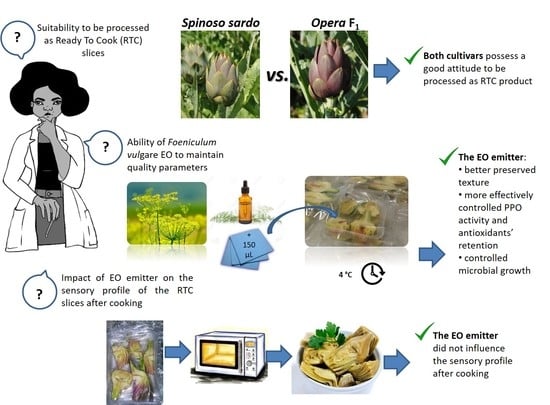
Active Packaging-Releasing System with Foeniculum vulgare Essential Oil for the Quality Preservation of Ready-to-Cook (RTC) Globe Artichoke Slices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Field, Plant Material, and Management Practices
2.2. Head Harvest, Post-Harvest Treatments, and Sampling
2.3. Weight Loss and Headspace Gas Composition
2.4. Color Analysis
2.5. Texture Analysis
2.6. Microbiological Analyses
2.7. Chemical and Enzymatic Analyses
2.8. Sensory Analyses
2.9. Statistical Analysis
3. Results and Discussion
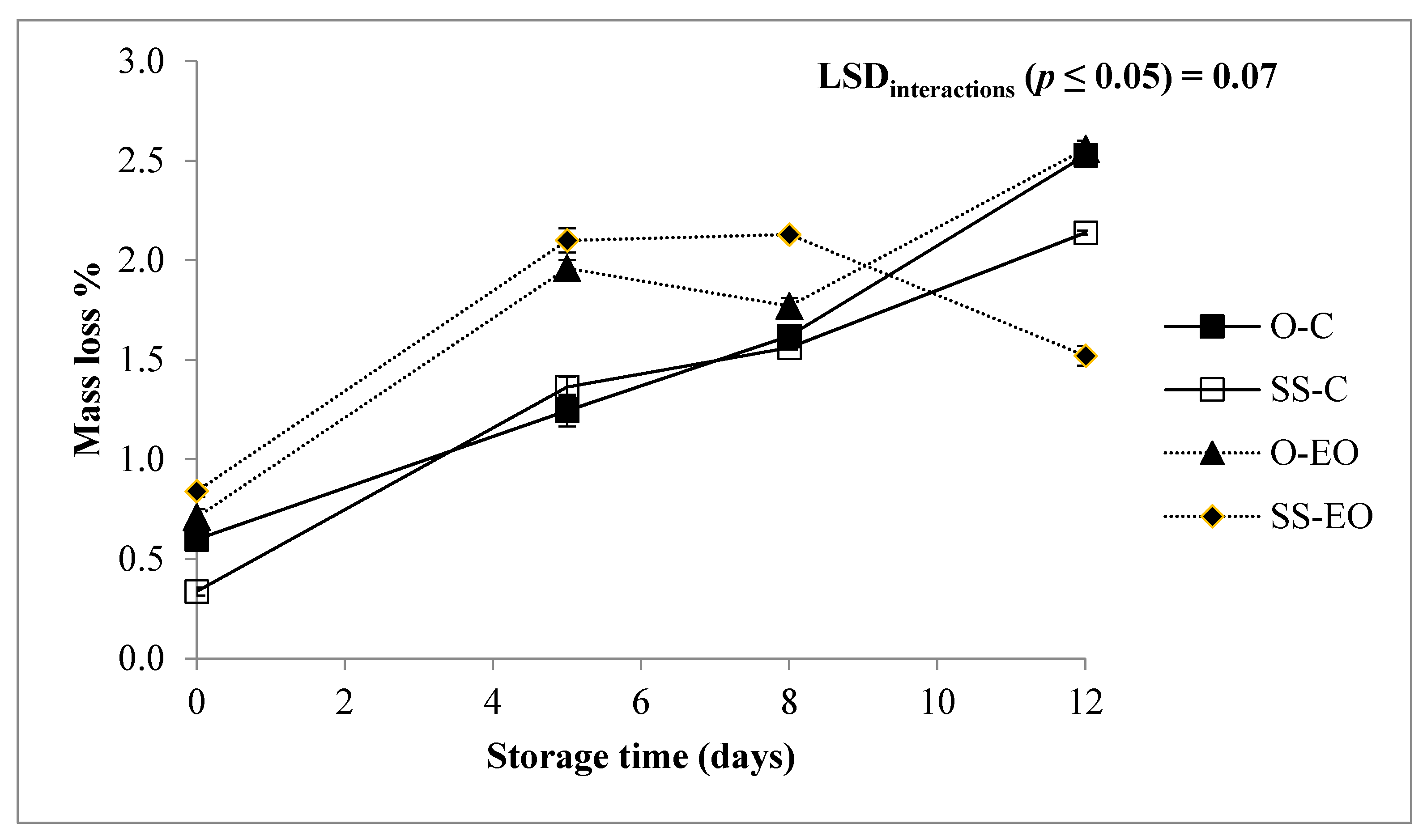
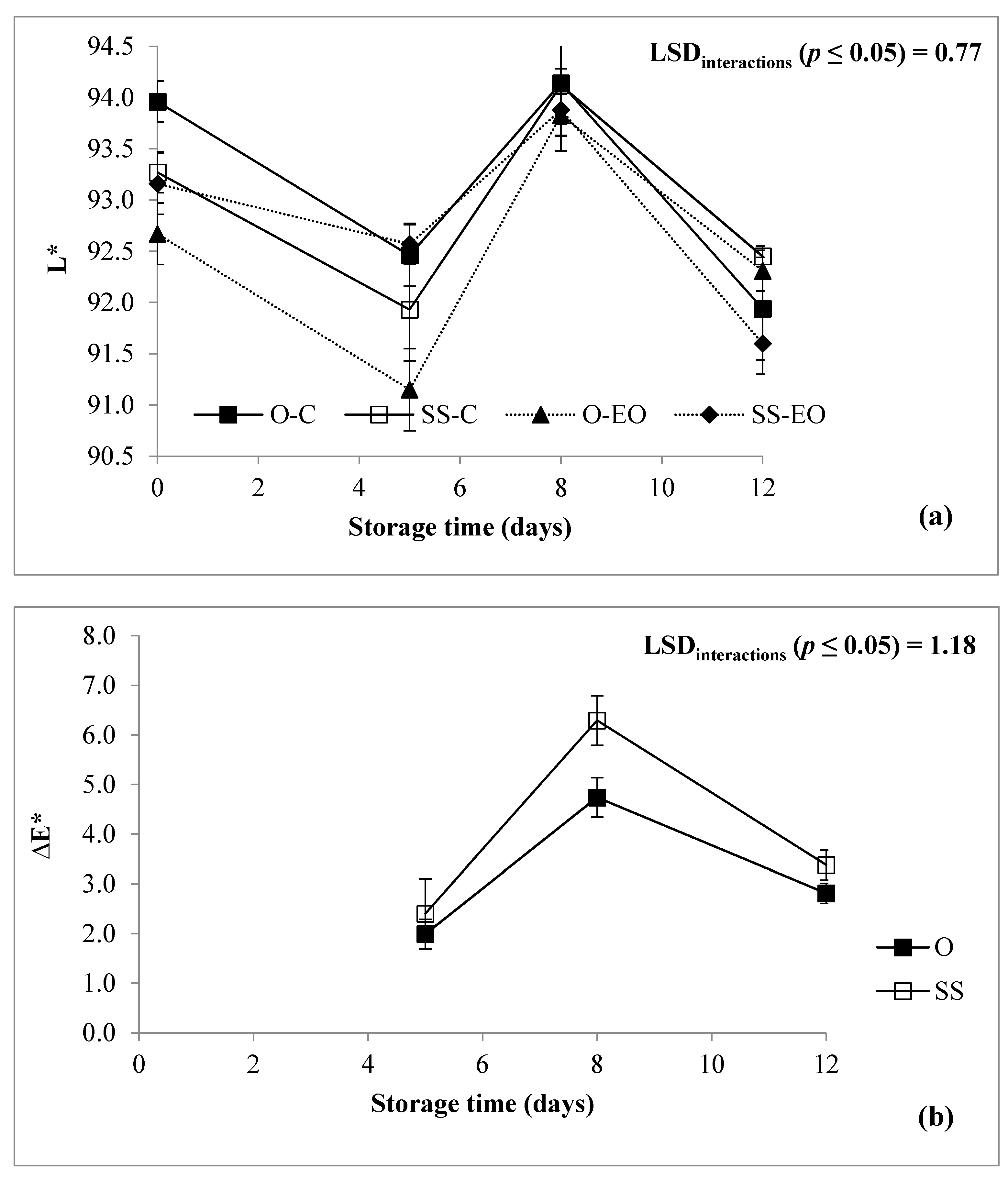
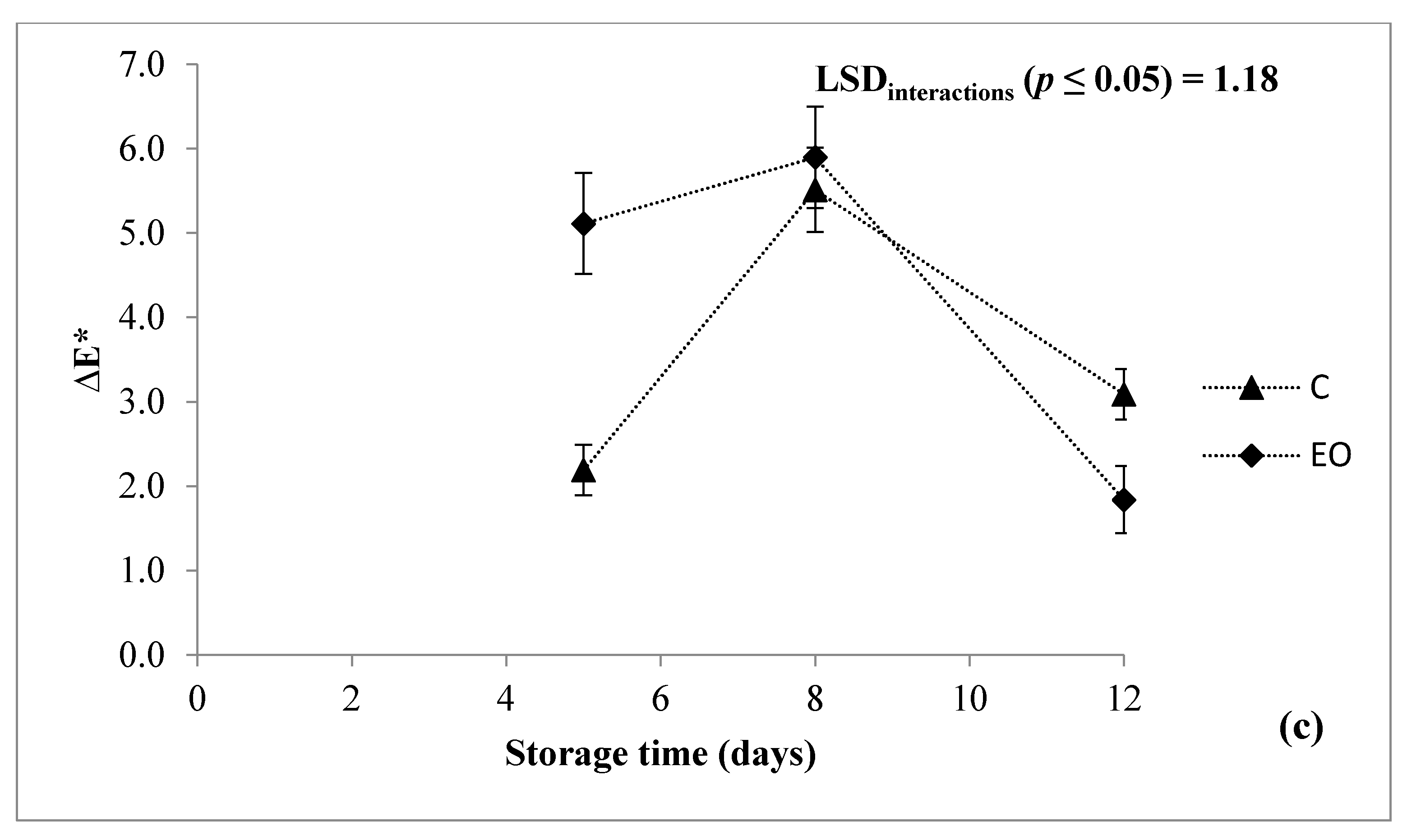
3.1. Fresh Weight Loss, Headspace Concentration, Color, and Texture
3.2. Antioxidant Compounds Content and Activity
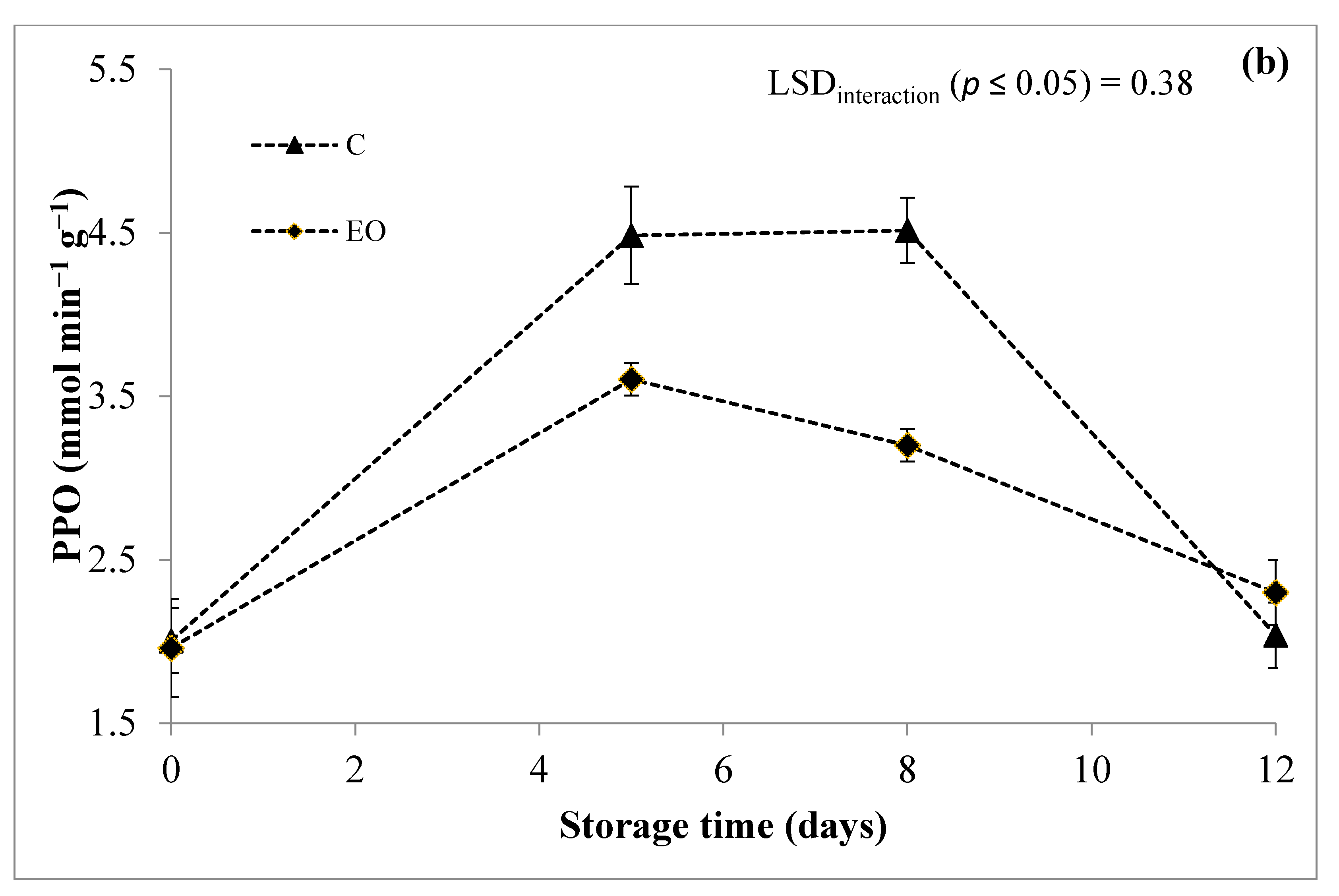
3.3. Polyphenol Oxidase (PPO) Activity
3.4. Microbiological Quality
3.5. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lombardo, S.; Pandino, G.; Mauromicale, G. The influence of pre-harvest factors on the quality of globe artichoke. Sci. Hortic. 2018, 233, 479–490. [Google Scholar] [CrossRef]
- Pandino, G.; Mauromicale, G. Globe artichoke and cardoon forms between traditional and modern uses. Acta Hortic. 2020, 1284, 1–18. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Moglia, A.; Portis, E.; Lanteri, S.; Mauromicale, G. Leaf polyphenol profile and SSR-based fingerprinting of new segregant Cynara cardunculus genotypes. Front. Plant Sci. 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Chemical and morphological characteristics of new clones and commercial varieties of globe artichoke (Cynara cardunculus var. scolymus). Plant Foods Hum. Nutr. 2011, 66, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Restuccia, C.; Muratore, G.; Barbagallo, R.N.; Licciardello, F.; Pandino, G.; Scifò, G.O.; Mazzaglia, A.; Ragonese, F.; Mauromicale, G. Effect of nitrogen fertilisation on the overall quality of minimally processed globe artichoke head. J. Sci. Food Agric. 2017, 97, 650–658. [Google Scholar] [CrossRef]
- Muratore, G.; Restuccia, C.; Licciardello, F.; Lombardo, S.; Pandino, G.; Mauromicale, G. Effect of packaging film and antibrowning solution on quality maintenance of minimally processed globe artichoke heads. Innov. Food Sci. Emerg. Technol. 2015, 31, 97–104. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Chisari, M.; Spagna, G. Enzymatic browning and softening in vegetable crops: Studies and experiences. Ital. J. Food Sci. 2009, 21, 3–16. [Google Scholar]
- Ricceri, J.; Barbagallo, R.N. Role of protease and oxidase activities involved in some technological aspects of the globe artichoke processing and storage. LWT Food Sci. Technol. 2016, 71, 196–201. [Google Scholar] [CrossRef]
- Licciardello, F.; Pandino, G.; Barbagallo, R.N.; Lombardo, S.; Restuccia, C.; Muratore, G.; Mazzaglia, A.; Strano, M.G.; Mauromicale, G. Quality traits of ready-to-use globe artichoke slices as affected by genotype, harvest time and storage time. Part II: Physiological, microbiological and sensory aspects. LWT Food Sci. Technol. 2017, 79, 554–560. [Google Scholar] [CrossRef]
- Pandino, G.; Barbagallo, R.N.; Lombardo, S.; Restuccia, C.; Muratore, G.; Licciardello, F.; Mazzaglia, A.; Ricceri, J.; Pesce, G.R.; Mauromicale, G. Quality traits of ready-to-use globe artichoke slices as affected by genotype, harvest time and storage time. Part I: Biochemical and physical aspects. LWT Food Sci. Technol. 2017, 76, 181–189. [Google Scholar] [CrossRef]
- Rizzo, V.; Lombardo, S.; Pandino, G.; Barbagallo, R.N.; Mazzaglia, A.; Restuccia, C.; Mauromicale, G.; Muratore, G. Shelf-life study of ready-to-cook slices of globe artichoke ‘Spinoso sardo’: Effects of anti-browning solutions and edible coating enriched with Foeniculum vulgare essential oil. J. Sci. Food Agric. 2019, 99, 5219–5228. [Google Scholar] [CrossRef]
- Rizzo, V.; Muratore, G. The Application of Essential Oils in Edible Coating: Case of Study on Two Fresh Cut Products. Int. J. Clin. Nutr. Diet. 2020, 6, 149. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.; Aggarwal, N.K. Efficacy of plant antimicrobials as preservative in food. In Food Preservation and Waste Exploitation; Socaci, S.A., Frca, A.C., Aussenac, T., Laguerre, J.-C., Eds.; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar] [CrossRef] [Green Version]
- Scavo, A.; Pandino, G.; Restuccia, C.; Parafati, L.; Cirvilleri, G.; Mauromicale, G. Antimicrobial activity of cultivated cardoon (Cynara cardunculus L. var. altilis DC.) leaf extracts against bacterial species of agricultural and food interest. Ind. Crops. Prod. 2019, 129, 206–211. [Google Scholar] [CrossRef]
- Wieczyńska, J.; Cavoski, I. Antimicrobial, antioxidant and sensory features of eugenol, carvacrol and trans-anethole in active packaging for organic ready-to-eat iceberg lettuce. Food Chem. 2018, 259, 251–260. [Google Scholar] [CrossRef] [PubMed]
- La Pergola, A.; Restuccia, C.; Napoli, E.; Bella, S.; Brighina, S.; Russo, A.; Suma, P. Commercial and wild sicilian Origanum vulgare essential oils: Chemical composition, antimicrobial activity and repellent effects. J. Essent. Oil Res. 2017, 29, 451–460. [Google Scholar] [CrossRef]
- Restuccia, C.; Conti, G.O.; Zuccarello, P.; Parafati, L.; Cristaldi, A.; Ferrante, M. Efficacy of different citrus essential oils to inhibit the growth and B1 aflatoxin biosynthesis of Aspergillus flavus. Environ. Sci. Pollut. Res. Int. 2019, 26, 31263–31272. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Pérez-Álvarez, J.A.; Fernández López, J. Chemical characterization and antibacterial activity of Thymus moroderi and Thymus piperella essential oils, two Thymus endemic species from southeast of Spain. Food Control 2012, 27, 294–299. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Salami, M.; Rahimmalek, M.; Ehtemam, M.H.; Szumny, A.; Fabian, S.; Matkowski, A. Essential oil composition, antimicrobial activity and anatomical characteristics of Foeniculum vulgare Mill. fruits from different regions of Iran. J. Essent. Oil Bear. Plants 2016, 19, 1614–1626. [Google Scholar] [CrossRef]
- Gulfraz, M.; Mehmood, S.; Minhas, N.; Jabeen, N.; Kausar, R.; Jabeen, K.; Arshad, G. Composition and antimicrobial properties of essential oil of Foeniculum vulgare. Afr. J. Biotechnol. 2008, 7, 4364–4368. Available online: http://www.academicjournals.org/AJB (accessed on 4 January 2021).
- Chang, S.; Abbaspour, H.; Nafchi, A.M.; Heydari, A.; Mohammadhosseini, M. Iranian Foeniculum vulgare essential oil and alcoholic extracts: Chemical composition, antimicrobial, antioxidant and application in olive oil preservation. J. Essent. Oil Bear. Plants 2016, 19, 1920–1931. [Google Scholar] [CrossRef]
- Belabdelli, F.; Piras, A.; Bekhti, N.; Falconieri, D.; Belmokhtar, Z.; Merad, Y. Chemical Composition and Antifungal Activity of Foeniculum vulgare Mill. Chem. Afr. 2020, 3, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; U.S. Gov. Print: Washington, DC, USA, 1999.
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Mauromicale, G. Choice of time of harvest influences the polyphenol profile of globe artichoke. J. Funct. Foods 2013, 5, 1822–1828. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauro, R.P.; Mauromicale, G. Variation in polyphenol profile and head morphology among clones of globe artichoke selected from a landrace. Sci. Hortic. 2012, 138, 259–265. [Google Scholar] [CrossRef]
- Mauromicale, G.; Ierna, A. Characteristics of heads of seed-grown globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori] as affected by harvest period, sowing date and gibberellic acid. Agronomie 2000, 20, 197–204. [Google Scholar] [CrossRef]
- Rizzo, V.; Amoroso, L.; Licciardello, F.; Mazzaglia, A.; Muratore, G.; Restuccia, C.; Lombardo, S.; Pandino, G.; Strano, M.G.; Mauromicale, G. The effect of sous vide packaging with rosemary essential oil on storage quality of fresh-cut potato. LWT-Food Sci. Technol. 2018, 94, 111–118. [Google Scholar] [CrossRef]
- Licciardello, F.; Lombardo, S.; Rizzo, V.; Pitino, I.; Pandino, G.; Strano, M.G.; Muratore, G.; Restuccia, C.; Mauromicale, G. Integrated agronomical and technological approach for the quality maintenance of ready-to-fry potato sticks during refrigerated storage. Postharvest Biol. Technol. 2018, 136, 23–30. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Espín, J.C.; Tudela, J.; García-Cánovas, F. Monophenolase activity of polyphenol oxidase from artichoke (Cynara scolymus, L.) heads. LWT Food Sci. Technol. 1997, 30, 819–825. [Google Scholar] [CrossRef]
- Espín, J.C.; Tudela, J.; García-Cánovas, F. 4-hydroxyanisole: The most suitable monophenolic substrate for determining spectrophotometrically the monophenolase activity of polyphenol oxidase from fruits and vegetables. Anal. Biochem. 1998, 259, 118–126. [Google Scholar] [CrossRef]
- UNI EN ISO 13299. Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile; Ente Nazionale Italiano di Unificazione: Milano, Italy, 2016.
- UNI EN ISO 8586. Sensory Analysis—General Guidelines for the Selection Training and Monitoring of Selected Assessors; Ente Nazionale Italiano di Unificazione: Milano, Italy, 2012.
- UNI EN ISO 8589. Analisi Sensoriale—Guida Generale per la Progettazione di Locali di Prova; Ente Nazionale Italiano di Unificazione: Milano, Italy, 2014.
- Ghidelli, C.; Mateos, M.; Rojas-Argudo, C.; Pérez-Gago, M.B. Novel approaches to control browning of fresh-cut artichoke: Effect of a soy protein-based coating and modified atmosphere packaging. Postharvest Biol. Technol. 2015, 99, 105–113. [Google Scholar] [CrossRef]
- Rico, D.; Martìn-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, S.; Restuccia, C.; Pandino, G.; Licciardello, F.; Muratore, G.; Mauromicale, G. Influence of an O3-atmosphere storage on microbial growth and antioxidant contents of globe artichoke as affected by genotype and harvest time. Innov. Food Sci. Emerg. Technol. 2015, 27, 121–128. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Ruta, C.; Mauromicale, G. In vitro micropropagation and mycorrhizal treatment influences the polyphenols content profile of globe artichoke under field conditions. Food Res. Int. 2017, 99, 385–392. [Google Scholar] [CrossRef]
- Restuccia, C.; Lombardo, S.; Pandino, G.; Licciardello, F.; Muratore, G.; Mauromicale, G. An innovative combined water ozonisation/O3-atmosphere storage for preserving the overall quality of two globe artichoke cultivars. Innov. Food Sci. Emerg. Technol. 2014, 21, 82–89. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaglione, D.; Reyes-Chin-Wo, S.; Acquadro, A.; Froenicke, L.; Portis, E.; Beitel, C.; Tirone, M.; Mauro, R.; Lo Monaco, A.; Mauromicale, G.; et al. The genome sequence of the outbreeding globe artichoke constructed de novo incorporating a phase-aware low-pass sequencing strategy of F1 progeny. Sci. Rep. 2016, 6, 19427. [Google Scholar] [CrossRef] [Green Version]
- Acquadro, A.; Barchi, L.; Portis, E.; Mangino, G.; Valentino, D.; Mauromicale, G.; Lanteri, S. Genome reconstruction in Cynara cardunculus taxa gains access to chromosome-scale DNA variation. Sci. Rep. 2017, 7, 5617. [Google Scholar] [CrossRef]
- Lattanzio, V.; Linsalata, V.; Palmeri, S.; Van Sumere, C.F. The beneficial effect of citric and ascorbic acid on the phenolic browning reaction in stored artichokes (Cynara scolymus L.) heads. Food Chem. 1989, 33, 93–106. [Google Scholar] [CrossRef]
- Leoni, O.; Calmieri, S.; Lattanzio, V.; Van Sumere, C.F. Polyphenol oxidase from artichoke (Cynara scolymus L.). Food Chem. 1990, 38, 27–39. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cardinali, A.; Di Venere, D.; Linsalata, V.; Palmieri, S. Browning phenomena in stored artichoke (Cynara scolymus L.) heads: Enzymic or chemical reactions? Food Chem. 1994, 50, 1–7. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, J.N.; Escribano, J.; Garcia-Canovas, F. A continuous spectrophotometric method for the determination of monophenolase activity of tyrosinase using 3-methyl-2-benzothiazolinone hydrazone. Anal. Biochem. 1994, 216, 205–212. [Google Scholar] [CrossRef] [PubMed]
- CNERNA-CNRS. Produits de la IVe gamme. In La Qualité Microbiologique des Aliments (Maîtrise et Critères), 2nd ed.; Jouve, J., Ed.; Polytechnica: Paris, France, 1996; pp. 73–98. [Google Scholar]
- Ministere de l’Economie des Finances et du Budget. Marché Consommation, Produits Vegetaux prets a L’emploi dits de la “IVemme Gamme”: Guide de Bonnes Pratique Hygieniques; Journal Officiel De La Republique Francaise: Paris, France, 1988; pp. 1621–1629. [Google Scholar]
- B.O.E. Real Decreto 3484/2000, de 29 de Diciembre, por el que se Establecen las Normas de Higiene para la Elaboración, Distribución y Comercio de Comidas Preparadas; Boletín Oficial del Estado: Madrid, Spain, 2001; p. 11. [Google Scholar]
- The Commission of the European Communities. Commission Regulation (EC) No 1441/2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuff; L 322/12; Official Journal of the European Union: Brussels, Belgium, 2007; pp. 12–29. [Google Scholar]






| (a) | Qualitative Trait | ||||||||||||
| Source of Variation | df | ML% | O2% | CO2% | TPC | AsAC | AA | Text | L* | a* | b* | ∆E | |
| Genotype (G) | 1 | 0.13 *** (2.46) | 4.20 NS (3.02) | 4.10 NS (5.84) | 43.01 * (4.75) | 2485 *** (15.24) | 304 *** (38.07) | 347.49 * (20.02) | 0.03 NS (0.3) | 1.87 NS (1.43) | 0.04 NS (0) | 0.81 NS (1.4) | |
| Treatment (T) | 1 | 0.6 *** (11.82) | 0.0 NS (0) | 20.11 * (28.66) | 118.20 ** (13.05) | 1300 *** (7.98) | 112 *** (14.10) | 89.28 NS (5.14) | 1.19 ** (6.2) | 5.74 NS (4.4) | 1.06 NS (2.3) | 2.11 NS (3.64) | |
| Storage time (St) | 3 | 3.54 *** (69.75) | 80.56 *** (57.95) | 30.31 ** (43.2) | 698.14 *** (77.10) | 11,577 *** (71) | 321 *** (40.25) | 113.75 NS (6.55) | 7.35 *** (71.78) | 104.1 ** (79.79) | 37.54 *** (81.38) | 45.41 *** (78.36) | |
| (G) × (T) | 1 | 0.0 NS (0) | 10.89 NS (7.83) | 2.28 NS (3.25) | 1.58 NS (0.17) | 4.5 NS (0.03) | 0.08 NS (0.01) | 10.27 NS (0.6) | 0.49 NS (4.79) | 0.31 NS (0.24) | 0.11 NS (0.24) | 0.77 NS (1.33) | |
| (G) × (St) | 3 | 0.33 *** (6.5) | 15.90 * (11.44) | 3.32 NS (4.73) | 33.23 * (3.67) | 118 NS (0.72) | 32 *** (4.04) | 333.91 * (19.24) | 0.14 NS (1.3) | 7.51 NS (5.8) | 2.88 NS (6.2) | 2.11 * (3.64) | |
| (T) × (St) | 3 | 0.36 *** (7.09) | 16.26 * (11.70) | 6.91 NS (9.85) | 6.45 * (0.71) | 815 *** (5) | 21 *** (2.64) | 338.91 * (19.52) | 0.09 NS (0.9) | 1.04 NS (0.8) | 0.40 NS (0.9) | 6.12 *** (10.56) | |
| (G) × (T) × (St) | 3 | 0.12 *** (2.36) | 11.21 NS (8.06) | 3.13 NS (4.46) | 4.89 NS (0.54) | 5.2 NS (0.03) | 7 NS (0.89) | 502.20 ** (28.93) | 0.95 ** (9.28) | 9.89 NS (7.58) | 4.10 NS (8.9) | 0.62 NS (1.1) | |
| Total mean square | 5.07 | 139.02 | 70.16 | 905.49 | 16,306 | 798 | 1735.81 | 10.24 | 130.46 | 46.13 | 57.95 | ||
| (b) | Qualitative Trait | ||||||||||||
| Source of Variation | df | MB | PB | YM | Enterobacteria | Pseudomonas | E. coli | PPO | |||||
| Genotype (G) | 1 | 11.59 *** (55) | 17.66 *** (61.71) | 33.84 *** (58.5) | 4.69 *** (24.41) | 4.13 *** (10.31) | 2.12 *** (4.35) | 4.14 *** (22.73) | |||||
| Treatment (T) | 1 | 1.06 *** (5) | 1.06 *** (3.7) | 0.77 *** (1.3) | 0.49 *** (2.55) | 1.75 *** (4.37) | 7.51 *** (15.42) | 1.58 *** (8.68) | |||||
| Storage time (St) | 3 | 7.74 *** (36.73) | 9.49 *** (33.16) | 22.70 *** (39.23) | 13.46 *** (70.07) | 30.07 *** (75.04) | 26.70 *** (54.81) | 9.19 *** (50.47) | |||||
| (G) × (T) | 1 | 8 × 10−4 NS (0) | 0.02 *** (0) | 0.03 *** (0) | 0.07 *** (0.36) | 0.29 *** (0.72) | 1.07 *** (2.2) | 0.09 NS (0.5) | |||||
| (G) × (St) | 3 | 0.53 *** (2.5) | 0.22 *** (0.7) | 0.40 *** (0.7) | 0.37 *** (1.93) | 3.49 *** (8.71) | 8.46 *** (17.37) | 2.07 *** (11.37) | |||||
| (T) × (St) | 3 | 0.12 *** (0.6) | 0.13 *** (0.45) | 0.09 *** (0.16) | 0.10 *** (0.5) | 0.16 *** (0.4) | 2.22 *** (4.56) | 1.12 *** (6.15) | |||||
| (G) × (T) × (St) | 3 | 0.03 *** (0.14) | 0.04 *** (0.14) | 0.03 *** (0) | 0.03 *** (0.16) | 0.18 *** (0.45) | 0.63 *** (1.29) | 0.02 NS (0.1) | |||||
| Total mean square | 21.07 | 28.62 | 57.86 | 19.21 | 40.07 | 48.71 | 18.21 | ||||||
| (a) | Main Factor | Qualitative Trait | ||||||||||
| ML% | O2% | CO2% | TPC (g kg−1 DM) | AsAC (mg kg−1 DM) | AA (% DPPH inhibition) | Text (N) | L* | a* | b* | ∆E | ||
| Genotype | ||||||||||||
| OPERA F1 | 1.62 a | 4.57 a | 12.26 a | 21.26 a | 118 a | 56.6 a | 63.83 a | 92.80 a | 2.17 a | 0.31 a | 2.79 a | |
| SPINOSO SARDO | 1.49 b | 5.29 a | 12.98 a | 18.94 b | 100 b | 50.4 b | 57.24 b | 92.87 a | 2.65 a | 0.24 a | 3.11 a | |
| Treatment | ||||||||||||
| Control | 1.42 b | 4.94 a | 13.41 a | 18.18 b | 103 b | 51.6 b | 62.20 a | 93.03 a | 1.98 a | 0.46 a | 2.69 a | |
| Essential oil | 1.69 a | 4.92 a | 11.83 b | 22.02 a | 116 a | 55.4 a | 58.86 a | 92.65 b | 2.83 a | 0.09 a | 3.21 a | |
| Storage time (day) | ||||||||||||
| 0 | 0.62 d | 9.24 a | 11.36 b | 31.6 a | 165 a | 61.7 a | 64.82 a | 93.26 b | 3.43 a | −0.53 b | - | |
| 5 | 1.56 c | 2.14 c | 14.77 a | 23.78 b | 102 b | 55.3 b | 55.80 a | 92.03 c | 4.47 a | −0.92 b | 3.65 b | |
| 8 | 1.77 b | 3.02 b,c | 13.77 a | 13.15 c | 90 c | 48.8 c | 59.79 a | 93.99 a | −2.93 b | 3.51 a | 5.70 a | |
| 12 | 2.18 a | 5.33 b | 10.61 b | 11.87 c | 80 d | 48.2 c | 61.71 a | 92.07 c | 4.68 a | −0.95 b | 2.46 c | |
| (b) | Main Factor | Qualitative Trait | ||||||||||
| MB | PB | YM | Enterobacteria | E. coli | Pseudomonas | PPO | ||||||
| (log CFU g−1) | (mmol min−1 g−1) | |||||||||||
| Genotype | ||||||||||||
| OPERA F1 | 3.89 b | 3.64 b | 3.13 b | 4.53 b | 1.64 b | 4.12 b | 2.65 b | |||||
| SPINOSO SARDO | 5.09 a | 5.13 a | 5.18 a | 5.30 a | 2.23 a | 4.84 a | 3.37 a | |||||
| Treatment | ||||||||||||
| Control | 4.68 a | 4.57 a | 4.31 a | 5.04 a | 2.15 a | 4.72 a | 3.23 a | |||||
| Essential oil | 4.31 b | 4.20 b | 4 b | 4.79 b | 1.72 b | 4.25 b | 2.79 b | |||||
| Storage time (day) | ||||||||||||
| 0 | 3.21 d | 3.23 d | 1.8 d | 3.07 d | 0.8 d | 1.63 c | 2.01 b | |||||
| 5 | 4.33 c | 3.99 c | 4.08 c | 4.96 c | 1.68 c | 4.93 b | 4.03 a | |||||
| 8 | 4.92 b | 4.51 b | 5.28 b | 5.70 b | 2.35 b | 5.70 a | 3.83 a | |||||
| 12 | 5.52 a | 5.82 a | 5.46 a | 5.92 a | 2.91 a | 5.68 a | 2.16 b | |||||
| Treatment | |||||
|---|---|---|---|---|---|
| Storage Time (d) | Attribute | C | EO | ||
| “Opera F1” | “Spinoso sardo” | “Opera F1” | “Spinoso sardo” | ||
| 0 | Fennel odor | 1.0 a,1 | 1.0 a | 3.2 b | 3.4 b |
| Fennel flavor | 1.0 a | 1.0 a | 3.4 b | 3.3 b | |
| 5 | Fennel odor | 1.0 a | 1.0 a | 3.6 b | 4.5 b |
| Bitter | 4.8 b | 2.7 a | 3.2 ab | 2.5 a | |
| Fennel flavor | 1.0 a | 1.0 a | 3.9 b | 4.3 b | |
| 8 | Fennel odor | 1.0 a | 1.0 a | 2.6 b | 4.5 c |
| Fennel flavor | 1.0 a | 1.0 a | 3.2 b | 5.1 c | |
| 12 | Fennel odor | 1.0 a | 1.0 a | 3.6 b | 3.3 b |
| Fennel flavor | 1.0 a | 1.0 a | 3.9 b | 3.8 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, V.; Lombardo, S.; Pandino, G.; Barbagallo, R.N.; Mazzaglia, A.; Restuccia, C.; Mauromicale, G.; Muratore, G. Active Packaging-Releasing System with Foeniculum vulgare Essential Oil for the Quality Preservation of Ready-to-Cook (RTC) Globe Artichoke Slices. Foods 2021, 10, 517. https://doi.org/10.3390/foods10030517
Rizzo V, Lombardo S, Pandino G, Barbagallo RN, Mazzaglia A, Restuccia C, Mauromicale G, Muratore G. Active Packaging-Releasing System with Foeniculum vulgare Essential Oil for the Quality Preservation of Ready-to-Cook (RTC) Globe Artichoke Slices. Foods. 2021; 10(3):517. https://doi.org/10.3390/foods10030517
Chicago/Turabian StyleRizzo, Valeria, Sara Lombardo, Gaetano Pandino, Riccardo N. Barbagallo, Agata Mazzaglia, Cristina Restuccia, Giovanni Mauromicale, and Giuseppe Muratore. 2021. "Active Packaging-Releasing System with Foeniculum vulgare Essential Oil for the Quality Preservation of Ready-to-Cook (RTC) Globe Artichoke Slices" Foods 10, no. 3: 517. https://doi.org/10.3390/foods10030517