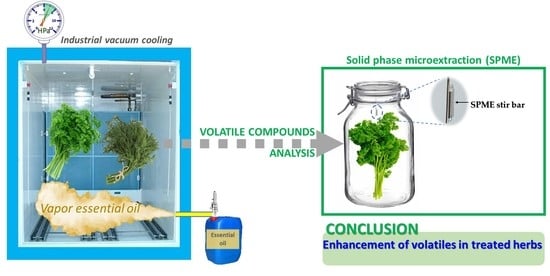
The Application of Essential Oil Vapors at the End of Vacuum Cooling of Fresh Culinary Herbs Promotes Aromatic Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Volatiles Analysis by Headspace Solid-Phase Microextraction (SPME)
2.3. Model of SPME Sorption of Volatiles from Aromatic Herbs
2.4. Effect of Vapor EOs Applied under Vacuum on Volatiles of Aromatic Herbs at Pilot Plant Scale
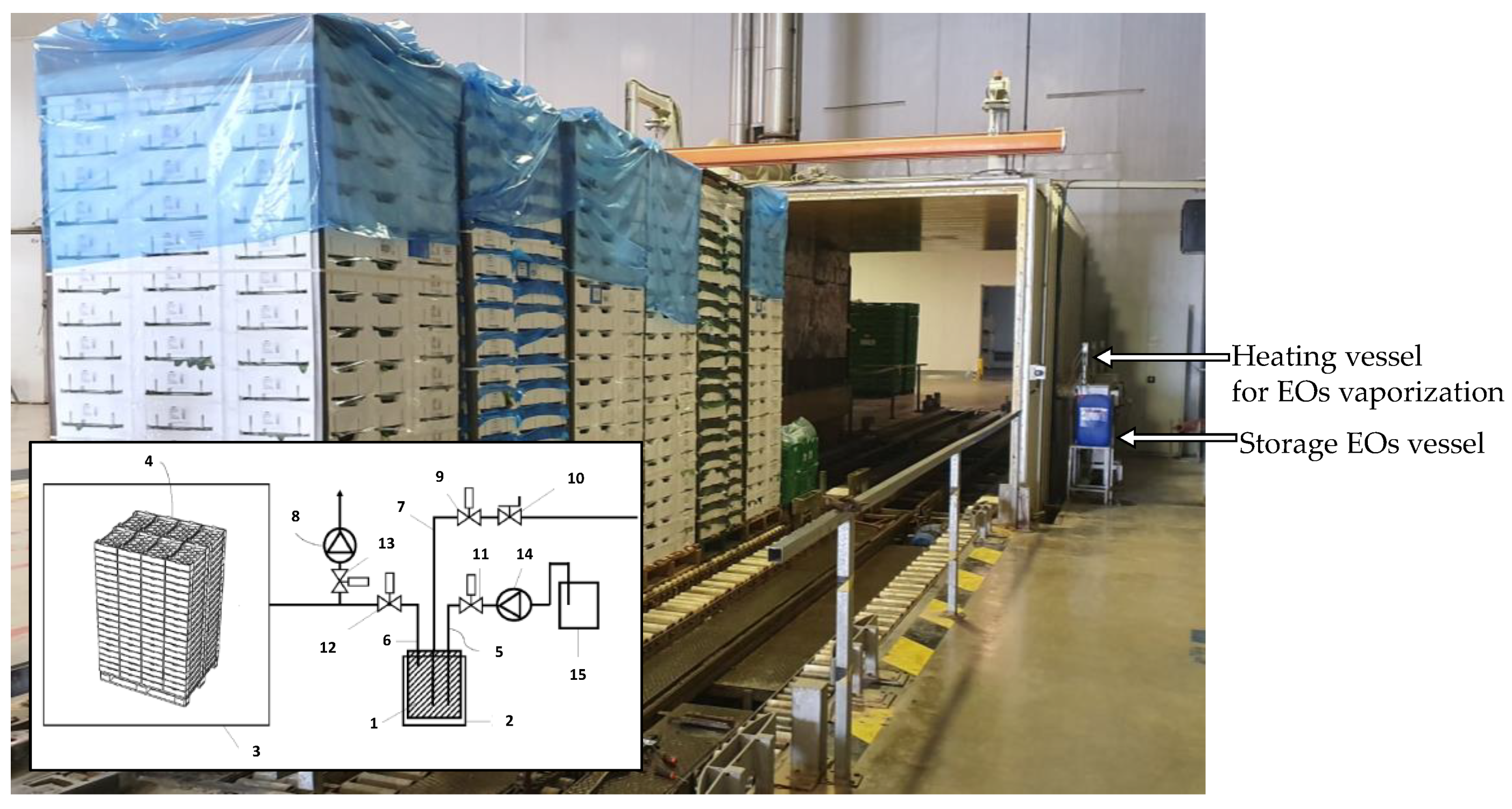
2.5. Validation at Industrial Scale of Aroma Recovery with Vapor EO Treatment of Aromatic Herbs
2.6. Statistical Analyses
3. Results and Discussion
3.1. SPME Sorption of Volatiles from Aromatic Herbs: Basil as a Model Herb
3.2. Volatiles Profiles of Aromatic Herbs
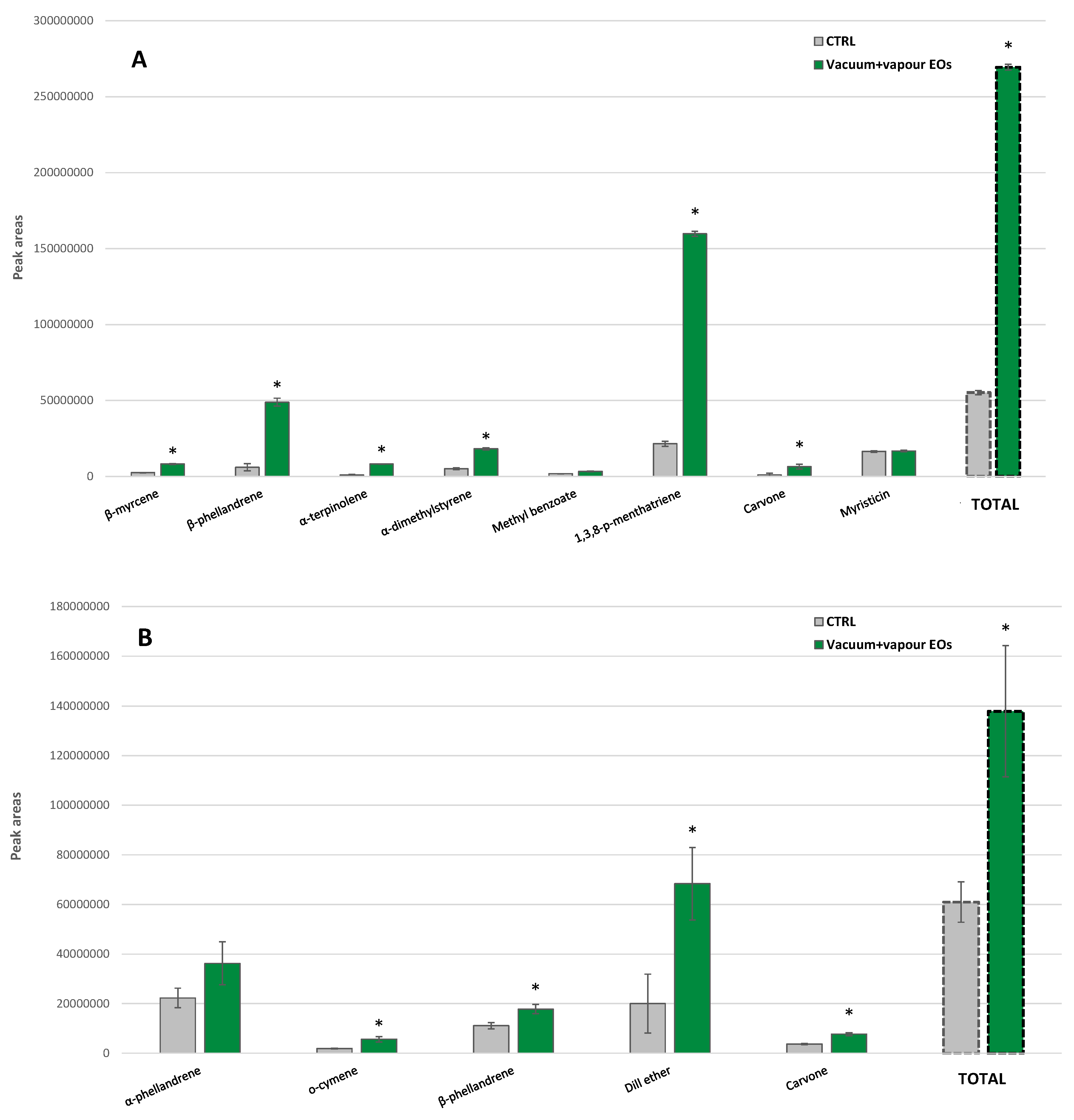
3.2.1. Curly Parsley
3.2.2. Dill
3.3. Enhancement of VOCs Biosynthesis after Vapor Essential Oils Applied under Vacuum Conditions at Pilot Plant Scale
3.4. Validation (Industrial Scale) of Aroma Recovery by Vapor Essential Oils Applied under Vacuum Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Gómez, A.; Ros-Chumillas, M.; Antolinos, V.; Buendía-Moreno, L.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; Soto-Jover, S. Fresh culinary herbs decontamination with essential oil vapours applied under vacuum conditions. Postharvest Biol. Technol. 2019, 156, 110942. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C.F.R. A comparison of the nutritional contribution of thirty-nine aromatic plants used as condiments and/or herbal infusions. Plant Foods Hum. Nutr. 2015, 70, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.I.; Reid, M.S. Postharvest physiology and handling of fresh culinary herbs. J. Herbs. Spices Med. Plants 1993, 1, 93–127. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F. Fresh-cut fruit and vegetables: Emerging eco-friendly techniques for sanitation and preserving safety. In Postharvest Handling; Kahramanoglu, I., Ed.; InTech: London, UK, 2017; pp. 7–45. [Google Scholar]
- Demyttenaere, J.C.R. The new European Union Flavouring Regulation and its impact on essential oils: Production of natural flavouring ingredients and maximum levels of restricted substances. Flavour Fragr. J. 2012, 27, 3–12. [Google Scholar] [CrossRef]
- FDA Food Additive Status List. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on 14 January 2021).
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Zaika, L.L. Spices and herbs: Their antimicrobial activity and its determination. J. Food Saf. 2007, 9, 97–118. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef]
- López−Gómez, A.; Navarro-Martínez, A.; Martínez−Hernández, G.B. Active paper sheets including nanoencapsulated essential oils: A green packaging technique to control ethylene production and maintain quality in fresh horticultural products. A case study in flat peaches. Foods 2020, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, A.; Ros-Chumillas, M.; Buendía-Moreno, L.; Martínez-Hernández, G.B. Active cardboard packaging with encapsulated essential oils for enhancing the shelf life of fruit and vegetables. Front. Nutr. 2020, 7, 275. [Google Scholar] [CrossRef]
- López-Gómez, A.; Ros-Chumillas, M.; Buendía-Moreno, L.; Navarro-Segura, L.; Martínez-Hernández, G.B. Active cardboard box with smart internal lining based on encapsulated essential oils for enhancing the shelf life of fresh mandarins. Foods 2020, 9, 590. [Google Scholar] [CrossRef]
- Buendía−Moreno, L.; Ros-Chumillas, M.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Soto-Jover, S.; Antolinos, V.; Martínez-Hernández, G.B.; López-Gómez, A. Effects of an active cardboard box using encapsulated essential oils on the tomato shelf life. Food Bioprocess Technol. 2019, 12, 1548–1558. [Google Scholar] [CrossRef]
- Method for the Surface Decontamination of Packaged Solid Food. Available online: https://patents.google.com/patent/WO2016146864A1/en (accessed on 25 February 2016).
- Álvarez, S.; Riera, F.A.; Álvarez, R.; Coca, J.; Cuperus, F.P.; Th Bouwer, S.; Boswinkel, G.; Van Gemert, R.W.; Veldsink, J.W.; Giorno, L.; et al. New integrated membrane process for producing clarified apple juice and apple juice aroma concentrate. J. Food Eng. 2000, 46, 109–125. [Google Scholar] [CrossRef]
- Sun, D.W.; Zheng, L. Vacuum cooling technology for the agri-food industry: Past, present and future. J. Food Eng. 2006, 77, 203–214. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Y.; Sun, D. Effects of initial temperatures on vacuum film cooling and vacuum spray cooling on apple juice and milk. J. Food Process. Preserv. 2020, 44. [Google Scholar] [CrossRef]
- Cagliero, C.; Mastellone, G.; Marengo, A.; Bicchi, C.; Sgorbini, B.; Rubiolo, P. Analytical strategies for in-vivo evaluation of plant volatile emissions-A review. Anal. Chim. Acta 2020. [Google Scholar] [CrossRef]
- Klimánková, E.; Holadová, K.; Hajšlová, J.; Čajka, T.; Poustka, J.; Koudela, M. Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008, 107, 464–472. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; Wiley-VCH: New York, NY, USA; Toronto, ON, Canada, 1997; ISBN 9780471190349. [Google Scholar]
- Ruiz-Hernández, V.; Roca, M.J.; Egea-Cortines, M.; Weiss, J. A comparison of semi-quantitative methods suitable for establishing volatile profiles. Plant Methods 2018, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Câmara, J.S. Profiling of volatiles in the leaves of Lamiaceae species based on headspace solid phase microextraction and mass spectrometry. Food Res. Int. 2013, 51, 378–387. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Öksüz, H.B.; Buzrul, S. Monte carlo analysis for microbial growth curves. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 418–423. [Google Scholar] [CrossRef]
- Modelling Microbial Growth under Static and Dynamic Conditions: Biogrowth. Available online: https://cran.r-project.org/web/packages/biogrowth/vignettes/biogrowth_basics.html (accessed on 25 January 2021).
- R-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Kim, N.S.; Lee, D.S. Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography-mass spectrometry. J. Chromatogr. A 2002, 982, 31–47. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Cabezudo, M.D. Headspace solid-phase microextraction analysis of volatile components of spices. Chromatographia 2002, 55, 723–728. [Google Scholar] [CrossRef]
- Kim, N.-S.; Lee, D.-S. Characterization of rosemary fragrances by Solid Phase Micro-extraction and GC-MS. In Proceedings of the Sixth Asian Conference on Analytical Sciences (ASIANALYSIS VI), Tokyo, Japan, 7–10 August 2001; The Japan Society for Analytical Chemistry: Tokyo, Japan, 2001; pp. a383–a386. [Google Scholar]
- Sheen, L.Y.; Ou, Y.H.T.; Tsai, S.J. Flavor characteristic compounds found in the essential oil of Ocimum basilicum L. with sensory evaluation and statistical analysis. J. Agric. Food Chem. 1991, 39, 939–943. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Wellington, G.A.; Cocchiara, J.; Lalko, J.; Letizia, C.S.; Api, A.M. Fragrance material review on methyl cinnamate. Food Chem. Toxicol. 2007, 45, S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Viña, A.; Murillo, E. Essential oil composition from twelve varieties of basil (Ocimum spp) grown in Colombia. J. Braz. Chem. Soc. 2003, 14, 744–749. [Google Scholar] [CrossRef]
- Lawrencet, B.M. Further examination of the variation of Ocimum basilicum L. In Developmenls in Food Science, Flavors and Fragrances: A World Perspecrive; Lawrence, B.M., Mookheyee, B.D., Willis, B.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 161–170. [Google Scholar]
- Simon, J.E.; Quinn, J. Characterization of essential oil of parsley. J. Agric. Food Chem. 1988, 36, 467–472. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Daferera, D.; Akoumianakis, C.A.; Passam, H.C.; Polissiou, M.G. The effect of sowing date and growth stage on the essential oil composition of three types of parsley (Petroselinum crispum). J. Sci. Food Agric. 2004, 84, 1606–1610. [Google Scholar] [CrossRef]
- Kasting, R.; Andersson, J.; von Sydow, E. Volatile constituents in leaves of parsley. Phytochemistry 1972, 11, 2277–2282. [Google Scholar] [CrossRef]
- Aziz, E.E.; Sabry, R.M.; Ahmed, S.S. Plant growth and essential oil production of sage (Salvia officinalis L.) and curly-leafed parsley (Petroselinum crispum ssp. crispum L.) cultivated under salt stress conditions. World Appl. Sci. J. 2013, 28, 785–796. [Google Scholar]
- Freeman, G.G.; Whenham, R.J.; Self, R.; Eagles, J. Volatile flavour components of parsley leaves (Petroselinum crispum (mill.) nyman). J. Sci. Food Agric. 1975, 26, 465–470. [Google Scholar] [CrossRef]
- Farouk, A.; Ali, H.; Al-Khalifa, A.R.; Mohsen, M.; Fikry, R. Aroma volatile compounds of parsley cultivated in the Kingdom of Saudi Arabia and Egypt extracted by hydrodistillation and headspace solid-phase microextraction. Int. J. Food Prop. 2017, 20, S2868–S2877. [Google Scholar] [CrossRef]
- Huopalahti, R. Gas chromatographic and sensory analyses in the evaluation of the aroma of dill herb, Anethum graveolens L. LWT-Food Sci. Technol. 1986, 19, 27. [Google Scholar]
- Blank, I.; Grosch, W. Evaluation of potent odorants in dill seed and dill herb (Anethum graveolens L.) by aroma extract dilution analysis. J. Food Sci. 1991, 56, 63–67. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á. Volatile composition of essential oils from different aromatic herbs grown in Mediterranean regions of Spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Kruma, Z.; Galoburda, R.; Sabovics, M.; Gramatina, I.; Skudra, I.; Dabina-Bicka, I. Aroma composition of microwave vacuum dried dill (Anethum graveolens L.) stems. Procedia Food Sci. 2011, 1, 1338–1343. [Google Scholar] [CrossRef] [Green Version]
- Cătunescu, G.M.; Socaci, S.A.; Rotar, I.; Vidican, R.; Bunghez, F.; Tofană, M.; Pleșa, A.; Muntean, M. Volatile profile of minimally processed herbs during cold storage. Rom. Biotechnol. Lett. 2016, 21, 1923–11931. [Google Scholar]
- Possell, M.; Loreto, F. The role of volatile organic compounds in plant resistance to abiotic stresses: Responses and mechanisms. In Tree Physiology; Part of the Tree Physiology book series (TREE, volume 5); Springer: Dordrecht, The Netherlands, 2013; pp. 209–235. [Google Scholar]
- Buendía−Moreno, L.; Soto−Jover, S.; Ros−Chumillas, M.; Antolinos−López, V.; Navarro−Segura, L.; Sánchez−Martínez, M.J.; Martínez−Hernández, G.B.; López−Gómez, A. An innovative active cardboard box for bulk packaging of fresh bell pepper. Postharvest Biol. Technol. 2020, 164, 111171. [Google Scholar] [CrossRef]
- Taheri, A.; Behnamian, M.; Dezhsetan, S.; Karimirad, R. Shelf life extension of bell pepper by application of chitosan nanoparticles containing Heracleum persicum fruit essential oil. Postharvest Biol. Technol. 2020, 170, 111313. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, J.; Zhang, R.; Kong, R.; Lu, W.; Wang, X. Characterization and application of the microencapsulated carvacrol/sodium alginate films as food packaging materials. Int. J. Biol. Macromol. 2019, 141, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Pinelli, P.; Manes, F.; Kollist, H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol. 2004, 24, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Porat, R.; Lichter, A.; Terry, L.A.; Harker, R.; Buzby, J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biol. Technol. 2018, 139, 135–149. [Google Scholar] [CrossRef] [Green Version]







| Compound | % |
|---|---|
| Carvone | 81.4 |
| Limonene | 4.78 |
| cis-dihydrocarvone | 2.30 |
| trans-dihydrocarvyl acetate | 1.93 |
| Menthone | 0.99 |
| cis-carvyl acetate | 0.34 |
| β-bourbonene | 0.12 |
| Octanol-3 | 0.11 |
| Sabinene hydrate | 0.02 |
| cis-jasmone | 0.01 |
| Viridiflorol | 0.01 |
| Specific Sorption Rate (min−1) | Cmax 1 (ln Peak Area) | RMSE 2 (ln Peak Area) | |
|---|---|---|---|
| Monoterpene hydrocarbons | |||
| α-pinene | 0.0063 ± 0.0020 | 2.76 ± 0.045 | 0.070 |
| Sabinene | 0.0042 ± 0.0009 | 3.17 ± 0.037 | 0.047 |
| β-pinene | 0.0052 ± 0.0020 | 2.91 ± 0.029 | 0.025 |
| β-myrcene | 0.0223 ± 0.0032 | 3.31 ± 0.009 | 0.036 |
| 3-carene | 0.0229 ± 0.0056 | 3.09 ± 0.014 | 0.038 |
| α-terpinene | 0.0420 ± 0.0067 | 3.09 ± 0.006 | 0.025 |
| Limonene | 0.0174 ± 0.0041 | 3.10 ± 0.008 | 0.025 |
| trans-ocimene | 0.0168 ± 0.0026 | 3.49 ± 0.010 | 0.033 |
| γ-terpinene | 0.0115 ± 0.0016 | 3.17 ± 0.009 | 0.027 |
| Oxygenated monoterpenes | |||
| 1,8-cineole | 0.0296 ± 0.0027 | 3.74 ± 0.005 | 0.016 |
| 4-thujanol | 0.0156 ± 0.0017 | 3.11 ± 0.008 | 0.023 |
| α-terpinolene | 0.0134 ± 0.0016 | 3.24 ± 0.010 | 0.031 |
| Linalool | 0.0079 ± 0.0011 | 3.79 ± 0.010 | 0.025 |
| Camphor | 0.0103 ± 0.0024 | 3.23 ± 0.012 | 0.034 |
| Borneol | 0.0119 ± 0.0018 | 2.77 ± 0.009 | 0.021 |
| 4-terpineol | 0.0085 ± 0.0009 | 3.34 ± 0.011 | 0.027 |
| α-terpineol | 0.0098 ± 0.0016 | 3.11 ± 0.012 | 0.033 |
| Bornyl acetate | 0.0137 ± 0.0026 | 3.1 ± 0.0076 | 0.020 |
| Sesquiterpene hydrocarbons | |||
| Bicycloelemene | 0.0130 ± 0.0019 | 3.05 ± 0.016 | 0.044 |
| α-copaene | 0.0120 ± 0.0018 | 3.16 ± 0.011 | 0.033 |
| α-cubebene | 0.0115 ± 0.0013 | 3.01 ± 0.009 | 0.030 |
| β-elemene | 0.0138 ± 0.0016 | 3.43 ± 0.010 | 0.035 |
| β-caryophyllene | 0.0132 ± 0.0015 | 3.24 ± 0.010 | 0.031 |
| β-cubebene | 0.0145 ± 0.0018 | 3.01 ± 0.009 | 0.031 |
| α-guaiene | 0.0123 ± 0.0017 | 3.25 ± 0.009 | 0.032 |
| β-bergamotene | 0.0186 ± 0.0020 | 3.01 ± 0.007 | 0.028 |
| β-farnesene | 0.0179 ± 0.0019 | 3.59 ± 0.008 | 0.030 |
| epi-bicyclo sesquiphellandrene | 0.0152 ± 0.0023 | 3.12 ± 0.013 | 0.064 |
| γ-muurolene | 0.0156 ± 0.0019 | 2.93 ± 0.008 | 0.030 |
| Germacrene D | 0.0192 ± 0.0026 | 3.58 ± 0.010 | 0.039 |
| α-bulnesene | 0.0133 ± 0.0017 | 3.45 ± 0.013 | 0.038 |
| δ-guaiene | 0.0151 ± 0.0017 | 3.39 ± 0.001 | 0.034 |
| γ-cadinene | 0.0144 ± 0.0016 | 3.43 ± 0.001 | 0.035 |
| Sesquisabinene | 0.0181 ± 0.0020 | 3.15 ± 0.001 | 0.036 |
| Oxygenated sesquiterpenes | |||
| T-cadinol | 0.0187 ± 0.0021 | 3.09 ± 0.013 | 0.042 |
| Aromatic compounds | |||
| Cis methyl cinnamate | 0.0095 ± 0.0012 | 3.36 ± 0.008 | 0.021 |
| Eugenol | 0.0260 ± 0.0052 | 3.48 ± 0.010 | 0.037 |
| trans methyl cinnamate | 0.0191 ± 0.0035 | 3.78 ± 0.006 | 0.023 |
| Methyleugenol | 0.0206 ± 0.0022 | 3.52 ± 0.007 | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Hernández, G.B.; Taboada-Rodríguez, A.; Garre, A.; Marín-Iniesta, F.; López-Gómez, A. The Application of Essential Oil Vapors at the End of Vacuum Cooling of Fresh Culinary Herbs Promotes Aromatic Recovery. Foods 2021, 10, 498. https://doi.org/10.3390/foods10030498
Martínez-Hernández GB, Taboada-Rodríguez A, Garre A, Marín-Iniesta F, López-Gómez A. The Application of Essential Oil Vapors at the End of Vacuum Cooling of Fresh Culinary Herbs Promotes Aromatic Recovery. Foods. 2021; 10(3):498. https://doi.org/10.3390/foods10030498
Chicago/Turabian StyleMartínez-Hernández, Ginés Benito, Amaury Taboada-Rodríguez, Alberto Garre, Fulgencio Marín-Iniesta, and Antonio López-Gómez. 2021. "The Application of Essential Oil Vapors at the End of Vacuum Cooling of Fresh Culinary Herbs Promotes Aromatic Recovery" Foods 10, no. 3: 498. https://doi.org/10.3390/foods10030498