Chemical, Physical, and Sensory Effects of the Use of Bentonite at Different Stages of the Production of Traditional Sparkling Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Winemaking and Experimental Design
2.2. Reagents and Standards
2.3. Volatile Compound Analysis
2.4. Determination of Foaming Properties
2.5. Sensorial Analyses
2.6. Statistics
3. Results and Discussion
3.1. Effects on Volatile Compound Profile
3.2. Impact on Foaming Properties
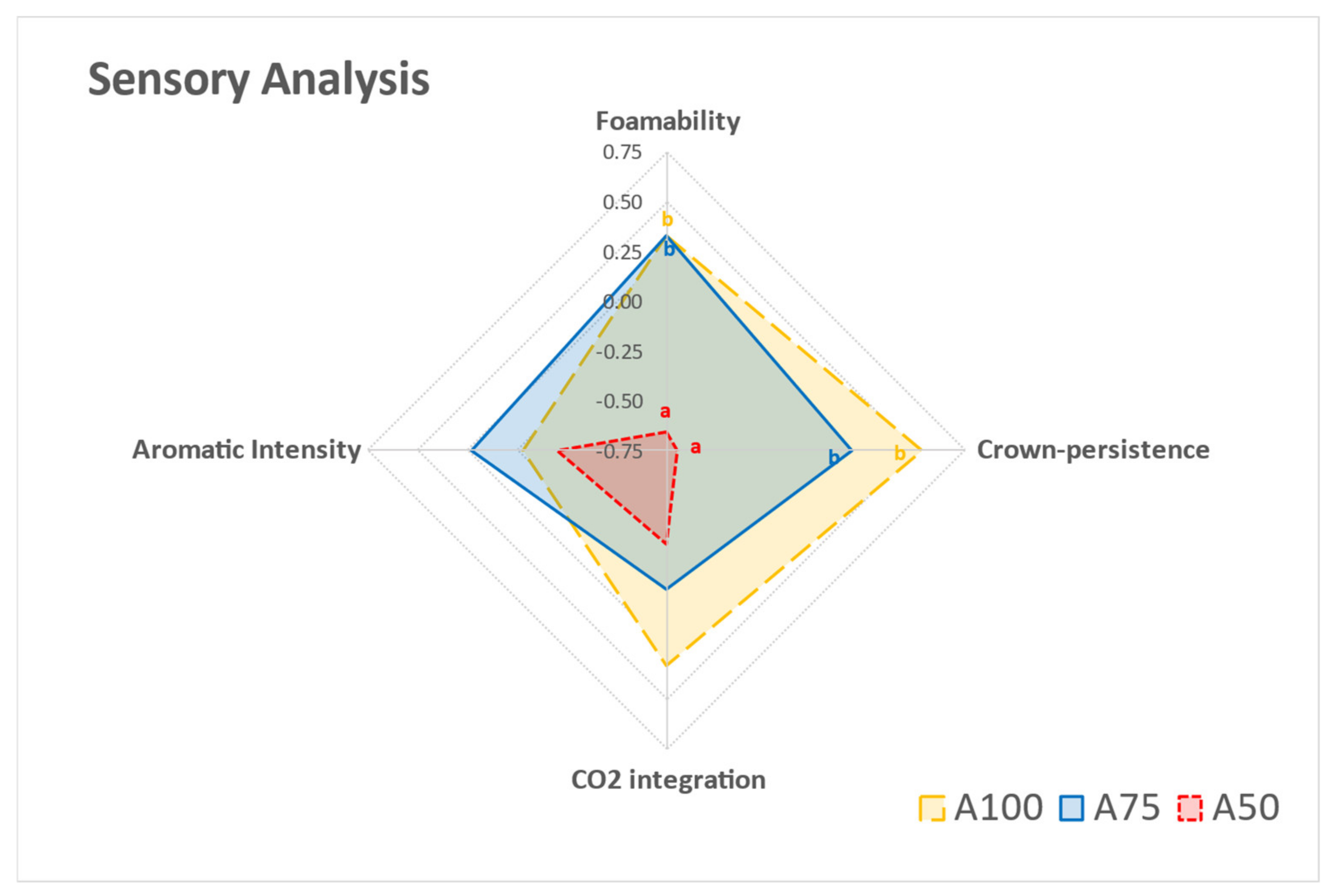
3.3. Sensory Effects
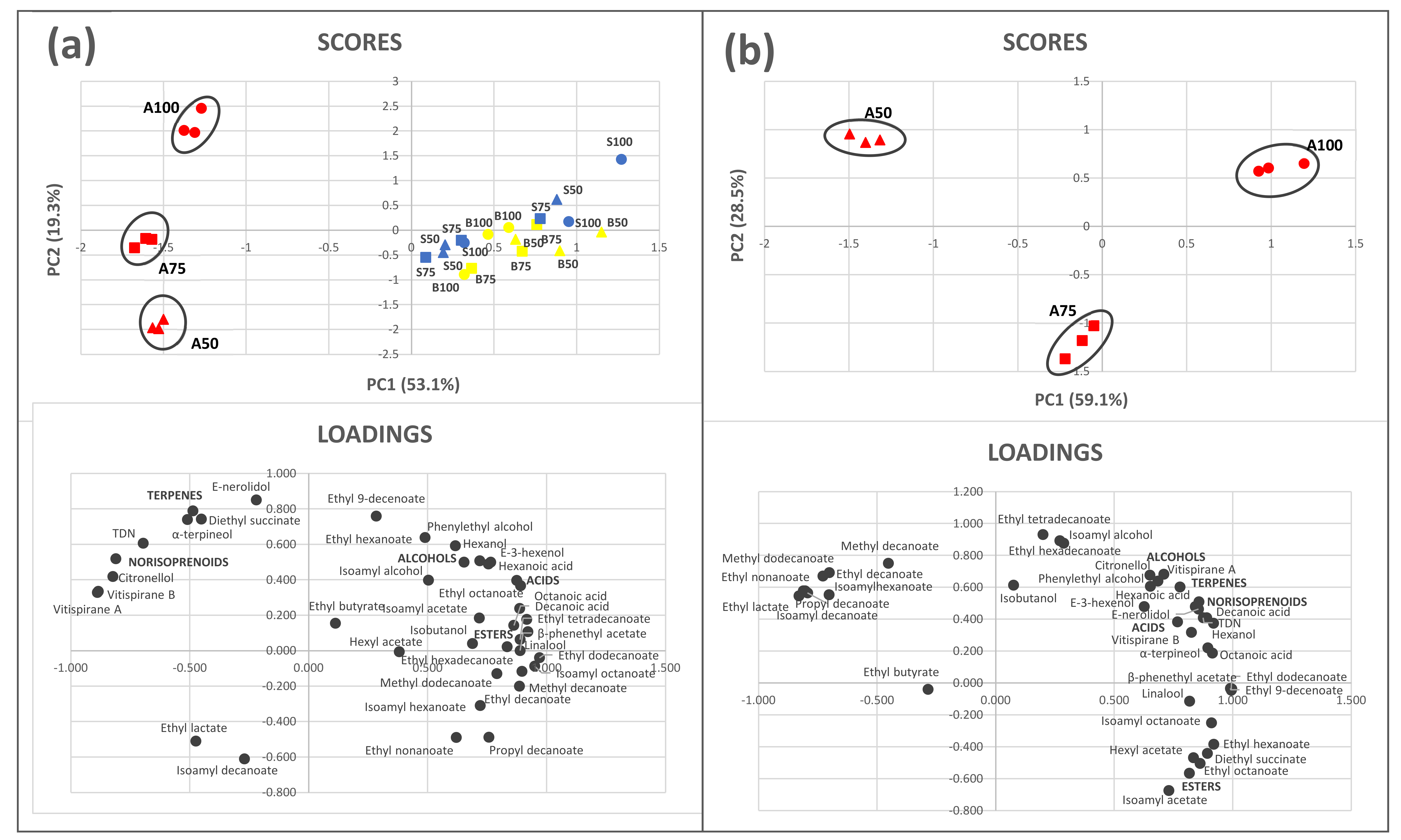
3.4. Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majewski, P.; Barbalet, A.; Waters, E.J. $1 billion hidden cost of bentonite fining. Aust. N. Z. Grapegrow. Winemak. 2011, 569, 58–62. [Google Scholar]
- Van Sluyter, S.C.; McRae, J.M.; Falconer, R.J.; Smith, P.A.; Bacic, A.; Waters, E.J.; Marangon, M. Wine protein haze: Mechanisms of formation and advances in prevention. J. Agric. Food Chem. 2015, 63, 4020–4030. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.J.; Alexander, G.; Muhlack, R.; Pocock, K.F.; Colby, C.; O’neill, B.K.; Høj, P.B.; Jones, P. Preventing protein haze in bottled white wine. Aust. J. Grape Wine Res. 2005, 11, 215–225. [Google Scholar] [CrossRef]
- Pocock, K.F.; Salazar, F.N.; Waters, E.J. The effect of bentonite fining at different stages of white winemaking on protein stability. Aust. J. Grape Wine Res. 2011, 17, 280–284. [Google Scholar] [CrossRef]
- Poinsaut, P.; Hardy, G. Bentonites. III. Utillisation des bentonites en oenologie. Revue des Oenologues 1995, 77, 29–34. [Google Scholar]
- Armada, L.; Falqué, E. Repercussion of the clarification treatment agents before the alcoholic fermentation on volatile composition of white wines. Eur. Food Res. Technol. 2007, 225, 553–558. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Effect of bentonite fining on odor-active compounds in two different white wine styles. Am. J. Enol. Vitic. 2010, 61, 225–233. [Google Scholar]
- Lambri, M.; Dordoni, R.; Giribaldi, M.; Violetta, M.R.; Giuffrida, M.G. Heat-unstable protein removal by different bentonite labels in white wines. LWT Food Sci. Technol. 2012, 46, 460–467. [Google Scholar] [CrossRef]
- Kemp, B.; Condé, B.; Jégou, S.; Howell, K.; Vasserot, Y.; Marchal, R. Chemical compounds and mechanisms involved in the formation and stabilization of foam in sparkling wines. Crit. Rev. Food Sci. Nutr. 2019, 59, 2072–2094. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Odor-active compound adsorption onto bentonite in a model white wine solution. Chem. Eng. Trans. 2013, 32, 1741–1746. [Google Scholar]
- Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A. Influence of yeast walls on the behavior of aroma compounds in a model wine. Am. J. Enol. Vitic. 1994, 45, 29–33. [Google Scholar]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of combined effect of proteins and bentonite fining on the wine aroma loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef]
- López-Barajas, M.; López-Tamames, E.; Buxaderas, S.; Suberbiola, G.; De la Torre-Boronat, M.C. Influence of wine polysaccharides of different molecular mass on wine foaming. Am. J. Enol. Vitic. 2001, 52, 146–150. [Google Scholar]
- Vincenzi, S.; Crapisi, A.; Curioni, A. Foamability of Prosecco wine: Cooperative effects of high molecular weight glycocompounds and wine PR-proteins. Food Hydrocoll. 2014, 34, 202–207. [Google Scholar] [CrossRef]
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Effect of production phase on bottle-fermented sparkling wine quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef]
- Vanrell, G.; Canals, R.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of the use of bentonite as a riddling agent on foam quality and protein fraction of sparkling wines (Cava). Food Chem. 2007, 104, 148–155. [Google Scholar] [CrossRef]
- Lira, E.; Rodríguez-Bencomo, J.J.; Salazar, F.N.; Orriols, I.; Fornos, D.; López, F. Impact of bentonite additions during vinification on protein stability and volatile compounds of Albariño wines. J. Agric. Food Chem. 2015, 63, 3004–3011. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Apolinar-Valiente, R.; Salmon, T.; Williams, P.; Nigen, M.; Sanchez, C.; Marchal, R.; Doco, T. Improvement of the foamability of sparkling base wines by the addition of Acacia gums. Food Chem. 2020, 313, 126062. [Google Scholar] [CrossRef]
- Chagas, R.; Monteiro, S.; Ferreira, R.B. Assessment of potential effects of common fining agents used for white wine protein stabilization. Am. J. Enol. Vitic. 2012, 63, 574–578. [Google Scholar] [CrossRef]
- Lira, E.; Salazar, F.N.; Rodríguez-Bencomo, J.J.; Vincenzi, S.; Curioni, A.; López, F. Effect of using bentonite during fermentation on protein stabilisation and sensory properties of white wine. Int. J. Food Sci. Technol. 2014, 49, 1070–1078. [Google Scholar] [CrossRef]
- Horvat, I.; Radeka, S.; Plavša, T.; Lukić, I. Bentonite fining during fermentation reduces the dosage required and exhibits significant side-effects on phenols, free and bound aromas, and sensory quality of white wine. Food Chem. 2019, 285, 305–315. [Google Scholar] [CrossRef]
- Esteruelas, M.; Poinsaut, P.; Sieczkowski, N.; Manteau, S.; Fort, M.F.; Canals, J.M.; Zamora, F. Characterization of natural haze protein in sauvignon white wine. Food Chem. 2009, 113, 28–35. [Google Scholar] [CrossRef]
- Ubeda, C.; Kania-Zelada, I.; del Barrio-Galán, R.; Medel-Marabolí, M.; Gil, M.; Peña-Neira, A. Study of the changes in volatile compounds, aroma and sensory attributes during the production process of sparkling wine by traditional method. Int. Food Res. J. 2019, 119, 554–563. [Google Scholar] [CrossRef]
- Maujean, A.; Poinsaut, P.; Dantan, H.; Brissonnet, F.; Cossiez, E. Study of the performance and quality of the foam in sparkling wines. 2. Perfecting of a measuring technique for foaming ability, performance and stability of the foam in sparkling wines [measurement of foam properties, bentonite, vegetable black]. Bull. de l’OIV 1991, 63, 405–427. [Google Scholar]
- Poinsaut, P. Le Mosalux, appareil de mesure du pouvoir moussant d’un vin. Revue des Oenologues 1991, 59, 35–43. [Google Scholar]
- Di Gaspero, M.; Ruzza, P.; Hussain, R.; Vincenzi, S.; Biondi, B.; Gazzola, D.; Siligardi, G.; Curioni, A. Spectroscopy reveals that ethyl esters interact with proteins in wine. Food Chem. 2017, 217, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Bayón, M.Á.; Hernández, M.T.; Martín-Álvarez, P.J.; Polo, M.C. Study of low molecular weight phenolic compounds during the aging of sparkling wines manufactured with red and white grape varieties. J. Agric. Food Chem. 2003, 51, 2089–2095. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. Hydrolysis and formation of volatile esters in New Zealand Sauvignon blanc wine. Food Chem. 2012, 135, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Chacon, J.J.; Vichi, S.; Lopez-Tamames, E.; Buxaderas, S. Changes in the sorption of diverse volatiles by Saccharomyces cerevisiae lees during sparkling wine aging. J. Agric. Food Chem. 2010, 58, 12426–12430. [Google Scholar] [CrossRef] [PubMed]
- Riu-Aumatell, M.; Bosch-Fusté, J.; López-Tamames, E.; Buxaderas, S. Development of volatile compounds of cava (Spanish sparkling wine) during long ageing time in contact with lees. Food Chem. 2006, 95, 237–242. [Google Scholar] [CrossRef]
- Ganss, S.; Kirsch, F.; Winterhalter, P.; Fischer, U.; Schmarr, H.G. Aroma changes due to second fermentation and glycosylated precursors in Chardonnay and Riesling sparkling wines. J. Agric. Food Chem. 2011, 59, 2524–2533. [Google Scholar] [CrossRef]
- Moio, L.; Ugliano, M.; Gambuti, A.; Genovese, A.; Piombino, P. Influence of clarification treatment on concentrations of selected free varietal aroma compounds and glycoconjugates in Falanghina (Vitis vinifera L.) must and wine. Am. J. Enol. Vitic. 2004, 55, 7–12. [Google Scholar]
- Torrens, J.; Riu-Aumatell, M.; Vichi, S.; Lopez-Tamames, E.; Buxaderas, S. Assessment of volatile and sensory profiles between base and sparkling wines. J. Agric. Food Chem. 2010, 58, 2455–2461. [Google Scholar] [CrossRef]
- Senée, J.; Robillard, B.; Vignes-Adler, M. Films and foams of Champagne wines. Food Hydrocoll. 1999, 13, 15–26. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, S.M.; Coimbra, M.A. Foamability and foam stability of molecular reconstituted model sparkling wines. J. Agric. Food Chem. 2011, 59, 8770–8778. [Google Scholar] [CrossRef] [PubMed]
- Jaeckels, N.; Tenzer, S.; Meier, M.; Will, F.; Dietrich, H.; Decker, H.; Fronk, P. Influence of bentonite fining on protein composition in wine. LWT Food Sci. Technol. 2017, 75, 335–343. [Google Scholar] [CrossRef]
- Kupfer, V.M.; Vogt, E.I.; Siebert, A.K.; Meyer, M.L.; Vogel, R.F.; Niessen, L. Foam-stabilizing properties of the yeast protein PAU5 and evaluation of factors that can influence its concentration in must and wine. Food Res. Int. 2017, 102, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. Olfactory impact of higher alcohols on red wine fruity ester aroma expression in model solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- De-La-Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.J.; Polo, M.C. Effect of the addition of bentonite to the tirage solution on the nitrogen composition and sensory quality of sparkling wines. Food Chem. 2003, 81, 383–388. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Polidori, G.; Zéninari, V. Unraveling the evolving nature of gaseous and dissolved carbon dioxide in Champagne wines: A state-of-the-art review, from the bottle to the tasting glass. Anal. Chim. Acta 2012, 732, 1–15. [Google Scholar] [CrossRef] [PubMed]

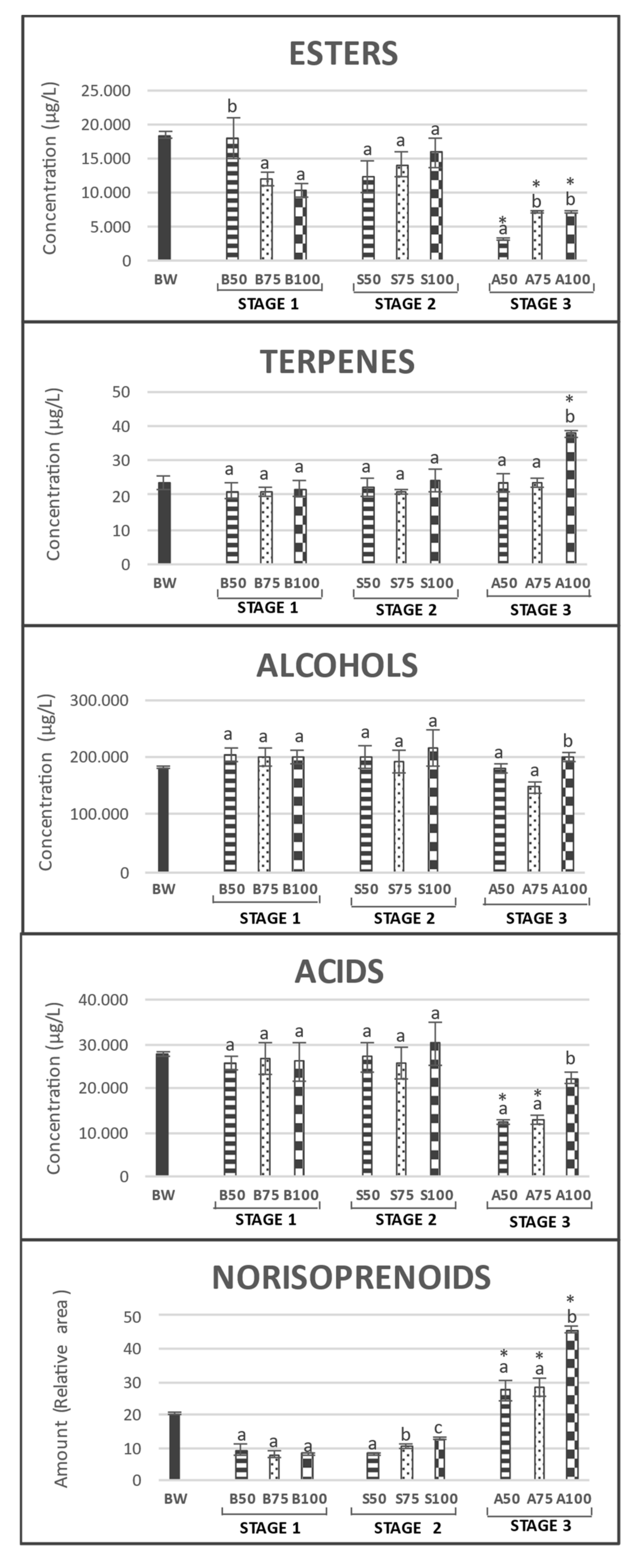

| Volatile Compounds | LRI | ID | Stage 0 | Stage 1 | Stage 2 | Stage 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | B50 | B75 | B100 | S50 | S75 | S100 | A50 | A75 | A100 | |||
| ESTERS | ||||||||||||
| Ethyl butyrate | 1055 | A | 798 ± 101 | 863 ± 245 b | 282 ± 56 a | 322 ± 88 a | 541 ± 139 a | 895 ± 221 a,b* | 1163 ± 80 b,* | 701 ± 59 a | 689 ± 62 a | 666 ± 88 a,* |
| Isoamyl acetate | 1122 | A | 10879 ± 19 | 8722 ± 1641 b | 4773 ± 1095 a | 3336 ± 829 a | 6019 ± 1166 a | 7311 ± 1187 a,* | 7873 ± 1093 a,* | 707 ± 12 a,* | 3677 ± 215 b | 3230 ± 144 b |
| Ethyl hexanoate | 1245 | A | 950 ± 70 | 988 ± 123 b | 597 ± 122 a | 653 ± 143 a | 836 ± 182 a | 804 ± 62 a | 963 ± 196 a | 6.47 ± 0.46 a,* | 758 ± 57 b | 844 ± 25 b |
| Hexyl acetate | 1285 | A | 608 ± 50 | 123 ± 21 a,b | 110 ± 19 a | 161 ± 35 b | 27.7 ± 3.8 a,* | 58.7 ± 5.5 b,* | 58.3 ± 14.2 b,* | 0.740 ± 0.062 a | 13.6 ± 3.0 b,* | 14.2 ± 1.0 b,* |
| Ethyl lactate | 1379 | A | 4.81 ± 1.40 | 4.66 ± 0.52 a | 4.91 ± 1.20 a | 5.13 ± 0.25 a | 5.11 ± 0.54 a | 5.93 ± 0.12 a | 5.41 ± 0.82 a | 103 ± 1 b,* | 6.11 ± 1.05 a | 10.1 ± 2.2 a |
| Ethyl octanoate | 1437 | A | 2440 ± 103 | 3166 ± 417 a | 2773 ± 146 a | 2596 ± 81 a | 2426 ± 419 a | 2449 ± 166 a | 2716 ± 397 a | 62 ± 23 a,* | 1516 ± 80 b,* | 1593 ± 37 b,* |
| Isoamyl hexanoate | 1468 | A | 3.73 ± 0.05 | 7.71 ± 0.58 b | 6.06 ± 1.11 a,b | 5.65 ± 0.92 a | 4.12 ± 0.56 a,* | 5.22 ± 0.89 a | 5.72 ± 1.73 a | 5.03 ± 1.81 b | 2.01 ± 0.09 a,* | 2.30 ± 0.10 a,* |
| Ethyl nonanoate | 1558 | A | 25.6 ± 0.7 | 43.9 ± 1.6 b | 35.4 ± 3.7 a | 32.1 ± 0.7 a | 35.3 ± 9.9 a | 36.7 ± 4.5 a | 38.7 ± 8.0 a | 49.9 ± 12.8 b | 4.51 ± 1.07 a,* | 6.54 ± 0.40 a,* |
| Methyl decanoate | 1600 | A | 13.8 ± 2.9 | 12.1 ± 1.5 a | 12.8 ± 0.6 a | 12.2 ± 2.4 a | 11.3 ± 2.2 a | 12.0 ± 0.3 a | 14.4 ± 3.8 a | 2.04 ± 0.07 b,* | 1.29 ± 0.29 a,* | 1.58 ± 0.21 a,b* |
| Ethyl decanoate | 1647 | A | 1548 ± 92 | 2773 ± 701 a | 2200 ± 252 a | 2141 ± 264 a | 1558 ± 393 a | 1608 ± 249 a,* | 1866 ± 485 a | 862 ± 74 b | 322 ± 54 a,* | 418 ± 18 a,* |
| Isoamy loctanoate | 1680 | A | 114 ± 31 | 205 ± 53 b | 146 ± 9 a,b | 117 ± 20 a | 123 ± 19 a | 93.3 ± 9.7 a,* | 137 ± 42 a | 6.15 ± 0.57 a,* | 13.5 ± 2.8 b,* | 16.2 ± 2.7 b,* |
| Diethy lsuccinate | 1675 | A | 11.5 ± 0.5 | 14.8 ± 2.6 a | 15.4 ± 3.2 a | 14.5 ± 3.7 a | 15.1 ± 1.9 a | 14.1 ± 1.9 a | 15.7 ± 2.7 a | nd a,* | 36.6 ± 3.2 b,* | 43.5 ± 2.0 c* |
| Ethyl 9-decenoate | 1698 | B | 11.4 ± 0.2 | 16.9 ± 3.5 a | 15.0 ± 1.2 a | 14.2 ± 0.6 a | 12.6 ± 1.8 a | 12.9 ± 0.8 a | 14.5 ± 1.6 a | 2.83 ± 0.37 a,* | 12.1 ± 0.9 b | 19.7 ± 1.4 c* |
| Propyl decanoate | 1725 | B | 4.88 ± 0.09 | 7.11 ± 1.22 a | 5.98 ± 1.03 a | 6.10 ± 0.67 a | 4.52 ± 0.77 a | 4.77 ± 0.28 a | 5.53 ± 0.96 a | 5.85 ± 0.73 b | 0.231 ± 0.031 a,* | 0.502 ± 0.031 a,* |
| Methyl dodecanoate | 1823 | B | 10.7 ± 2.4 | 4.55 ± 1.03 a | 6.57 ± 1.05 a,b | 8.51 ± 1.82 b | 7.39 ± 1.79 a | 7.63 ± 1.34 a | 8.90 ± 1.24 a | 2.06 ± 0.09 c* | 0.673 ± 0.091 a,* | 0.903 ± 0.032 b,* |
| β-phenethyl acetate | 1851 | A | 146 ± 7 | 108 ± 10 a | 120 ± 7 a | 120 ± 12 a | 78.4 ± 7.0 a,* | 89.3 ± 9.6 a,* | 95.2 ± 10.5 a,* | 1.91 ± 0.11 a,* | 21.7 ± 1.1 b,* | 38.9 ± 1.5 c* |
| Ethyl dodecanoate | 1864 | B | 769 ± 30 | 773 ± 72 a | 658 ± 100 a | 679 ± 65 a | 549 ± 108 a | 577 ± 86 a | 733 ± 111 a | 3.62 ± 1.20 a,* | 52.3 ± 6.8 b,* | 95.2 ± 6.4 c* |
| Isoamyl decanoate | 1888 | A | 10.2 ± 1.2 | 12.8 ± 2.4 a | 10.4 ± 2.1 a | 11.3 ± 1.6 a | 8.33 ± 2.71 a | 9.40 ± 1.33 a | 14.7 ± 2.7 b | 102 ± 10 b,* | 0.396 ± 0.015 a,* | 1.03 ± 0.18 a,* |
| Ethyl tetradecanoate | 2041 | B | 37.0 ± 8.7 | 37.6 ± 10.4 a | 34.2 ± 4.3 a | 25.1 ± 8.6 a | 29.7 ± 6.9 a | 31.6 ± 6.2 a | 32.8 ± 6.0 a | 18.3 ± 4.1 b | 7.26 ± 0.32 a,* | 19.8 ± 1.6 b |
| Ethyl hexadecanoate | 2235 | B | 29.6 ± 7.5 | 37.3 ± 10.8 a | 28.1 ± 7.4 a | 23.3 ± 4.6 a | 27.0 ± 7.5 a | 27.2 ± 7.3 a | 36.0 ± 2.0 a,* | 10.6 ± 3.6 b | 4.26 ± 0.60 a,* | 12.7 ± 0.9 b,* |
| ALCOHOLS | ||||||||||||
| Isobutanol | 1074 | A | 23,739 ± 2359 | 34,191 ± 2111 a | 35,845 ± 14 a | 33,422 ± 5141 a | 35,867 ± 2617 a | 30,621 ± 5268 a | 36,070 ± 4798 a | 27,497 ± 639 a,* | 22,265 ± 3510 a,* | 27,906 ± 6129 a |
| Isoamyl alcohol | 1200 | A | 118,133 ± 865 | 125,616 ± 10,888 a | 121,876 ± 9627 a | 124,744 ± 4829 a | 120,297 ± 13,684 a | 119,670 ± 11,248 a | 131,238 ± 19,875 a | 122,046 ± 2687 b | 96,350 ± 5931 a,* | 127,663 ± 11,937 b |
| Hexanol | 1375 | A | 6115 ± 172 | 7088 ± 435 a | 7113 ± 1177 a | 7044 ± 1239 a | 7584 ± 1221 a | 7176 ± 952 a | 8037 ± 1607 a | 3612 ± 118 a,* | 4688 ± 1077 a,b | 6511 ± 232 b |
| E-3-Hexenol | 1366 | A | 9002 ± 41 | 9693 ± 150 a | 9558 ± 964 a | 9756 ± 1126 a | 10,312 ± 1212 a | 10,184 ± 485 a | 11,116 ± 1881 a | 7607 ± 2026 a | 7318 ± 1286 a | 9784 ± 522 a |
| Phenylethyl alcohol | 1940 | A | 24,449 ± 181 | 26,394 ± 612 a | 26,299 ± 3228 a | 25,880 ± 4477 a | 26,412 ± 2834 a | 24,905 ± 3354 a | 28,541 ± 4636 a | 19,282 ± 4706 a,* | 16,019 ± 996 a,* | 28,327 ± 2418 b |
| TERPENES | ||||||||||||
| Linalool | 1555 | A | 5.86 ± 0.61 | 6.01 ± 0.58 a | 6.01 ± 0.79 a | 5.87 ± 1.32 a | 6.17 ± 0.51 a | 5.84 ± 0.57 a | 6.61 ± 0.94 a | 3.16 ± 0.08 a,* | 3.55 ± 0.30 a,* | 3.74 ± 0.17 a,* |
| α-terpineol | 1693 | A | 4.20 ± 0.20 | 3.11 ± 0.77 a | 3.17 ± 0.75 a | 3.49 ± 1.10 a | 3.80 ± 0.55 a | 3.74 ± 0.45 a | 4.19 ± 1.13 a | 3.80 ± 1.11 a | 4.25 ± 0.55 a | 6.49 ± 0.40 b |
| Citronellol | 1785 | A | 3.37 ± 0.48 | 3.19 ± 0.14 a | 3.23 ± 0.07 a | 3.41 ± 0.66 a | 3.01 ± 0.30 a | 2.91 ± 0.36 a | 3.42 ± 0.43 a | 8.82 ± 2.98 a,* | 7.47 ± 0.40 a,* | 13.0 ± 0.3 b,* |
| E-nerolidol | 2056 | A | 10.1 ± 0.6 | 8.70 ± 1.75 a | 8.54 ± 0.72 a | 9.13 ± 0.69 a | 9.12 ± 1.59 a | 8.44 ± 0.53 a | 9.82 ± 0.90 a | 7.90 ± 1.34 a | 8.53 ± 0.73 a | 14.4 ± 1.1 b,* |
| ACIDS | ||||||||||||
| Hexanoic acid | 1880 | A | 10,775 ± 196 | 10,428 ± 337 a | 11,431 ± 1808 a | 11,225 ± 2013 a | 11,777 ± 1122 a | 11,158 ± 1593 a | 12,604 ± 1792 a | 6053 ± 350 a,* | 5132 ± 275 a,* | 10,959 ± 797 b |
| Octanoic acid | 2076 | A | 12,124 ± 220 | 11,470 ± 655 a | 11,689 ± 1355 a | 11,071 ± 1642 a | 11,839 ± 1702 a | 11,387 ± 1532 a | 13,122 ± 2352 a | 5481 ± 761 a,* | 6533 ± 996 a,* | 9236 ± 407 b |
| Decanoic acid | 2339 | A | 4889 ± 25 | 3620 ± 587 a | 3696 ± 376 a | 3708 ± 619 a | 3414 ± 369 a | 3375 ± 491 a | 4474 ± 741 a | 1040 ± 112 a,* | 1190 ± 41 a,* | 1972 ± 77 b,* |
| NORISOPRENOIDS | ||||||||||||
| Vitispirane a | 1518 | B | 4.53 ± 0.40 | 2.13 ± 0.32 a | 1.86 ± 0.21 a | 1.81 ± 0.01 a | 2.21 ± 0.31 a | 2.59 ± 0.05 b | 3.14 ± 0.42 b | 9.93 ± 0.81 a,* | 8.73 ± 2.15 a,* | 8.56 ± 0.46 a,* |
| Vitispirane b | 1522 | B | 3.53 ± 0.82 | 1.50 ± 0.01 a | 1.65 ± 0.10 a | 1.44 ± 0.24 a | 1.50 ± 0.43 a | 1.93 ± 0.42 a | 2.27 ± 0.56 a | 6.62 ± 0.31 a,* | 6.88 ± 1.79 a,* | 5.77 ± 0.11 a,* |
| TDN | 1745 | B | 12.2 ± 0.1 | 5.69 ± 1.44 a | 4.53 ± 0.59 a | 4.82 ± 0.46 a | 4.69 ± 0.24 a | 6.04 ± 0.12 a,b | 7.43 ± 0.45 b | 10.9 ± 2.6 a | 12.4 ± 1.1 a,* | 16.2 ± 1.44 b,* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ubeda, C.; Lambert-Royo, M.I.; Gil i Cortiella, M.; Del Barrio-Galán, R.; Peña-Neira, Á. Chemical, Physical, and Sensory Effects of the Use of Bentonite at Different Stages of the Production of Traditional Sparkling Wines. Foods 2021, 10, 390. https://doi.org/10.3390/foods10020390
Ubeda C, Lambert-Royo MI, Gil i Cortiella M, Del Barrio-Galán R, Peña-Neira Á. Chemical, Physical, and Sensory Effects of the Use of Bentonite at Different Stages of the Production of Traditional Sparkling Wines. Foods. 2021; 10(2):390. https://doi.org/10.3390/foods10020390
Chicago/Turabian StyleUbeda, Cristina, María Ignacia Lambert-Royo, Mariona Gil i Cortiella, Rubén Del Barrio-Galán, and Álvaro Peña-Neira. 2021. "Chemical, Physical, and Sensory Effects of the Use of Bentonite at Different Stages of the Production of Traditional Sparkling Wines" Foods 10, no. 2: 390. https://doi.org/10.3390/foods10020390
APA StyleUbeda, C., Lambert-Royo, M. I., Gil i Cortiella, M., Del Barrio-Galán, R., & Peña-Neira, Á. (2021). Chemical, Physical, and Sensory Effects of the Use of Bentonite at Different Stages of the Production of Traditional Sparkling Wines. Foods, 10(2), 390. https://doi.org/10.3390/foods10020390






