Recent Developments in Solid Lipid Microparticles for Food Ingredients Delivery
Abstract
1. Introduction
1.1. Food Additives
1.2. Encapsulation of Phytochemicals and Food Additives
1.3. Examples of Microencapsulation of Bioactives in Foods
1.4. Microencapsulation of Phytochemicals and Food Additives Using Polysaccharides and Proteins
2. Solid Lipid Encapsulation
3. Ingredients Used for the Preparation of SLM
4. Methods for the Solid Lipid Microparticles Preparation
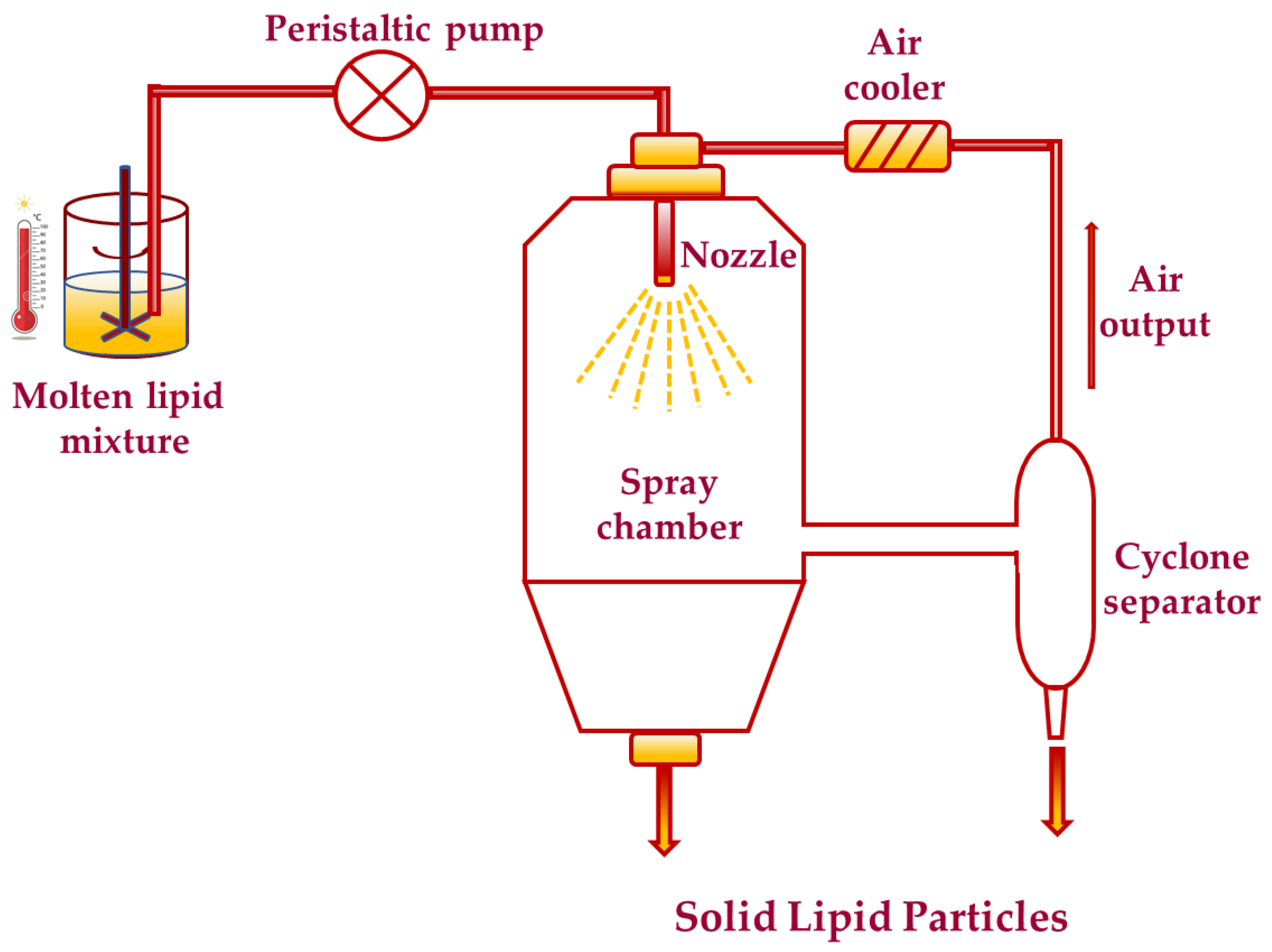
4.1. Spray Drying Technique
4.2. Spray Chilling Technique
4.3. High-Shear Homogenization
4.4. Melt Extrusion Technique
5. Characterization of SLMs
5.1. Particle Size and Size Distribution
5.2. Morphology Analysis
5.3. Encapsulation Efficiency
5.4. Stability of Encapsulated Bioactives
5.5. Gastrointestinal Stability of Encapsulated Phytochemicals and Food Additives
6. Examples of Encapsulated Bio-Additives
6.1. Essential Oils (EOs)
6.2. Flavors
6.2.1. Coffee
6.2.2. Ginger Oleoresin (GO)
6.3. Antioxidants
6.3.1. Quercetin
6.3.2. Cinnamon
6.3.3. Guaraná
6.3.4. Curcumin
6.4. Natural Colorants
6.4.1. β-Carotene
6.4.2. Lycopene
6.4.3. Genipap
6.4.4. Anthocyanin/Norbixin
6.5. Probiotics
6.6. Fatty Acids
6.6.1. Fish Oil
6.6.2. Chia Oil
6.7. Vitamins
6.7.1. Vitamin D
6.7.2. Vitamin B12
6.7.3. Vitamin C (Ascorbic Acid)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Overview of Food Ingredients, Additives & Colors, FDA. Available online: https://www.fda.gov (accessed on 9 February 2021).
- Augustin, M.A.; Sanguansri, L. Introduction to microencapsulation of functional ingredients in food products—short course on micro- and nano-encapsulation of functional ingredients in food products. In Proceedings of the World Congress on Oils & Fats and 31st Lectureship Series, Rosario, Argentina, 31 October–4 November 2015. [Google Scholar]
- Branen, A.L.; Davidson, P.M.; Salminen, S.; Thorngate, J.H., III. Food Additives, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Encyclopedia Britannica. Available online: https://www.britannica.com/topic/food-additive (accessed on 9 February 2021).
- Gaonkar, A.; Vasisht, N.; Khare, A.; Sobel, R. Microencapsulation in the Food Industry, 1st ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Nedovica, V.; Kalusevica, A.; Manojlovicb, V.; Levica, S.; Bugarskib, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Poncelet, D.; Picot, A.; El Mafadi, S. Encapsulation: An essential technology for functional food applications. Innov. Food Technol. 2011, 32–34. Available online: www.innovationsfood.com (accessed on 9 February 2021).
- James, H.P.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Food Encapsulation Market by Shell Material (Polysaccharides, Emulsifiers, Lipids, Proteins), Technology (Microencapsulation, Nanoencapsulation, Hybrid encapsulation), Application, Method, Core Phase, and Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/food-encapsulation-advanced-technologies-and-global-market-68.html (accessed on 9 February 2021).
- Gharsallaoui, A. Ingredients: Microencapsulation of Food Ingredients: Still a Field to Be Explored? Available online: https://www.newfoodmagazine.com/ (accessed on 9 February 2021).
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Shu, B.; Yu, W.; Zhao, Y.; Liu, X. Study on microencapsulation of lycopene by spray-drying. J. Food Eng. 2006, 76, 664–669. [Google Scholar] [CrossRef]
- Poncelet, D. Microencapsulation: Fundamentals, Methods and Applications. In Surface Chemistry in Biomedical and Environmental Science; Springer: Heidelberg, Germany, 2006; pp. 23–34. [Google Scholar]
- Pimparade, M.B.; Morott, J.T.; Park, J.B.; Kulkarni, V.I.; Majumdar, S.; Murthy, S.N.; Lian, Z.; Pinto, E.; Bi, V.; Durig, T.; et al. Development of taste masked caffeine citrate formulations utilizing hot melt extrusion technology and in vitro-in vivo evaluations. Int. J. Pharm. 2015, 487, 167–176. [Google Scholar] [CrossRef]
- Zhao, X.-H.; Tang, C.-H. Spray-drying microencapsulation of CoQ10 in olive oil for enhanced water dispersion, stability and bioaccessibility: Influence of type of emulsifiers and/or wall materials. Food Hydrocoll. 2016, 61, 20–30. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr. Polym. 2015, 128, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Domb, A.J. Lipsopheres for Controlled Delivery Substances. U.S. Patent 5,188,837A, 13 November 1989. [Google Scholar]
- Domb, A.J. Long-acting injectable oxytetracycline-liposphere formulations. Int. J. Pharm. 1995, 124, 271–278. [Google Scholar] [CrossRef]
- Masters, D.B.; Domb, A.J. Liposphere local anesthetic timed-release for perineural site application. Pharm. Res. 1998, 15, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Bekerman, T.; Golenser, J.; Domb, A. Cyclosporin nanoparticulate lipospheres for oral administration. J. Pharm. Sci. 2004, 93, 1264–1270. [Google Scholar] [CrossRef]
- Elgart, A.; Cherniakova, I.; Aldouby, Y.; Domb, A.J.; Hoffman, A. lipospheres and pro-nano lipospheres for delivery of poorly water-soluble compounds. Chem. Phys. Lipids 2012, 165, 438–453. [Google Scholar] [CrossRef]
- Avramoff, A.; Khan, W.; Ezra, A.; Elgart, A.; Hoffman, A.; Domb, A.J. Cyclosporin pro-dispersion liposphere formulation. J. Control. Release 2012, 160, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Cherniakov, I.; Domb, A.J.; Hoffman, A. Self-nano-emulsifying drug delivery systems: An update of the biopharmaceutical aspects. Expert Opin. Drug Deliv. 2014, 12, 1121–1133. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A.J.; Hoffman, A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J. Control. Release 2017, 266, 1–7. [Google Scholar] [CrossRef]
- Izgelov, D.; Cherniakov, I.; Bier, G.A.; Domb, A.J.; Hoffman, A. The effect of piperine pro-nano lipospheres on direct intestinal phase ii metabolism: The raloxifene paradigm of enhanced oral bioavailability. Mol. Pharm. 2018, 15, 1548–1555. [Google Scholar] [CrossRef]
- Atsmon, J.; Cherniakov, I.; Izgelov, D.; Hoffman, A.; Domb, A.J.; Deutsch, L.; Deutsch, F.; Heffetz, D.; Sacks, H. PTL401, a new formulation based on pro-nano dispersion technology, improves oral cannabinoids bioavailability in healthy volunteers. J. Pharm. Sci. 2018, 107, 1423–1429. [Google Scholar] [CrossRef]
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Cazado, C.P.S.; de Pinho, S.C. Effect of different stress conditions on the stability of quercetin-loaded lipid microparticles produced with babacu (Orbignya speciosa) oil: Evaluation of their potential use in food applications. Food Sci. Technol. Camp. 2016, 36, 9–17. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Muthukumar, S.P.; Anandharamakrishnan, C. Solid lipid nanoparticle enhances bioavailability of hydroxycitric acid compared to a microparticle delivery system. RSC Adv. 2016, 6, 53784–53793. [Google Scholar] [CrossRef]
- Albertinia, B.; Bertonia, S.; Perissuttib, B.; Passerinia, N. An investigation into the release behavior of solid lipid microparticles in different simulated gastrointestinal fluids. Colloids Surf. B 2019, 173, 276–285. [Google Scholar] [CrossRef]
- Rosiaux, Y.; Jannin, V.; Hughes, S.; Marchaud, D. Solid lipid excipients—Matrix agents for sustained drug delivery. J. Control. Release 2014, 28, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid-based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar]
- Đorđević, V.; Paraskevopoulou, A.; Mantzouridou, F.; Lalou, S.; Pantić, M.; Bugarski, B.; Nedović, V. Encapsulation technologies for food industry. In Emerging and Traditional Technologies for Safe, Healthy and Quality Food; Food Engineering Series; Nedović, V., Raspor, P., Lević, J., Tumbas Šaponjac, V., Barbosa-Cánovas, G., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Robin, A.L.; Sankhla, D. European legislative framework controlling the use of food additives. In Essential Guide to Food Additives; Saltmarsh, M., Ed.; RSC Publishing: Cambridge, UK, 2013; pp. 44–53. [Google Scholar]
- Silva, M.P.; Tulini, F.L.; Matos, F.E., Jr.; Oliveira, M.G.; Thomazini, M.; Favaro-Trindade, C.S. Application of spray chilling and electrostatic interaction to produce lipid microparticles loaded with probiotics as an alternative to improve resistance under stress conditions. Food Hydrocoll. 2018, 83, 109–117. [Google Scholar] [CrossRef]
- Paucar, O.C.; Tulini, F.L.; Thomazini, M.; Balieiro, J.C.C.; Pallonec, E.M.J.A.; Favaro-Trindade, C.S. Production by spray chilling and characterization of solid lipid microparticles loaded with vitamin D3. Food Bioprod. Process. 2016, 100, 344–350. [Google Scholar] [CrossRef]
- Brito-Oliveira, T.C.; Molina, C.V.; Netto, F.M.; Pinho, S.C. Encapsulation of beta-carotene in lipid microparticles stabilized with hydrolyzed soy protein isolate: Production parameters, alpha-tocopherol coencapsulation and stability under stress conditions. J. Food Sci. 2017, 82, 659–669. [Google Scholar] [CrossRef]
- Molina, C.V.; Lima, J.G.; Moraes, I.C.F.; Pinho, S.C. Physicochemical characterization and sensory evaluation of yogurts incorporated with beta-carotene-loaded solid lipid microparticles stabilized with hydrolyzed soy protein isolate. Food Sci. Biotechnol. 2019, 28, 59–66. [Google Scholar] [CrossRef]
- Silva, M.P.; Thomazinia, M.; Holkema, A.T.; Pinhoa, L.S.; Genoveseb, M.I.; Fávaro-Trindade, C.S. Production and characterization of solid lipid microparticles loaded with guaraná (Paullinia cupana) seed extract. Food Res. Int. 2019, 123, 144–152. [Google Scholar] [CrossRef]
- da Rocha, B.A.; Lopes Francisco, C.R.; de Almeida, M.; Ames, F.Q.; Bona, E.; Leimann, F.V.; Gonçalves, O.H.; Bersani-Amado, C.A. Antiinflammatory activity of carnauba wax microparticles containing curcumin. J. Drug Deliv. Sci. Technol. 2020, 59, 101918. [Google Scholar] [CrossRef]
- Khor, C.M.; Ng, W.K.; Kanaujia, P.; Chan, K.P.; Dong, Y. Hot-melt extrusion microencapsulation of quercetin for taste-masking. J. Microencapsul. 2017, 34, 29–37. [Google Scholar] [CrossRef]
- Oriani, V.B.; Alvim, I.D.; Consoli, L.; Molina, G.; Pastore, G.M.; Hubinger, M.D. Solid lipid microparticles produced by spray chilling technique to deliver ginger oleoresin: Structure and compound retention. Food Res. Int. 2016, 80, 41–49. [Google Scholar] [CrossRef]
- Oriania, V.B.; Alvimb, I.D.; Paulinoc, B.N.; Procópioa, F.R.; Pastorec, G.M.; Hubinger, M.D. The influence of the storage temperature on the stability of lipid microparticles containing ginger oleoresin. Food Res. Int. 2018, 109, 472–480. [Google Scholar] [CrossRef]
- Sartori, T.; Consoli, L.; Hubinger, M.D.; Menegalli, F.C. Ascorbic acid microencapsulation by spray chilling: Production and characterization. Food Sci. Technol. 2015, 63, 353–360. [Google Scholar] [CrossRef]
- Yang, J.; Ciftci, O.N. Encapsulation of fish oil into hollow solid lipid micro- and nanoparticles using carbon dioxide. Food Chem. 2017, 231, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.P.; Geremias-Andrade, I.M.; Ferreira, L.d.S.; Brito-Oliveira, T.C.; de Pinho, S.C. Technological and sensory feasibility of enrichment of low-sugar mango jams with curcumin encapsulated in lipid microparticles. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- de Lima, J.G.; Brito-Oliveira, T.C.; de Pinho, S.C. Characterization and evaluation of sensory acceptability of ice creams incorporated with beta-carotene encapsulated in solid lipid microparticles. Food Sci. Technol. 2016, 36, 664–671. [Google Scholar] [CrossRef]
- Holkem, A.T.; Favaro-Trindade, C.S. Potential of solid lipid microparticles covered by the protein-polysaccharide complex for protection of probiotics and proanthocyanidin-rich cinnamon extract. Food Res. Int. 2020, 136, 109520. [Google Scholar] [CrossRef] [PubMed]
- Mazzocato, M.C.; Thomazini, M.; Favaro-Trindade, C.S. Improving stability of Vitamin B12 (Cyanocobalamin) using microencapsulation by spray chilling technique. Food Res. Int. 2019, 126, 108663. [Google Scholar] [CrossRef]
- Souza, M.F.; Francisco, C.R.L.; Sanchez, J.L.; Guimarães-Inácio, A.; Valderrama, P.; Bona, E.; Tanamati, A.A.C.; Leimann, F.V.; Gonçalves, O.H. Fatty acids profile of chia oil-loaded lipid microparticles. Braz. J. Chem. Eng. 2016, 34, 659–669. [Google Scholar] [CrossRef]
- Diasa, M.I.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef]
- Manufactory Site. Available online: http://sonarome.com/2014/12/02/spray-drying-technology/ (accessed on 9 February 2021).
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in spray-drying encapsulation of food bioactive ingredients: From microcapsules to nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Lee, J.; Lim, S.; Kang, H.; Ahn, S.-I.; Jhoo, J.-W.; Ra, C.-S. Microencapsulation of caramel flavor and properties of ready-to-drink milk beverages supplemented with coffee containing these microcapsules. Food Sci. Anim. Resour. 2019, 39, 780–791. [Google Scholar] [CrossRef]
- Ravanfar, R.; Comunian, T.A.; Abbaspourrad, A. Thermoresponsive, water-dispersible microcapsules with a lipidpolysaccharide shell to protect heat-sensitive colorants. Food Hydrocoll. 2018, 81, 419–428. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Alvim, I.D.; Stein, M.A.; Koury, I.P.; Dantas, F.B.H.; Cruz, C.L.d.C.V. Comparison between the spray drying and spray chilling microparticles contain ascorbic acid in a baked product application. Food Sci. Technol. 2016, 65, 689–694. [Google Scholar] [CrossRef]
- Matos, F.E., Jr.; Di Sabatino, M.; Passerini, N.; Favaro-Trindade, C.S.; Albertini, B. Development and characterization of solid lipid microparticles loaded with ascorbic acid and produced by spray congealing. Food Res. Int. 2015, 67, 52–59. [Google Scholar] [CrossRef]
- Fávaro-Trindade, C.S.; Okuro, P.K.; Matos, F.E., Jr. Encapsulation via spray chilling/cooling/congealing. In Handbook of Encapsulation and Controlled Release; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Consoli, L.; Grimaldi, R.; Sartori, T.; Menegalli, F.C.; Hubinger, M.D. Gallic acid microparticles produced by spray chilling technique: Production and characterization. Food Sci. Technol. 2016, 65, 79–87. [Google Scholar] [CrossRef]
- Queirós, M.d.S.; Viriato, R.L.S.; Ribeiro, A.P.B.; Gigante, M.L. Dairy-based solid lipid microparticles: A novel approach. Food Res. Int. 2020, 131, 109009. [Google Scholar] [CrossRef]
- Cordeiro, P.; Temtem, M.; Winters, C. Spray congealing: Applications in the Pharmaceutical Industry. Chem. Today 2013, 31, 69–72. [Google Scholar]
- Sartori, T.; Menegalli, F.C. Development and characterization of unripe banana starch films incorporated with solid lipid microparticles containing ascorbic acid. Food Hydrocoll. 2016, 55, 210–219. [Google Scholar] [CrossRef]
- Gottschalk, P.; Brodesser, B.; Poncelet, D.; Jaeger, H.; Rennhofer, H.; Cole, S. Formation of essential oil containing microparticles comprising a hydrogenated vegetable oil matrix and characterisation thereof. J. Microencapsul. 2018, 35, 513–521. [Google Scholar] [CrossRef]
- Bampi, G.B.; Backes, G.T.; Cansian, R.L.; de Matos, F.E., Jr.; Ansolin, I.M.A.; Poleto, B.C.; Corezzolla, L.R.; Favaro-Trindade, C.S. Spray chilling microencapsulation of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis and its use in the preparation of savory probiotic cereal bars. Food Bioprocess. Technol. 2016, 9, 1422–1428. [Google Scholar] [CrossRef]
- Shah, M.K.; Khatri, P.; Vora, N.; Patel, N.K.; Jain, S.; Lin, S. Chapter 5—Lipid nanocarriers: Preparation, characterization and absorption mechanism and applications to improve oral bioavailability of poorly water-soluble drugs. In Biomedical Applications of Nanoparticles; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 117–147. [Google Scholar]
- Chaves, M.A.; de Pinho, S.C. Effect of production parameters and stress conditions on beta-carotene-loaded lipid particles produced with palm stearin and whey protein isolate. Braz. J. Food Technol. 2018, 21, 2017035. [Google Scholar] [CrossRef]
- Brito-Oliveira, T.C.; Bispo, M.; Moraes, I.C.F.; Campanella, O.H.; Pinho, S.C. Stability of curcumin encapsulated in solid lipid microparticles incorporated in cold-set emulsion filled gels of soy protein isolate and xanthan gum. Food Res. Int. 2017, 102, 759–767. [Google Scholar] [CrossRef]
- Castro, N.; Durrieu, V.; Raynaud, C.; Rouilly, A.; Rigal, L.; Quellet, C. Melt extrusion encapsulation of flavors: A review. Polym. Rev. 2016, 56, 137–186. [Google Scholar] [CrossRef][Green Version]
- SuKo Polymer Machine Tech. Available online: www.sukoptfe.com (accessed on 9 February 2021).
- Hansen, L.T.; Allan-Wojtas, P.M.; Jin, Y.-L.; Paulson, A.T. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol. 2002, 19, 35–45. [Google Scholar] [CrossRef]
- Salvim, M.O.; Thomazini, M.; Pelaquim, F.P.; Urbano, A.; Moraes, I.C.F.; Favaro-Trindade, C.S. Production and structural characterization of solid lipid microparticles loaded with soybean protein hydrolysate. Food Res. Int. 2015, 76, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the AOAC, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1997; Volume 2. [Google Scholar]
- Tulini, F.L.; Souza, V.B.; Echalar-Barrientos, M.A.; Thomazini, M.; Pallone, E.M.J.A.; Favaro-Trindade, C.S. Development of solid lipid microparticles loaded with a proanthocyanidinrich cinnamon extract (Cinnamomum zeylanicum): Potential for increasing antioxidant content in functional foods for diabetic population. Food Res. Int. 2016, 85, 10–18. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, R.; Williams, P.A.; Zhong, F. Factors affecting the bioaccessibility of β-carotene in lipid-based microcapsules: Digestive conditions, the composition, structure and physical state of microcapsules. Food Hydrocoll. 2018, 77, 187–203. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Donsì, F.; Barba, F.J.; Jambrak, A.R. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents. Food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ciftci, O.N. Development of free-flowing peppermint essential oil-loaded hollow solid lipid micro- and nanoparticles via atomization with carbon dioxide. Food Res. Int. 2016, 87, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Abd El Kader, A.E.; Abu Hashish, H.M. Encapsulation techniques of food bioproduct. Egypt. J. Chem. 2020, 63, 1881–1909. [Google Scholar] [CrossRef]
- Yonekura, L.; Martins, C.A.; Sampaio, G.R.; Monteiro, M.P.; César, L.A.; Mioto, B.M.; Mori, C.S.; Mendes, T.M.; Ribeiro, M.L.; Arçari, D.P.; et al. Bioavailability of catechins from guaraná (Paullinia cupana) and its effect on antioxidant enzymes and other oxidative stress markers in healthy human subjects. Food Funct. 2016, 7, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Mudgil, P.; Gani, A.; Hamed, F.; Masoodi, F.A.; Maqsood, S. Nano-encapsulation of catechin in starch nanoparticles: Characterization, release behavior and bioactivity retention during simulated in-vitro digestion. Food Chem. 2019, 270, 95–104. [Google Scholar] [CrossRef]
- Ye, K.-X.; Fan, T.-T.; Keen, L.J.; Han, B.-N. A review of pigments derived from marine natural products. Isr. J. Chem. 2019, 59, 327–338. [Google Scholar] [CrossRef]
- Carochoa, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Pelissari, J.R.; Souza, V.B.; Pigoso, A.A.; Tulini, F.L.; Thomazini, M.; Rodrigues, C.E.C.; Urbano, A.; Favaro-Trindade, C.S. Production of solid lipid microparticles loaded with lycopene by spray chilling: Structural characteristics of particles and lycopene stability. Food Bioprod. Process. 2016, 98, 86–94. [Google Scholar] [CrossRef]
- Liang, X.; Ma, C.; Yan, X.; Liu, X.; Liu, F. Advances in research on bioactivity, metabolism, stability and delivery systems of lycopene. Trends Food Sci. Technol. 2019, 93, 185–196. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; DellaTorre, A.; Oriani, V.B.; Franch, G.C.; Angolini, C.F.F.; Hubinger, M.D.; Gois Ruiz, A.L.T.; Pastore, G.M. In vitro bioactivity approach of unripe genipap (Genipa americana L.; Rubiaceae) fruit extract and its solid lipid microparticle. Food Res. Int. 2020, 127, 108720. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; He, J. Microencapsulation of vitamins in food applications to prevent losses in processing and storage: A review. Food Res. Int. 2020, 137, 109326. [Google Scholar] [CrossRef]
- Maurya, V.K.; Bashirb, K.; Aggarwal, M. Vitamin D microencapsulation and fortification: Trends and technologies. J. Steroid Biochem. Mol. Biol. 2020, 196, 105489. [Google Scholar] [CrossRef] [PubMed]
- Dary, O.; Hurrel, R. Guidelines on Food Fortification with Micronutrients; World Health Organization—Food and Agricultural Organization of the United Nations: Geneva, Switzerland, 2006. [Google Scholar]
- Fennema, O.R.; Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry, 4th ed.; Taylor & Francis: Porto Alegre, Brazil, 2010; pp. 399–401. [Google Scholar]




| Anti-Ageing | Digestive Health | Immunity | Cognitive Health | Energy |
|---|---|---|---|---|
| Herbs/Botanicals | Probiotics | Herbs/Botanicals | Omega-3 | Vitamins |
| Antioxidants | Herbs/Botanicals | Probiotics | Herbs/Botanicals | CoQ10 |
| Omega-3 | Enzymes | Antioxidants | Antioxidants | Herbs/Botanicals |
| Vitamin C | Fiber | Vitamin C | Ginger | Proteins |
| Vitamin E | Omega-3 | Beta-sitosterol | Lecithin | Guarana |
| Lipids | Chemical Composition | Properties * | Examples |
|---|---|---|---|
| Waxes | Esters of fatty acids and long chain alcohols | Hydrophobic mp = 62–86 °C | Carnauba wax, Candelilla wax, beeswax, solid paraffin, Rice Bran wax |
| Vegetable oils | Mixture of triglycerides, free fatty acids, phospholipids | Often digestible mp = 60–71 °C | Hydrogenated soybean oil, hydrogenated palm oil (Softisan 154) |
| Fatty acids | Long chain fatty acids | mp = 60–90 °C | Palmitic acid, Stearic acid, Behenic acid, Lauric acid |
| Triglycerides | Monoacid triglycerides | mp = 46–73 °C | Glyceryl tripalmitate (Dynasan 116), Glyceryl trimyristate, Glyceryl trilaurate |
| Fatty alcohol | Mixture of fatty alcohols | mp = 48–56 °C | Cetyl alcohol, Lauryl alcohol, Stearyl alcohol, Oleyl alcohol |
| Method | Principles | Comments |
|---|---|---|
| Spray drying | Core material is dispersed into an aqueous encapsulant solution forming an emulsion or dispersion, followed by homogenization of the liquid, and then the atomization of the mixture into the drying chamber | The oldest and most commonly used method, flexible and lucrative process |
| Spray Chilling | Core material is dispersed into a coating solution and sprayed into a cold environment to solidify the carrier material | Suitable for temperature-sensitive cores |
| Extrusion | Emulsion dispersion containing core material passes through a die at high temperature and pressure into a bath for solidification of particles | Used primarily for encapsulation of flavors and other volatile cores in glassy matrices |
| High-shear homogenization | Dispersion of the additive-loaded lipid in hot aqueous surfactant solution, followed by high shear homogenization at a temperature above the melting point of lipid carrier and quick cooling | Cold homogenization for hydrophilic core material, hot for lipophilic core |
| Method | Size Distribution | Morphology | References |
|---|---|---|---|
| Spray drying | 1.1 and 10.6 µm 10–50 µm—2–3 mm | Hollow, low density spherical particles with an irregular geometry | [57] [54] |
| Spray Chilling | 10 µm 90–160 µm 200 µm | Dense particles with a spherical shape and smooth and continuous surface | [50] [60] [68] |
| Extrusion | 0.3–5 mm 500–1000 µm | High density granules, sheets or strands can be obtained | [42] [54] |
| High-shear homogenization | 1.09 µm 20 µm | Spherical particles with different density | [29] [41] |
| Food Additive | Examples | Reason for Encapsulation | Encapsulation Material | Ref. |
|---|---|---|---|---|
| Antioxidants | Cinnamon (proantho-cyanidins) Quercetin Curcumin Guarana | Low stability, unpleasant taste, rapid inactivation, degradation by proteolytic enzymes Low oral bioavailability, bitterness High hydrophobicity, poor absorption, low bioavailability, spicy flavor, high sensitivity to light Unstable in food processing and gastrointestinal conditions | Vegetable fat Babacu oil, Carnauba wax Babacu oil, Tristearin, Palm stearin Vegetable fat | [76] [29,42] [47,70] [40] |
| Essential oils (EOs) | Lemon, Peppermint, Carvacrol, Thymol | Storage stability, oxidation, controlled release, hydrophobicity | Hydrogenated vegetable fat Fully hydrogenated soybean oil | [66] [82] |
| Fatty acids | Fish oil (EPA & DHA) Chia oil | Low aqueous solubility, susceptibility to oxidation | Fully hydrogenated soybean oil Stearic acid | [46] [51] |
| Flavors | Coffee, Caramel Ginger oleoresin (GO) | Loss of volatile compounds, oxidation, degradation High viscosity, volatility, heat and light degradation | Medium-chain triglycerides (MCT) Palmitic acid with oleic acid or palm fat | [56] [43,44] |
| Natural colorants | Lycopene β-Carotene Genipap (Genipa americana L.) Anthocyanin, Norbixin | Oxidative degradation, cis-trans isomerization, low water-solubility, low bioavailability Low stability and bioavailability, high hydrophobicity, and sensitivity Thermal degradation, photodegradation Thermal and photodegradation | Hydrogenated and interesterified cottonseed, soy and palm oils Palm stearin Stearic acid Glycerol mono-oleate (GMO) and soy lecithin | [88,89] [39,77] [90,91] [57] |
| Probiotics | Lactobacillus acidophilus (LA), Bifidobacterium animalis subsp. lactis (BL) | Maintaining viability during processing and throughout the product’s shelf life | Vegetable fat | [36,67] |
| Vitamins | Ascorbic acid (Vitamin C) Vitamin D Vitamin B12 | High instability by high temperature, high pH, and the presence of oxygen, metallic ions, UV and X-rays Poor water solubility, rapid degradation, and low absorption Degraded by light, heat, acid and basic media, and oxidizing agents | Lauric acid (LA)/oleic acid (OA) mixtures, vegetable glycerol monostearate, stearic acid—hydrogenated vegetable fat Vegetable fat Vegetable fat | [59,60] [37,92] [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahum, V.; Domb, A.J. Recent Developments in Solid Lipid Microparticles for Food Ingredients Delivery. Foods 2021, 10, 400. https://doi.org/10.3390/foods10020400
Nahum V, Domb AJ. Recent Developments in Solid Lipid Microparticles for Food Ingredients Delivery. Foods. 2021; 10(2):400. https://doi.org/10.3390/foods10020400
Chicago/Turabian StyleNahum, Victoria, and Abraham J. Domb. 2021. "Recent Developments in Solid Lipid Microparticles for Food Ingredients Delivery" Foods 10, no. 2: 400. https://doi.org/10.3390/foods10020400
APA StyleNahum, V., & Domb, A. J. (2021). Recent Developments in Solid Lipid Microparticles for Food Ingredients Delivery. Foods, 10(2), 400. https://doi.org/10.3390/foods10020400







