilization of oils using hydrogels as strategy to replace animal fats and improve the healthiness of meat products. Curr. Opin. Food Sci. 2021, 37, 135–144. [Google Scholar] [CrossRef]
- Lucas-González, R.; Roldán-Verdu, A.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of emulsion gels formulated with chestnut (Castanea sativa M.) flour and chia (Salvia hispanica L.) oil as partial fat replacers in pork burger formulation. J. Sci. Food Agric. 2020, 100, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M. Raman spectroscopy a promising technique for quality assessment of meat and fish: A review. Food Chem. 2008, 107, 1642–1651. [Google Scholar] [CrossRef]
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases; WHO Technical Report Series No. 916; WHO: Geneva, Switzerland, 2003; pp. 1–149. [Google Scholar]
- Paglarini, C.S.; Vidal, V.A.S.; Martini, S.; Cunha, R.L.; Rosiane, L.; Pollonio, M.A.R. Protein-based hydrogelled emulsions and their application as fat replacers in meat products. A review. Crit. Rev. Food Sci. 2020, 1–16. [Google Scholar] [CrossRef]
- Zong, G.; Li, Y.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Willett, W.C.; Hu, F.B.; Sun, Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: Two prospective longitudinal cohort studies. BMJ 2016, 355, i5796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. American heart association. dietary fats and cardiovascular disease: A presidential advisory from the american heart association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.W.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M.K. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef]
- FAO/INFOODS. FAO/INFOODS Guidelines for Checking Food Composition Data Prior to the Publication of a User Table/Database-Version 1.0; FAO: Rome, Italy, 2012; Available online: http://www.fao.org/fileadmin/templates/food_composition/documents/pdf/Guidelines_data_checking2012.pdf (accessed on 21 December 2020).
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Triki, M.; Herrero, A.M.; Rodríguez-Salas, L.; Ruiz-Capillas, C. Healthy oil combination stabilized in a konjac matrix as pork fat replacement in low-fat, PUFA-enriched, dry fermented sausages. LWT Food Sci. Technol. 2013, 51, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Heck, R.; Saldaña, E.; Lorenzo, J.; Correa, L.; Fagundes, M.; Cichoski, A.; de Menezes, C.; Wagner, R.; Campagnol, P. Hydrogelled emulsion from chia and linseed oils: A promising strategy to produce low-fat burgers with a healthier lipid profile. Meat Sci. 2019, 156, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Schrader, B. Infrared and Raman Spectroscopy. In Methods and Applications; Weinheim, V.C.H., Ed.; Verlagsgesellschaft mbH: Weinheim, Germany, 1995. [Google Scholar]
- Herrero, A.M. Raman spectroscopy for monitoring protein structure in muscle food systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 512–523. [Google Scholar] [CrossRef]
- Damez, J.L.; Clerjon, S. Meat quality assessment using biophysical methods related to meat structure. Meat Sci. 2008, 80, 132–149. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Applications of Vibrational Spectroscopy to Study Protein Structural Changes in Muscle and Meat Batter Systems. In Applications of Vibrational Spectroscopy to Food Science; Chalmers, J., Griffiths, P., Li-Chan, E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 315–328. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta. (BBA) 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillén, M.D.; Cabo, N. Characterization of edible oils and lard by Fourier transform infrared spectroscopy. Relationships between composition and frequency of concrete bands in the fingerprint region. J. Am. Oil Chem. Soc. 1997, 74, 1281–1286. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- van de Voort, F.R.; Sedman, J.; Russin, T. Lipids analysis by vibrational spectroscopy. Eur. J. Lipid Sci. Technol. 2001, 103, 815–840. [Google Scholar] [CrossRef]
- Fraile, M.V.; Patrón-Gallardo, B.; López-Rodríguez, G.; Carmona, P. FT-IR study of multilamellar lipid dispersions containing cholesteryl linoleate and dipalmitoylphosphatidylcholine. Chem. Phys. Lipids 1999, 97, 119–128. [Google Scholar] [CrossRef]
- Tuma, R. Raman spectroscopy of proteins: From peptides to large assemblies. J. Raman Spectrosc. 2005, 36, 307–319. [Google Scholar] [CrossRef]
- Carmona, P.; Ramos, J.M.; de Cózar, M.; Monreal, J. Conformational features of lipids and proteins in myelin membranes using Raman and infrared spectroscopy. J. Raman Spectrosc. 1987, 18, 473–476. [Google Scholar] [CrossRef]
- Levin, I.W.; Lewis, E.N. Fourier transform Raman spectroscopy of biological materials. Anal. Chem. 1990, 62, 1101A–1111A. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, S.E.M.; Nielsen, O.F. Low-frequency Raman spectroscopy. J. Mol. Struct. 1995, 347, 267–284. [Google Scholar] [CrossRef]
- Maeda, Y.; Kitano, H. The structure of water in polymer systems as revealed by Raman spectroscopy. Spectrochim. Acta Part A 1995, 51A, 2433–2446. [Google Scholar] [CrossRef]
- Beattie, J.R.; Bell, S.E.J.; Borgaard, C.; Fearon, A.; Moss, B.W. Prediction of adipose tissue composition using Raman spectroscopy: Average properties and individual fatty acids. Lipids 2006, 41, 287–294. [Google Scholar] [CrossRef]
- Olsen, E.F.; Rukke, E.O.; Flatten, A.; Isaksson, T. Quantitative determination of saturated-, monounsaturated- and polyunsaturated fatty acids in pork adipose tissue with non-destructive Raman spectroscopy. Meat Sci. 2007, 76, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Ju-Yong, L.; Jin-Ho, P.; Hyoyoung, M.; Won-Bo, S.; Sang-Hyun, L.; Min-Gon, K. Quantitative analysis of lard in animal fat mixture using visible Raman spectroscopy. Food Chem. 2018, 254, 109–114. [Google Scholar]
- Tao, F.F.; Ngadi, M. Recent advances in rapid and nondestructive determination of fat content and fatty acids composition of muscle foods. Crit. Rev. Food Sci. 2018, 58, 1565–1593. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M.Z. Perspective of vibrational spectroscopy analytical methods in on-field/official control of olives and virgin olive oil. Eur. J. Lipid Sci. Technol. 2017, 119, 1600148. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J.; Paradkar, M.M. Discriminant analysis of edible oils and fats by FTIR, FT-NIR and FT-Raman spectroscopy. Food Chem. 2005, 93, 25–32. [Google Scholar] [CrossRef]
- Fang, Y.; Dalgleish, D.G. The conformation of α-lactalbumin as a function of pH, heat treatment and adsorption at hydrophobic surfaces studied by FTIR. Food Hydrocoll. 1998, 12, 121–126. [Google Scholar] [CrossRef]
- Lefèvre, T.; Subirade, M. Formation of intermolecular β-sheet structures: A phenomenon relevant to protein film structure at oil–water interfaces of emulsions. J. Coll. Interface Sci. 2003, 263, 59–67. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Pintado, T.; Jiménez-Colmenero, F.; Ruíz-Capillas, C. Infrared spectroscopic analysis of structural features and interactions in olive oil-in-water emulsions stabilized with soy protein. Food Res. Int. 2011, 44, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Herrero, A.M.; Carmona, P.; Pintado, T.; Jiménez-Colmenero, F.; Ruíz-Capillas, C. Olive oil-in-water emulsions stabilized with caseinate: Elucidation of protein-lipid interactions by infrared spectroscopy. Food Hydrocoll. 2011, 25, 12–18. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Ruiz-Capillas, C.; Salvador, M.; Herrero, A.M. Emulsion gels as delivery systems for phenolic compounds: Nutritional, technological and structural properties. Food Chem. 2021, 339, 128049. [Google Scholar] [CrossRef] [PubMed]
- Carmona, P.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Pintado, T.; Herrero, A.M. Infrared study of structural characteristics of frankfurters formulated with olive oil-in-water emulsions stabilized with casein as pork backfat replacer. J. Agric. Food Chem. 2011, 59, 12998–13003. [Google Scholar] [CrossRef]
- Herrero, A.M.; Ruiz-Capillas, C.; Pintado, T.; Carmona, P.; Jiménez-Colmenero, F. Infrared spectroscopy used to determine effects of chia and olive oil incorporation strategies on lipid structure of reduced-fat frankfurters. Food Chem. 2017, 221, 1333–1339. [Google Scholar] [CrossRef]
- Pintado, T.; Muñoz-González, I.; Salvador, M.; Ruiz-Capillas, C.; Herrero, A.M. Phenolic compounds in emulsion gel-based delivery systems applied as animal fat replacers in frankfurters: Physico-chemical, structural and microbiological approach. Food Chem. 2021, 340, 128095. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Capillas, C.; Herrero, A.M. Development of Meat Products with Healthier Lipid Content: Vibrational Spectroscopy. Foods 2021, 10, 341. https://doi.org/10.3390/foods10020341
Ruiz-Capillas C, Herrero AM. Development of Meat Products with Healthier Lipid Content: Vibrational Spectroscopy. Foods. 2021; 10(2):341. https://doi.org/10.3390/foods10020341
Chicago/Turabian StyleRuiz-Capillas, Claudia, and Ana M. Herrero. 2021. "Development of Meat Products with Healthier Lipid Content: Vibrational Spectroscopy" Foods 10, no. 2: 341. https://doi.org/10.3390/foods10020341
APA StyleRuiz-Capillas, C., & Herrero, A. M. (2021). Development of Meat Products with Healthier Lipid Content: Vibrational Spectroscopy. Foods, 10(2), 341. https://doi.org/10.3390/foods10020341