Effect of Storage on the Nutritional Quality of Queen Garnet Plum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Physicochemical Properties
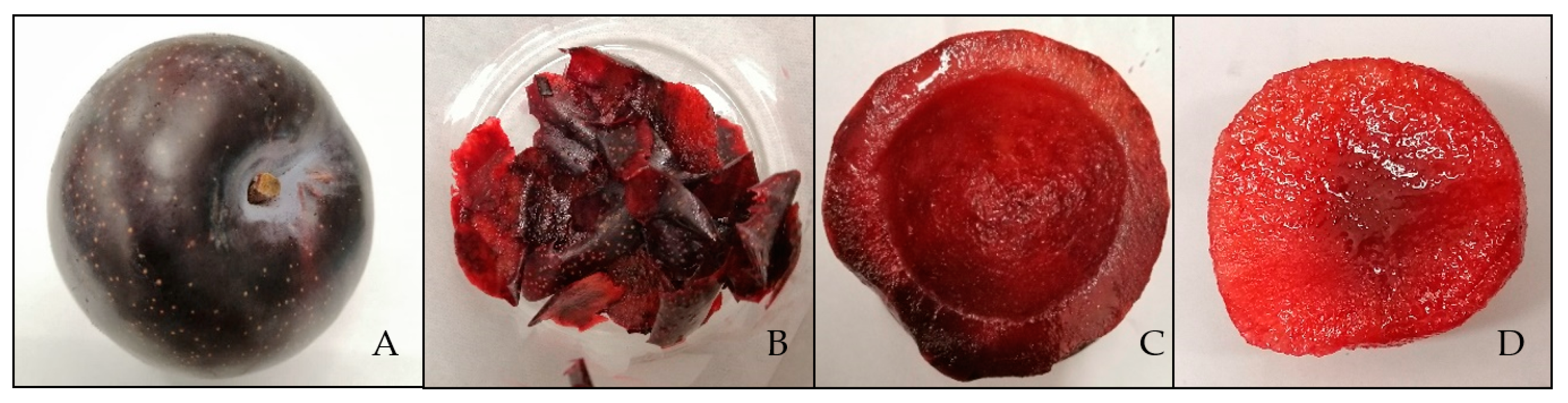
2.2.1. Color Measurement
2.2.2. Total Soluble Solids (TSS) and Titratable Acidity (TA)
2.3. Determination of Total Phenolic Content and Analysis of Anthocyanins
2.3.1. Extraction
2.3.2. TPC
2.3.3. Anthocyanins
2.4. Analysis of Carotenoids
2.4.1. Extraction
2.4.2. Analysis
2.5. Analysis of Sugars
2.5.1. Extraction
2.5.2. Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Phytochemicals
3.2.1. Total Phenolics
3.2.2. Anthocyanins
3.2.3. Carotenoids
3.3. Sugar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes—A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Heidor, R.; Miranda, M.L.P.; Ong, T.P.; Moreno, F.S. Bioactive Compounds from Fruits and Vegetables and Cancer Prevention. In Nutrition and Cancer Prevention: From Molecular Mechanisms to Dietary Recommendations; The Royal Society of Chemistry: London, UK, 2000; pp. 13–26. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.-Y.; Li, H.-B. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Sharp, S.J.; Imamura, F.; Chowdhury, R.; Gundersen, T.E.; Steur, M.; Sluijs, I.; van der Schouw, Y.T.; Agudo, A.; Aune, D.; et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ 2020, 370, m2194. [Google Scholar] [CrossRef]
- Fanning, K.J.; Topp, B.; Russell, D.; Stanley, R.; Netzel, M. Japanese plums (Prunus salicina Lindl.) and phytochemicals-breeding, horticultural practice, postharvest storage, processing and bioactivity. J. Sci. Food Agric. 2014, 94, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.M.; Rizwan, H.M.; Li, P.; Luo, S.X.; Sherameti, I.; Wu, W.F.; Lin, J.; Zheng, S.X.; Oelmüller, R.; Chen, F.X. Comparative studies on the physiochemical properties, phenolic compounds and antioxidant activities in 13 Japanese plum cultivars grown in the subtropical region of China. Appl. Ecol. Environ. Res. 2020, 18, 3147–3159. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food. Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Lozano, M.; Vidal-Aragón, M.C.; Hernández, M.T.; Ayuso, M.C.; Bernalte, M.J.; García, J.; Velardo, B. Physicochemical and nutritional properties and volatile constituents of six Japanese plum (Prunus salicina Lindl.) cultivars. Eur. Food Res. Technol. 2009, 228, 403–410. [Google Scholar] [CrossRef]
- Bobrich, A.; Fanning, K.J.; Rychlik, M.; Russell, D.; Topp, B.; Netzel, M. Phytochemicals in Japanese plums: Impact of maturity and bioaccessibility. Food Res. Int. 2014, 65, 20–26. [Google Scholar] [CrossRef]
- Fanning, K.; Edwards, D.; Netzel, M.; Stanley, R.; Netzel, G.; Russell, D.; Topp, B. Increasing Anthocyanin Content in Queen Garnet Plum and Correlations with In-Field Measures. Acta Hortic. 2013, 985, 97–104. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Srivastav, M.; Varghese, E. Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Sci. Hortic. 2017, 226, 104–109. [Google Scholar] [CrossRef]
- Wang, J.; Pan, H.; Wang, R.; Hong, K.; Cao, J. Patterns of flesh reddening, translucency, ethylene production and storability of ‘Friar’ plum fruit harvested at three maturity stages as affected by the storage temperature. Postharvest Biol. Technol. 2016, 121, 9–18. [Google Scholar] [CrossRef]
- Russell, D.; Topp, B. Queen Garnet. Plant Var. 2011, 24, 141–143. [Google Scholar]
- Netzel, M.; Fanning, K.; Netzel, G.; Zabaras, D.; Karagianis, G.; Treloar, T.; Russell, D.; Stanley, R. Urinary excretion of antioxidants in healthy humans following queen garnet plum juice ingestion: A new plum variety rich in antioxidant compounds. J. Food Biochem. 2012, 36, 159–170. [Google Scholar] [CrossRef]
- Bhaswant, M.; Fanning, K.; Netzel, M.; Mathai, M.L.; Panchal, S.K.; Brown, L. Cyanidin 3-glucoside improves diet-induced metabolic syndrome in rats. Pharmacol. Res. 2015, 102, 208–217. [Google Scholar] [CrossRef]
- Ghattamaneni, N.K.R.; Panchal, S.K.; Brown, L. Cyanidin 3-glucoside from Queen Garnet plums and purple carrots attenuates DSS-induced inflammatory bowel disease in rats. J. Funct. Foods 2019, 56, 194–203. [Google Scholar] [CrossRef]
- Igwe, E.O.; Charlton, K.E.; Roodenrys, S.; Kent, K.; Fanning, K.; Netzel, M.E. Anthocyanin-rich plum juice reduces ambulatory blood pressure but not acute cognitive function in younger and older adults: A pilot crossover dose-timing study. Nutr. Res. 2017, 47, 28–43. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Kundur, A.R.; Sabapathy, S.; Stanley, R.; Singh, I. The potential of anthocyanin-rich Queen Garnet plum juice supplementation in alleviating thrombotic risk under induced oxidative stress conditions. J. Funct. Foods 2015, 14, 747–757. [Google Scholar] [CrossRef]
- Guerra, M.; Casquero, P.A. Effect of harvest date on cold storage and postharvest quality of plum cv. Green Gage. Postharvest Biol. Technol. 2008, 47, 325–332. [Google Scholar] [CrossRef]
- Pan, H.; Wang, L.; Wang, R.; Xie, F.; Cao, J. Modifications of cell wall pectin in chilling-injured ’Friar’ plum fruit subjected to intermediate storage temperatures. Food Chem. 2018, 242, 538–547. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M. Changes in physicochemical and nutritive parameters and bioactive compounds during development and on-tree ripening of eight plum cultivars: A comparative study. J. Sci. Food Agric. 2008, 88, 2499–2507. [Google Scholar] [CrossRef]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Anthocyanin composition and changes during kernel development in purple-pericarp supersweet sweetcorn. Food Chem. 2020, 315. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical Characteristics and Antimicrobial Activity of Australian Grown Garlic (Allium Sativum L.) Cultivars. Foods 2019, 8, 358. [Google Scholar] [CrossRef]
- Calvo-Brenes, P.; Fanning, K.; O’Hare, T. Does kernel position on the cob affect zeaxanthin, lutein and total carotenoid contents or quality parameters, in zeaxanthin-biofortified sweet-corn? Food Chem. 2019, 277, 490–495. [Google Scholar] [CrossRef]
- Hong, H.T.; Phan, A.D.T.; O’Hare, T.J. Temperature and Maturity Stages Affect Anthocyanin Development and Phenolic and Sugar Content of Purple-Pericarp Supersweet Sweetcorn during Storage. J. Agric. Food. Chem. 2021, 69, 922–932. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Garner, D.; Crisosto, G.M.; Bowerman, E. Increasing ‘Blackamber’ plum (Prunus salicina Lindell) consumer acceptance. Postharvest Biol. Technol. 2004, 34, 237–244. [Google Scholar] [CrossRef]
- Myracle, A.D.; Castonguay, Z.J.; Elwell, A.; Moran, R.E. Fruit Quality and Consumer Acceptability of Three Plum Types and 14 Plum Cultivars Grown in Maine for a Local Market. HortTechnology 2018, 28, 230–238. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Echeverria, G.; Puy, J. Segregation of plum and pluot cultivars according to their organoleptic characteristics. Postharvest Biol. Technol. 2007, 44, 271–276. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Singh, Z.; Khan, A.S. Physiology of plum fruit ripening. Stewart Postharvest Rev. 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Voss, D.H. Relating colorimeter measurement of plant color to the royal-horticultural-society color chart. Hortscience 1992, 27, 1256–1260. [Google Scholar] [CrossRef]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Changes in hydrophilic and lipophilic antioxidant activity and related bioactive compounds during postharvest storage of yellow and purple plum cultivars. Postharvest Biol. Technol. 2009, 51, 354–363. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Byrne, D.; Okie, W.R.; Cisneros-Zevallos, L. Selecting new peach and plum genotypes rich in phenolic compounds and enhanced functional properties. Food Chem. 2006, 96, 273–280. [Google Scholar] [CrossRef]
- Wang, L.; Sang, W.; Xu, R.; Cao, J. Alteration of flesh color and enhancement of bioactive substances via the stimulation of anthocyanin biosynthesis in ’Friar’ plum fruit by low temperature and the removal. Food Chem. 2020, 310. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Veberič, R.; Štampar, F. Quality changes during ripening of plums (Prunus domestica L.). Food Chem. 2008, 111, 830–836. [Google Scholar] [CrossRef]
- Chaves-Silva, S.; Santos, A.L.d.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants—Tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yuan, C.; Chen, Y.C.; Li, H.; Liu, J.H. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vicente, A.R.; Crisosto, C.H.; Labavitch, J.M. Effect of delayed storage and continuous ethylene exposure on flesh reddening of ‘Royal Diamond’ plums. J. Sci. Food Agric. 2008, 88, 2180–2185. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z.; Swinny, E.E. Sugars and organic acids in Japanese plums (Prunus salicina Lindell) as influenced by maturation, harvest date, storage temperature and period. Int. J. Food Sci. Technol. 2009, 44, 1973–1982. [Google Scholar] [CrossRef]
- Martínez-Madrid, M.C.; López-Gómez, E.; Pretel, M.T.; Sánchez-Bel, P.; Egea, I.; García-Legaz, M.F. Changes in organic acid and sugars at ripening and after storage of three local plums. In Proceedings of the VIII International Postharvest Symposium, Cartagena, Spain, 21–24 June 2018; pp. 1025–1032. [Google Scholar]





| Physicochemical Property | Storage Temperature (°C) | Storage Time (Days) | ||||
|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 10 | 14 | ||
| Peel chroma | 4 | 11.35 ± 0.80 a,b,A | 12.56 ± 0.77 a,A | 11.89 ± 0.92 a,A | 9.82 ± 0.33 b,A | 11.25 ± 0.68 a,b,A |
| 23 | 11.35 ± 0.80 a,A | 7.85 ± 0.43 b,B | 5.87 ± 0.49 c,B | 4.82 ± 0.33 c,d,B | 3.58 ± 0.31 d,B | |
| OF chroma | 4 | 15.44 ± 0.83 b,A | 18.93 ± 0.97 a,A | 22.15 ± 0.77 a,A | 21.41 ± 1.69 a,A | 19.48 ± 1. 26 a,A |
| 23 | 15.44 ± 0.83 a,b,A | 15.78 ± 1.57 a,b,A | 18.21 ± 1.17 a,B | 12.86 ± 0.96 b,c,B | 10.46 ± 0.96 c,B | |
| IF chroma | 4 | 18.97 ± 1.15 b,A | 23.34 ± 0.62 a,A | 25.40 ± 1.29 a,A | 24.48 ± 1.45 a,A | 24.79 ± 1.21 a,A |
| 23 | 18.97 ± 1.15 a,A | 19.09 ± 1.34 a,B | 19.05 ± 1.29 a,B | 17.70 ± 1.20 a,B | 16.69 ± 1.18 a,B | |
| Peel hue | 4 | 9.28 ± 1.30 a,b,A | 12.06 ± 1.18 a,A | 7.21 ± 1.33 b,A | 6.32 ± 0.46 b,c,A | 8.56 ± 1.10 a,b,A |
| 23 | 9.28 ± 1.30 b,c,A | 7.75 ± 1.68 c,B | 14.58 ± 1.84 a,B | 12.03 ± 1.15 a,b,B | 14.21 ± 1.42 a,B | |
| OF hue | 4 | 17.83 ± 0.39 c,A | 18.41 ± 0.65 b,c,A | 20.55 ± 0.56 a,A | 19.82 ± 0.73 a,b,A | 19.94 ± 0.06 a,b,A |
| 23 | 17.83 ± 0.39 a,A | 17.72 ± 2.79 a,A | 15.60 ± 0.54 b,B | 13.57 ± 0.49 d,B | 15.17 ± 0.84 b,c,d,A | |
| IF hue | 4 | 31.16 ± 3.70 a,A | 27.19 ± 2.29 a,bA | 24.20 ± 1.68 b,A | 21.93 ± 0.72 b,A | 22.72 ± 1.18 b,A |
| 23 | 31.16 ± 3.70 a,A | 25.13 ± 3.04 a,A | 21.29 ± 0.89 b,A | 15.54 ± 1.72 b,c,B | 13.33 ± 0.76 c,B | |
| OF TSS | 4 | 18.5 ± 0.2 a,A | 18.2 ± 0.3 a,b,A | 16.7 ± 0.4 c,A | 17.3 ± 0.5 b,c,A | 16.8 ± 0.4 c,A |
| 23 | 18.5 ± 0.2 a,A | 18.5 ± 0.4 a,A | 18.7 ± 0.3 a,B | 18.7 ± 0.3 a,B | 17.8 ± 0.4 a,A | |
| IF TSS | 4 | 18.8 ± 0.2 a,A | 18.7 ± 0.7 a,A | 17.4 ± 0.4 b,A | 17.9 ± 0.4 b,A | 17.4 ± 0.3 b,A |
| 23 | 18.8 ± 0.2 a,A | 18.6 ± 0.3 a,A | 18.8 ± 0.3 a,B | 18.5 ± 0.2 a,A | 17.5 ± 0.3 b,A | |
| OF TA | 4 | 0.74 ± 0.05 c,A | 0.75 ± 0.02 c,b,A | 0.89 ± 0.04 a,A | 0.87 ± 0.03 a,A | 0.89 ± 0.04 a,A |
| 23 | 0.74 ± 0.05 a,A | 0.70 ± 0.05 a,A | 0.70 ± 0.03 a,B | 0.63 ± 0.03 a,b,B | 0.54 ± 0.00 b,B | |
| IF TA | 4 | 0.46 ± 0.04 c,A | 0.56 ± 0.03 b,A | 0.68 ± 0.02 a,A | 0.60 ± 0.03 b,A | 0.61 ± 0.03 a,b,A |
| 23 | 0.46 ± 0.04 a,b,A | 0.51 ± 0.06 a,A | 0.41 ± 0.02 c,b,B | 0.39 ± 0.02 c,b,B | 0.36 ± 0.02 c,B | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodagoda, G.; Hong, H.T.; O’Hare, T.J.; Sultanbawa, Y.; Topp, B.; Netzel, M.E. Effect of Storage on the Nutritional Quality of Queen Garnet Plum. Foods 2021, 10, 352. https://doi.org/10.3390/foods10020352
Kodagoda G, Hong HT, O’Hare TJ, Sultanbawa Y, Topp B, Netzel ME. Effect of Storage on the Nutritional Quality of Queen Garnet Plum. Foods. 2021; 10(2):352. https://doi.org/10.3390/foods10020352
Chicago/Turabian StyleKodagoda, Gethmini, Hung T. Hong, Tim J. O’Hare, Yasmina Sultanbawa, Bruce Topp, and Michael E. Netzel. 2021. "Effect of Storage on the Nutritional Quality of Queen Garnet Plum" Foods 10, no. 2: 352. https://doi.org/10.3390/foods10020352
APA StyleKodagoda, G., Hong, H. T., O’Hare, T. J., Sultanbawa, Y., Topp, B., & Netzel, M. E. (2021). Effect of Storage on the Nutritional Quality of Queen Garnet Plum. Foods, 10(2), 352. https://doi.org/10.3390/foods10020352










