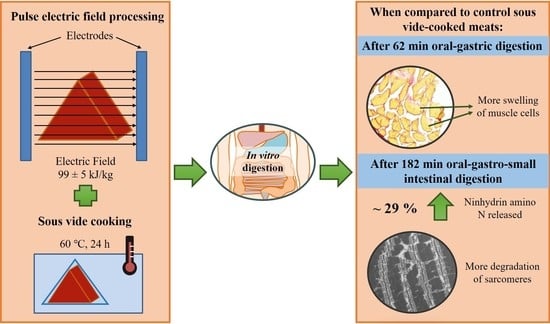
Effects of Pulsed Electric Field Processing and Sous Vide Cooking on Muscle Structure and In Vitro Protein Digestibility of Beef Brisket
Abstract
:1. Introduction
2. Materials and Methods
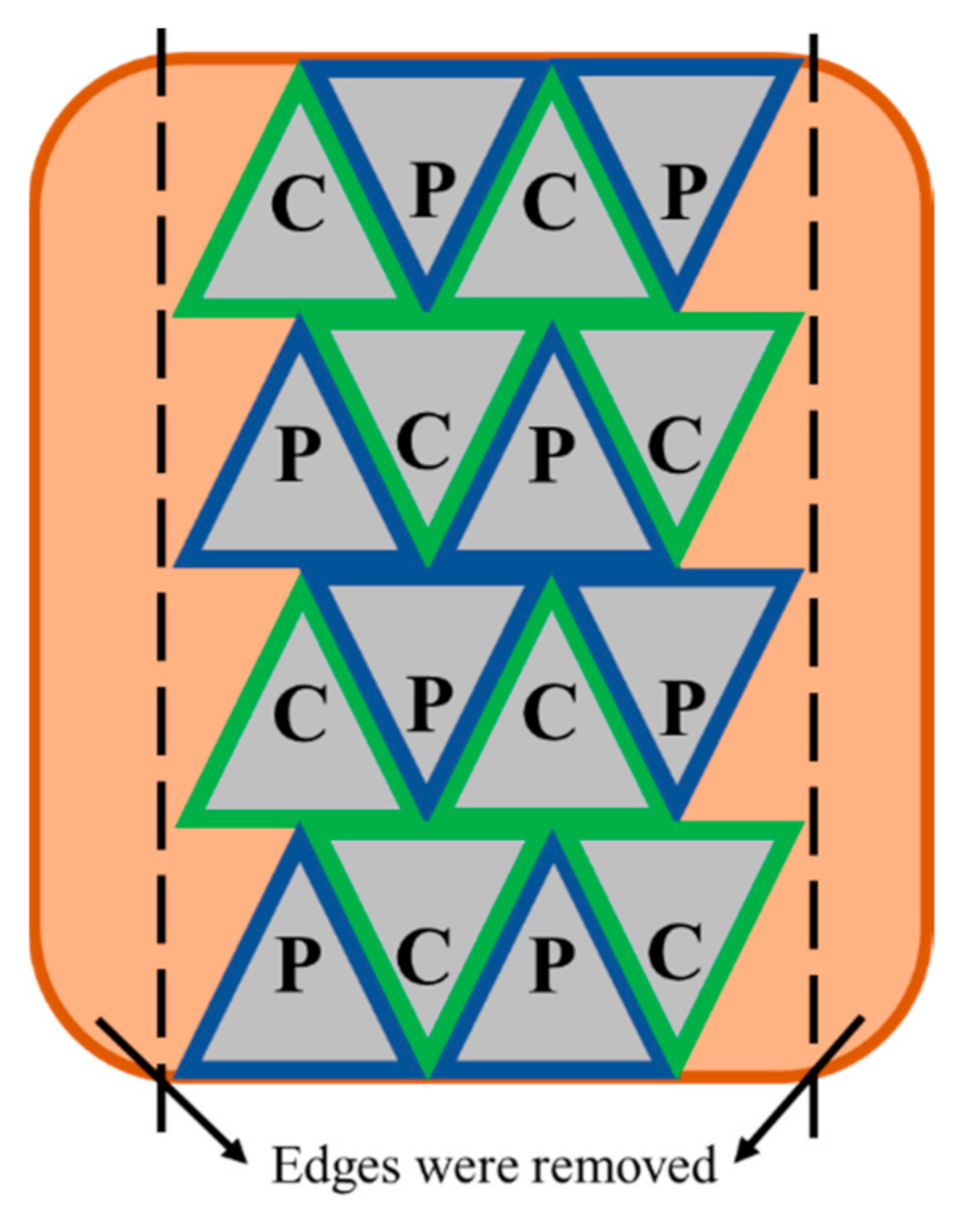
2.1. Pulsed Electric Field Processing and Sous Vide Cooking of Beef Brisket
2.2. In Vitro Protein Digestibility
2.2.1. In Vitro Digestion
2.2.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Ninhydrin-Reactive Amino Nitrogen Analysis
2.3. Microscopy Analysis of Muscles
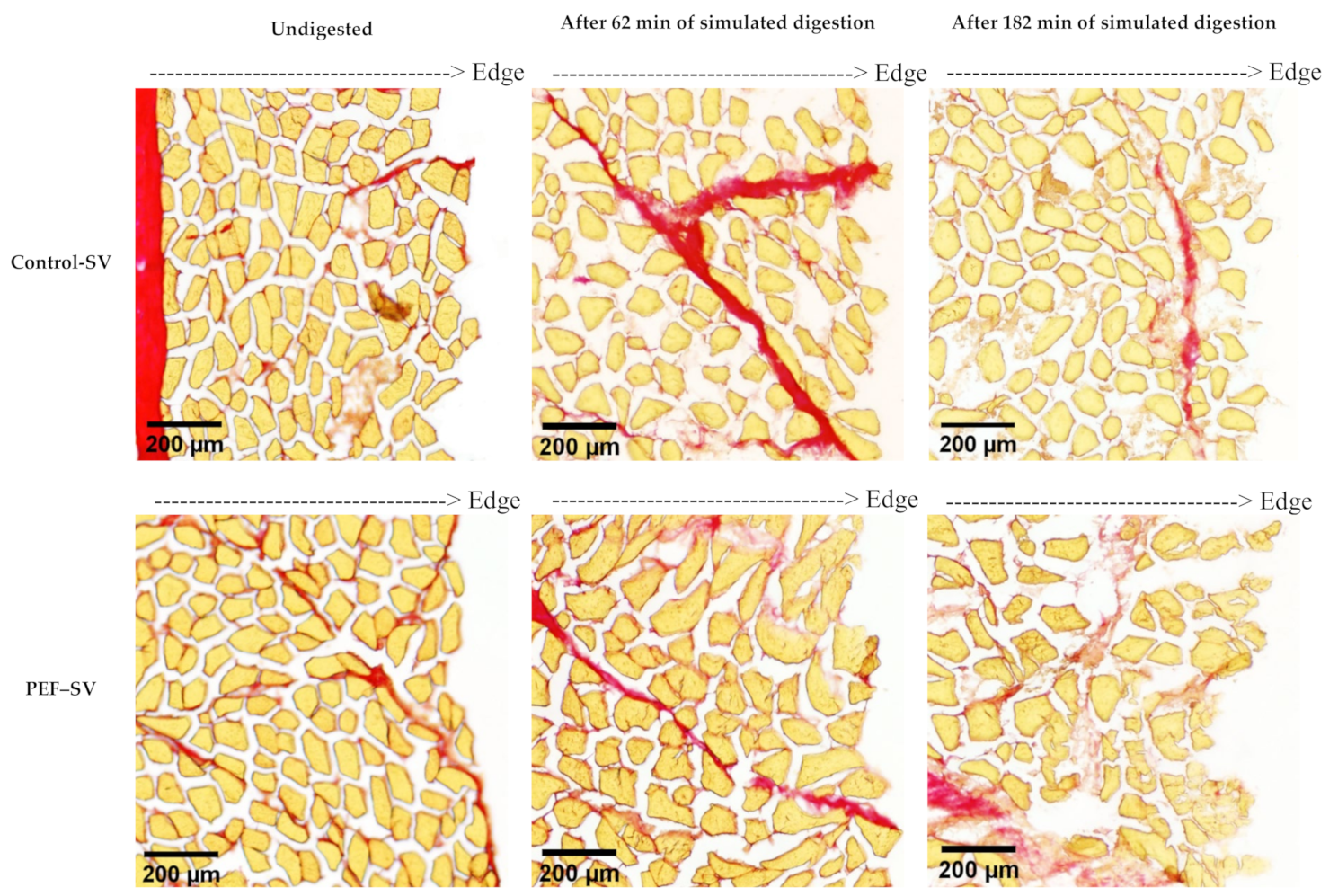
2.3.1. Histochemical Analysis
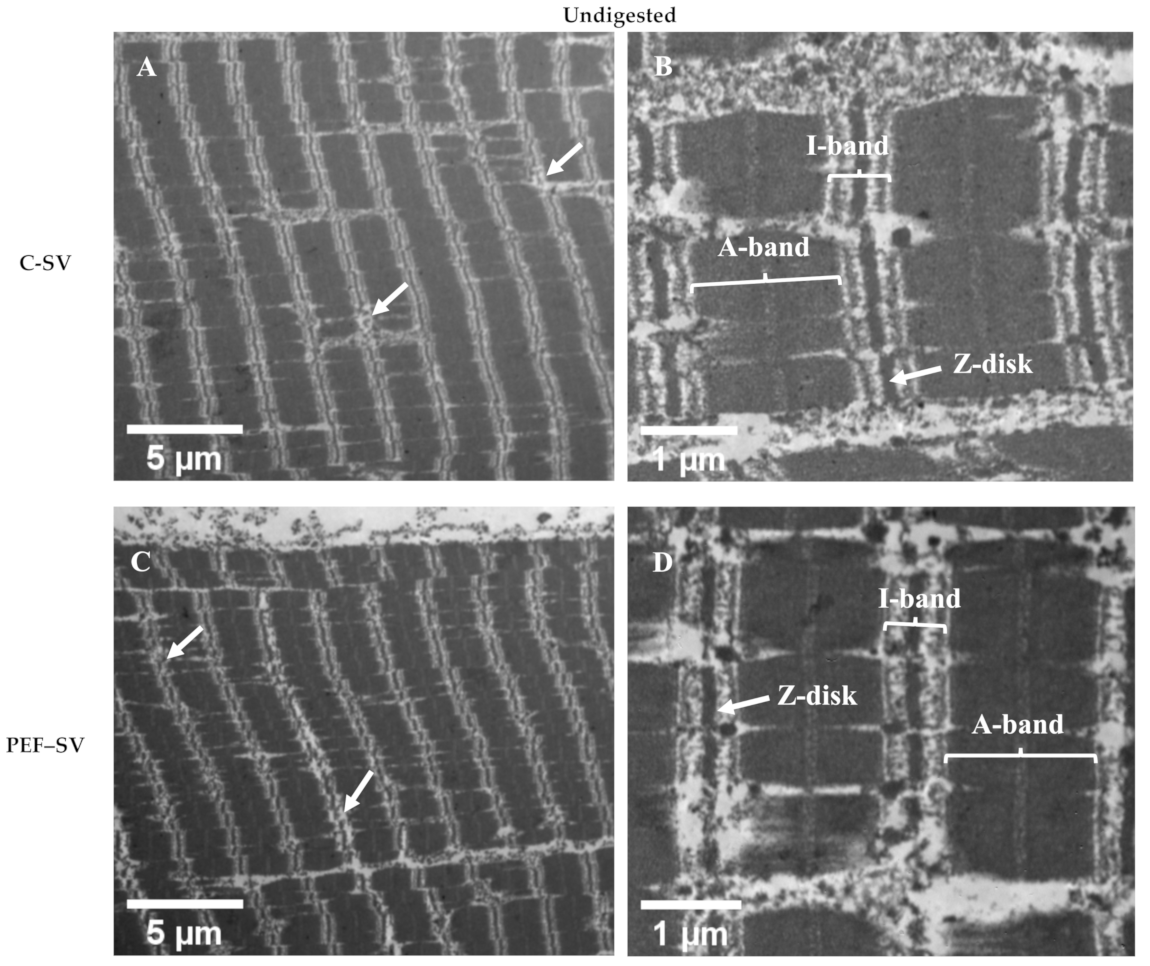
2.3.2. Transmission Electron Microscopy (TEM) Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Protein Digestibility
3.1.1. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
3.1.2. Ninhydrin-Reactive Amino Nitrogen Analysis
3.2. Structural Changes of Meat Samples during Simulated Digestion
3.2.1. Microstructure Studied Using Histochemical Analysis
3.2.2. Ultrastructure Studied Using TEM Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toepfl, S.; Heinz, V.; Knorr, D. High intensity pulsed electric fields applied for food preservation. Chem. Eng. Process. Process. Intensif. 2007, 46, 537–546. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Oey, I.; Bremer, P.; Silcock, P. Process optimisation of pulsed electric fields pre-treatment to reduce the sous vide processing time of beef briskets. Int. J. Food Sci. Technol. 2019, 54, 823–834. [Google Scholar] [CrossRef]
- Faridnia, F.; Ma, Q.L.; Bremer, P.J.; Burritt, D.J.; Hamid, N.; Oey, I. Effect of freezing as pre-treatment prior to pulsed electric field processing on quality traits of beef muscles. Innov. Food Sci. Emerg. Technol. 2015, 29, 31–40. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Oey, I.; Bremer, P.; Silcock, P. Quality and Safety Considerations of Incorporating Post-PEF Ageing into the Pulsed Electric Fields and Sous Vide Processing Chain. Food Bioprocess Technol. 2019, 12, 852–864. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Calpain activity, myofibrillar protein profile, and physico-chemical properties of beef Semimembranosus and Biceps femoris from culled dairy cows during aging. J. Food Process. Preserv. 2018, 42, 13835. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Oey, I.; Bremer, P.; Silcock, P. Optimisation of Sous Vide Processing Parameters for Pulsed Electric Fields Treated Beef Briskets. Food Bioprocess Technol. 2018, 11, 2055–2066. [Google Scholar] [CrossRef]
- Hung, Y.; De Kok, T.M.; Verbeke, W. Consumer attitude and purchase intention towards processed meat products with natural compounds and a reduced level of nitrite. Meat Sci. 2016, 121, 119–126. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Jayawardena, S.R.; Bekhit, A.E.D.A. Pulsed electric field: A new way to im-prove digestibility of cooked beef. Meat Sci. 2019, 155, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, M.; Hafsteinsson, H. Effect of electric field pulses on microstructure of muscle foods and roes. Trends Food Sci. Technol. 2001, 12, 122–128. [Google Scholar] [CrossRef]
- Chian, F.M.; Kaur, L.; Oey, I.; Astruc, T.; Hodgkinson, S.; Boland, M. Effect of Pulsed Electric Fields (PEF) on the ultrastructure and in vitro protein digestibility of bovine longissimus thoracis. LWT 2019, 103, 253–259. [Google Scholar] [CrossRef]
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Lhoutellier, S.V.; Astruc, T.; Marinova, P.; Greve, E.; Gatellier, P. Effect of Meat Cooking on Physicochemical State and in Vitro Digestibility of Myofibrillar Proteins. J. Agric. Food Chem. 2008, 56, 1488–1494. [Google Scholar] [CrossRef]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.; Dellarosa, N.; Laghi, L.; Carrière, F.; Capozzi, F.; Dupont, D.; Deglaire, A. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official method 981.10 crude protein in meat: Block digestion method. In Official Methods of Analysis of AOAC; AOAC: Rockville, MD, USA, 1981; Volume 2, pp. 7–8. [Google Scholar]
- Moore, S. Amino Acid Analysis: Aqueous Dimethyl Sulfoxide as Solvent for the Ninhydrin Reaction. J. Biol. Chem. 1968, 243, 6281–6283. [Google Scholar] [CrossRef]
- Flint, F.O.; Pickering, K. Demonstration of collagen in meat products by an improved picro-Sirius Red polarisation method. Analyst 1984, 109, 1505–1506. [Google Scholar] [CrossRef]
- Chian, F.M.; Kaur, L.; Astruc, T.; Vénien, A.; Stübler, A.S.; Aganovic, K.; Loison, O.; Hodgkinson, S.; Boland, M. Shockwave processing of beef brisket in conjunction with sous vide cooking: Effects on protein structural characteristics and muscle microstructure. Food Chem. 2021, 343, 128500. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The Use of Lead Citrate at High Ph as an Electron-Opaque Stain in Electron Microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Kaur, L.; Maudens, E.; Haisman, D.R.; Boland, M.J.; Singh, H. Microstructure and protein digestibility of beef: The effect of cooking conditions as used in stews and curries. LWT 2014, 55, 612–620. [Google Scholar] [CrossRef]
- Boland, M.; Kaur, L.; Chian, F.M.; Astruc, T. Muscle proteins. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 164–179. [Google Scholar]
- Obinata, T.; Maruyama, K.; Sugita, H.; Kohama, K.; Ebashi, S. Dynamic aspects of structural proteins in vertebrate skeletal muscle. Muscle Nerve 1981, 4, 456–488. [Google Scholar] [CrossRef] [PubMed]
- Fadda, S.; Chambon, C.; Vergès, C.M.; Talon, R.; Vignolo, G. Lactobacillus role during conditioning of refrigerated and vacuum-packaged Argentinean meat. Meat Sci. 2008, 79, 603–610. [Google Scholar] [CrossRef]
- Lindahl, G.; Lagerstedt, Å.; Ertbjerg, P.; Sampels, S.; Lundström, K. Ageing of large cuts of beef loin in vacuum or high oxygen modified atmosphere–Effect on shear force, calpain activity, desmin degradation and protein oxidation. Meat Sci. 2010, 85, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Astruc, T.; Vénien, A.; Loison, O.; Cui, J.; Irastorza, M.; Boland, M. High pressure processing of meat: Effects on ultrastructure and protein digestibility. Food Funct. 2016, 7, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S. Methodology for determining degree of hydrolysis of proteins in hydrolysates: A review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef] [Green Version]
- Bordoni, A.; Laghi, L.; Babini, E.; Di Nunzio, M.; Picone, G.; Ciampa, A.; Valli, V.; Danesi, F.; Capozzi, F. The foodomics approach for the evaluation of protein bioaccessibility in processed meat upon in vitro digestion. Electrophoresis 2014, 35, 1607–1614. [Google Scholar] [CrossRef]
- Astruc, T. Muscle structure and digestive enzyme bioaccessibility to intracellular compartments. In Food Structures, Digestion and Health; Boland, M., Golding, M., Singh, H., Eds.; Academic Press: London, UK, 2014; pp. 193–222. [Google Scholar]
- Ke, S.; Huang, Y.; Decker, E.A.; Hultin, H.O. Impact of citric acid on the tenderness, microstructure and oxidative sta-bility of beef muscle. Meat Sci. 2009, 82, 113–118. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Faridnia, F.; Bremer, P.J.; Silcock, P.; Oey, I. Pulsed Electric Fields Effects on Meat Tissue Quality and Functionality. In Handbook of Electroporation; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–21. [Google Scholar]
- Zhu, X.; Kaur, L.; Staincliffe, M.; Boland, M. Actinidin pretreatment and sous vide cooking of beef brisket: Effects on meat microstructure, texture and in vitro protein digestibility. Meat Sci. 2018, 145, 256–265. [Google Scholar] [CrossRef]
- Leander, R.C.; Hedrick, H.B.; Brown, M.F.; White, J.A. Comparison of structural changes in bovine longissimus and semitendinosus muscles during cooking. J. Food Sci. 1980, 45, 1–6. [Google Scholar] [CrossRef]
- Knöll, R.; Buyandelger, B.; Lab, M. The Sarcomeric Z-Disc and Z-Discopathies. J. Biomed. Biotechnol. 2011, 2011, 569628. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.; Oiseth, S.; Hughes, J.; Warner, R. The structural basis of cooking loss in beef: Variations with temperature and ageing. Food Res. Int. 2016, 89, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Martens, H.; Stabursvik, E. Texture and Colour Changes in Meat During Cooking Related to Thermal Denaturation of Muscle Proteins. J. Texture Stud. 1982, 13, 291–309. [Google Scholar] [CrossRef]
- Ouali, A. Meat tenderization: Possible Causes and Mechanisms. A review. J. Muscle Foods 1990, 1, 129–165. [Google Scholar] [CrossRef]
- Hernandez, D.E.; Salaseviciene, A.; Ertbjerg, P. Low-temperature long-time cooking of meat: Eating quality and underlying mechanisms. Meat Sci. 2018, 143, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, L.; Ertbjerg, P.; Løje, H.; Risbo, J.; Berg, F.W.J.V.D.; Christensen, M. Relationship between meat toughness and properties of connective tissue from cows and young bulls heat treated at low temperatures for prolonged times. Meat Sci. 2013, 93, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Hui, S.X.; Boland, M. Changes in Cathepsin Activity during Low-Temperature Storage and Sous Vide Processing of Beef Brisket. Food Sci. Anim. Resour. 2020, 40, 415–425. [Google Scholar] [CrossRef]
- Christensen, L.; Ertbjerg, P.; Aaslyng, M.D.; Christensen, M. Effect of prolonged heat treatment from 48°C to 63°C on toughness, cooking loss and color of pork. Meat Sci. 2011, 88, 280–285. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef]
- Sen, A.R.; Sharma, N. Effect of Freezing and Thawing on the Histology and Ultrastructure of Buffalo Muscle. Asian-Australas. J. Anim. Sci. 2004, 17, 1291–1295. [Google Scholar] [CrossRef]
- McGuire, M.; Beerman, K.A. Protein. In Nutritional Sciences: From Fundamentals to Food, 3rd ed.; Cengage Learning: Wadsworth, OH, USA, 2012; pp. 161–216. [Google Scholar]


| Digestion Phase | Enzyme Types | Enzyme Concentrations | Digestion Duration (min) | Cumulative Digestion Time (min) | Sampling Time (min) Based on Cumulative Digestion Time |
|---|---|---|---|---|---|
| Oral (pH 7 ± 0.1) | α-amylase (10025, Sigma Aldrich, Saint Louis, MO, USA) | 1.25 × 10−6 katal /mL bolus | 2 | 2 | No |
| Gastric (pH 3 ± 0.1) | Pepsin (P7125, Sigma Aldrich, Saint Louis, MO, USA) | 1.33 × 10−7 katal/mg meat protein | 60 | 62 | 2, 32, 62 |
| Small intestinal (pH 7 ± 0.1) | Pancreatin (P1750, Sigma Aldrich, Saint Louis, MO, USA) | 1:100 pancreatin to meat protein ratio | 120 | 182 | 122, 182 |
| Cumulative Digestion Time (min) | 2 | 32 | 62 | 122 | 182 | |
|---|---|---|---|---|---|---|
| Ninhydrin-reactive amino nitrogen (%) | Control–SV | 1.9 ± 0.0 a,A | 2.3 ± 0.4 a,A | 2.7 ± 1.0 a,A | 8.1 ± 1.2 b,A | 9.8 ± 0.6 c,A |
| PEF–SV | 1.9 ± 0.0 a,A | 2.7 ± 0.6 a,b,A | 3.4 ± 1.0 b,A | 8.4 ± 0.4 c,A | 12.6 ± 0.7 d,B | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chian, F.M.; Kaur, L.; Oey, I.; Astruc, T.; Hodgkinson, S.; Boland, M. Effects of Pulsed Electric Field Processing and Sous Vide Cooking on Muscle Structure and In Vitro Protein Digestibility of Beef Brisket. Foods 2021, 10, 512. https://doi.org/10.3390/foods10030512
Chian FM, Kaur L, Oey I, Astruc T, Hodgkinson S, Boland M. Effects of Pulsed Electric Field Processing and Sous Vide Cooking on Muscle Structure and In Vitro Protein Digestibility of Beef Brisket. Foods. 2021; 10(3):512. https://doi.org/10.3390/foods10030512
Chicago/Turabian StyleChian, Feng Ming, Lovedeep Kaur, Indrawati Oey, Thierry Astruc, Suzanne Hodgkinson, and Mike Boland. 2021. "Effects of Pulsed Electric Field Processing and Sous Vide Cooking on Muscle Structure and In Vitro Protein Digestibility of Beef Brisket" Foods 10, no. 3: 512. https://doi.org/10.3390/foods10030512
APA StyleChian, F. M., Kaur, L., Oey, I., Astruc, T., Hodgkinson, S., & Boland, M. (2021). Effects of Pulsed Electric Field Processing and Sous Vide Cooking on Muscle Structure and In Vitro Protein Digestibility of Beef Brisket. Foods, 10(3), 512. https://doi.org/10.3390/foods10030512