Curcumin-Loaded Pickering Emulsion Formed by Ultrasound and Stabilized by Metal Organic Framework Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Synthesis of UiO-66-NH2
2.4. Emulsion Preparation
2.5. Encapsulation Properties
2.6. Statistical Analysis
3. Results and Discussion
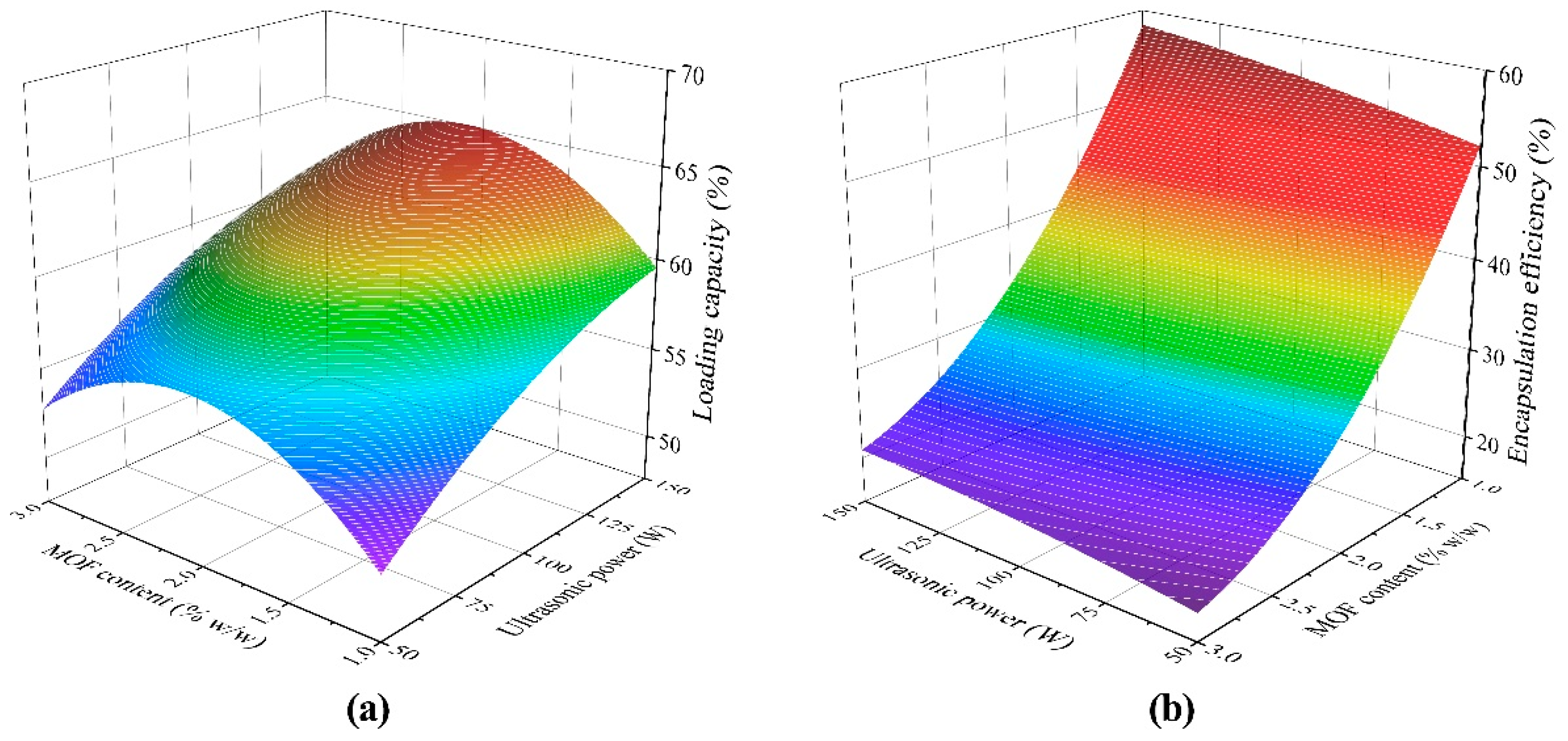
3.1. Mutual Effect of Parameters
3.2. Optimization of Synthesis Conditions
3.3. Model Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sui, X.; Bi, S.; Qi, B.; Wang, Z.; Zhang, M.; Li, Y.; Jiang, L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocoll. 2017, 63, 727–734. [Google Scholar] [CrossRef]
- Leong, T.S.; Martin, G.J.; Ashokkumar, M. Ultrasonic encapsulation–a review. Ultrason. Sonochemistry 2017, 35, 605–614. [Google Scholar] [CrossRef]
- Johansson, L.; Singh, T.; Leong, T.; Mawson, R.; McArthur, S.; Manasseh, R.; Juliano, P. Cavitation and non-cavitation regime for large-scale ultrasonic standing wave particle separation systems–In Situ gentle cavitation threshold determination and free radical related oxidation. Ultrason. Sonochemistry 2016, 28, 346–356. [Google Scholar] [CrossRef]
- McClements, D. Enhanced delivery of lipophilic bioactives using emulsions: A review of major factors affecting vitamin, nutraceutical, and lipid bioaccessibility. Food Funct. 2018, 9, 22–41. [Google Scholar] [CrossRef]
- Meroni, D.; Gasparini, C.; Di Michele, A.; Ardizzone, S.; Bianchi, C.L. Ultrasound-assisted synthesis of ZnO photocatalysts for gas phase pollutant remediation: Role of the synthetic parameters and of promotion with WO3. Ultrason. Sonochemistry 2020, 66, 105119. [Google Scholar] [CrossRef]
- Wang, P.-L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal–Organic Frameworks for Food Safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial applications of metal–organic frameworks and their composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Luo, Y.; Zhou, B.; Mei, L.; Wang, Q.; Zhang, B. Porous metal-organic framework (MOF) Carrier for incorporation of volatile antimicrobial essential oil. Food Control 2019, 98, 174–178. [Google Scholar] [CrossRef]
- Kagan, C.R. At the Nexus of Food Security and Safety: Opportunities for Nanoscience and Nanotechnology; ACS Publications: Washington, DC, USA, 2016. [Google Scholar]
- Dong, Z.; Sun, Y.; Chu, J.; Zhang, X.; Deng, H. Multivariate metal–organic frameworks for dialing-in the binding and programming the release of drug molecules. J. Am. Chem. Soc. 2017, 139, 14209–14216. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, P.; Li, A.; Wiggin, S.; Tao, A.; Maloney, A.; Wood, P.; Ward, S.; Fairen-Jimenez, D. Development of a Cambridge Structural Database Subset: A Collection of Metal-Organic Frameworks for Past, Present, and Future. Chem. Mater. 2017, 29, 2618–2625. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Gualdron, D.; Moghadam, P.; Hupp, J.; Farha, O.; Snurr, R. Application of Consistency Criteria To Calculate BET Areas of Micro- And Mesoporous Metal-Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 215–224. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Wang, X.; Chai, Z.; Shi, W.; Hou, J.; Hayat, T.; Alsaedi, A.; Wang, X. Heteroaggregation behavior of graphene oxide on Zr-based metal-organic frameworks in aqueous solutions: A combined experimental and theoretical study. J. Mater. Chem. A 2017, 5, 20398–20406. [Google Scholar] [CrossRef]
- Orellana-Tavra, C.; Baxter, E.; Tian, T.; Bennett, T.; Slater, N.; Cheetham, A.; Fairen-Jimenez, D. Amorphous metal-organic frameworks for drug delivery. Chem. Commun. 2015, 51, 13878–13881. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chen, J.; Hu, Y.; Zhang, N.; Fu, Y.; Chen, X. Tuning complexation of carboxymethyl cellulose/cationic chitosan to stabilize Pickering emulsion for curcumin encapsulation. Food Hydrocoll. 2021, 110, 106135. [Google Scholar] [CrossRef]
- Lv, P.; Wang, D.; Dai, L.; Wu, X.; Gao, Y.; Yuan, F. Pickering emulsion gels stabilized by high hydrostatic pressure-induced whey protein isolate gel particles: Characterization and encapsulation of curcumin. Food Res. Int. 2020, 132, 109032. [Google Scholar] [CrossRef]
- Shi, A.; Feng, X.; Wang, Q.; Adhikari, B. Pickering and high internal phase Pickering emulsions stabilized by protein-based particles: A review of synthesis, application and prospective. Food Hydrocoll. 2020, 69, 106117. [Google Scholar] [CrossRef]
- Kang, J.; Kim, Y.H.; Choi, S.J.; Rho, S.J.; Kim, Y.R. Improving the Stability and Curcumin Retention Rate of Curcumin-Loaded Filled Hydrogel Prepared Using 4αGTase-Treated Rice Starch. Foods 2021, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, S.; Shiroodi, S.; Schwarz, M.H.; Nitin, N.; Ovissipour, R. Inactivation of Aeromonas hydrophila and Vibrio parahaemolyticus by Curcumin-Mediated Photosensitization and Nanobubble-Ultrasonication Approaches. Foods 2020, 9, 1306. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fang, Q.; Li, P.; Zhang, C.; Yuan, Y.; Zhuang, H. Effects of Emulsifier Type and Post-Treatment on Stability, Curcumin Protection, and Sterilization Ability of Nanoemulsions. Foods 2021, 10, 149. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Zhou, W.; Liu, W.; Liu, C.; McClements, D.J. Encapsulation of lipophilic polyphenols into nanoliposomes using pH-driven method: Advantages and disadvantages. J. Agric. Food Chem. 2019, 67, 7506–7511. [Google Scholar] [CrossRef]
- Ma, P.; Zhang, J.; Liu, P.; Wang, Q.; Zhang, Y.; Song, K.; Li, R.; Shen, L. Computer-assisted design for stable and porous metal organic framework (MOF) as a carrier for curcumin delivery. LWT 2020, 120, 108949. [Google Scholar] [CrossRef]
- Leong, T.; Wooster, T.; Kentish, S.; Ashokkumar, M. Minimising oil droplet size using ultrasonic emulsification. Ultrason. Sonochemistry 2009, 16, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Development of stable curcumin nanoemulsions: Effects of emulsifier type and surfactant-to-oil ratios. J. Food Sci. Technol. Mysore 2018, 55, 3485–3497. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Preparation of curcumin-loaded emulsion using high pressure homogenization: Impact of oil phase and concentration on physicochemical stability. LWT Food Sci. Technol. 2017, 84, 34–46. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chen, J.-H.; Chang, C.; Shieh, C.-J. Optimization of ultrasound-accelerated synthesis of enzymatic caffeic acid phenethyl ester by response surface methodology. Ultrason. Sonochemistry 2011, 18, 455–459. [Google Scholar] [CrossRef]
- Tikekar, R.; Pan, Y.; Nitin, N. Fate of curcumin encapsulated in silica nanoparticle stabilized Pickering emulsion during storage and simulated digestion. Food Res. Int. 2013, 51, 370–377. [Google Scholar] [CrossRef]


| No. | Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| Ultrasonic Power (W) | Ultrasonic Power (min) | Oil Content (% w/w) | MOF * Content (% w/w) | Loading Capacity (%) | S.D. * | Encapsulation Efficiency (%) | S.D. * | |
| 1 | 100 | 10 | 30 | 3 | 4.86 | 0.38 | 17.93 | 0.10 |
| 2 | 100 | 5 | 22.5 | 3 | 6.79 | 0.23 | 19.36 | 4.78 |
| 3 | 150 | 5 | 22.5 | 2 | 5.33 | 0.30 | 32.89 | 1.48 |
| 4 | 100 | 15 | 15 | 2 | 7.01 | 0.41 | 30.01 | 0.30 |
| 5 | 100 | 15 | 22.5 | 1 | 2.31 | 0.17 | 54.73 | 1.45 |
| 6 | 100 | 10 | 30 | 1 | 1.61 | 0.11 | 50.27 | 4.28 |
| 7 | 100 | 5 | 30 | 2 | 3.56 | 0.23 | 28.71 | 3.19 |
| 8 | 50 | 5 | 22.5 | 2 | 4.30 | 0.18 | 26.53 | 3.79 |
| 9 | 150 | 10 | 22.5 | 3 | 6.93 | 0.41 | 19.76 | 3.84 |
| 10 | 100 | 10 | 22.5 | 2 | 5.05 | 0.34 | 31.17 | 3.21 |
| 11 | 100 | 10 | 15 | 3 | 8.87 | 0.57 | 17.86 | 4.39 |
| 12 | 150 | 10 | 30 | 2 | 3.85 | 0.05 | 31.02 | 4.86 |
| 13 | 100 | 10 | 22.5 | 2 | 4.96 | 0.03 | 30.62 | 4.11 |
| 14 | 100 | 10 | 22.5 | 2 | 5.10 | 0.16 | 31.46 | 0.73 |
| 15 | 150 | 10 | 15 | 2 | 7.15 | 0.57 | 30.59 | 0.67 |
| 16 | 100 | 15 | 22.5 | 3 | 6.89 | 0.02 | 19.67 | 3.06 |
| 17 | 50 | 10 | 22.5 | 1 | 2.26 | 0.14 | 53.41 | 3.17 |
| 18 | 100 | 10 | 22.5 | 2 | 5.14 | 0.16 | 31.68 | 4.91 |
| 19 | 100 | 5 | 15 | 2 | 6.30 | 0.41 | 26.96 | 2.80 |
| 20 | 100 | 15 | 30 | 2 | 3.38 | 0.17 | 27.19 | 3.50 |
| 21 | 150 | 10 | 22.5 | 1 | 2.47 | 0.04 | 58.41 | 0.59 |
| 22 | 50 | 10 | 30 | 2 | 3.35 | 0.25 | 26.95 | 3.56 |
| 23 | 50 | 15 | 22.5 | 2 | 4.25 | 0.35 | 26.22 | 3.82 |
| 24 | 50 | 10 | 15 | 2 | 6.17 | 0.13 | 26.39 | 0.25 |
| 25 | 150 | 15 | 22.5 | 2 | 5.26 | 0.40 | 32.43 | 4.40 |
| 26 | 50 | 10 | 22.5 | 3 | 6.35 | 0.40 | 18.11 | 2.46 |
| 27 | 100 | 5 | 22.5 | 1 | 2.29 | 0.17 | 54.24 | 0.92 |
| 28 | 100 | 10 | 15 | 1 | 3.49 | 0.26 | 56.28 | 3.08 |
| 29 | 100 | 10 | 22.5 | 2 | 5.08 | 0.13 | 31.33 | 3.65 |
| Response Factor | Model | Degree of Freedom | p-Value | Std. Deviation | R2 |
|---|---|---|---|---|---|
| LC | Linear | 20 | 0.0056 | 3.4853 | 0.4432 |
| 2FI | 14 | 0.9022 | 3.8099 | 0.5009 | |
| Quadratic | 10 | <0.0001 | 1.9172 | 0.9017 | |
| Cubic | 2 | 0.0138 | 0.9031 | 0.9906 | |
| EE | Linear | 20 | <0.0001 | 4.1877 | 0.9027 |
| 2FI | 14 | 0.9911 | 4.7351 | 0.9067 | |
| Quadratic | 10 | <0.0001 | 1.0366 | 0.9965 | |
| Cubic | 2 | 0.0066 | 0.4280 | 0.9997 |
| Response Factor | Factor | Sum of Squares | p-Value |
|---|---|---|---|
| LC | Ultrasonic Power (X1) | 18.5340 | 5.0422 |
| Ultrasonic Time (X2) | 0.6960 | 0.19 | |
| Oil Phase Concentration (X3) | 2.9304 | 0.8 | |
| MOF Concentration (X4) | 9.4696 | 2.58 | |
| EE | Ultrasonic Power (X1) | 62.98 | <0.0001 |
| Ultrasonic Time (X2) | 0.2 | 0.6726 | |
| Oil Phase Concentration (X3) | 3.01 | 0.1164 | |
| MOF Concentration (X4) | 3839.55 | <0.0001 |
| LC | EE | |||
|---|---|---|---|---|
| Model Parameters | Coefficient Estimate | p-Value | Coefficient Estimate | p-Value |
| X1 | 0.0172 | <0.0001 | 0.1263 | <0.0001 |
| X2 | 0.2419 | NS | 1.5573 | NS |
| X3 | 0.2901 | NS | 1.5314 | 0.1164 |
| X4 | 1.2325 | 0.1308 ^ | −47.1652 | <0.0001 |
| X1*X2 | −0.0003 | NS | −0.0002 | NS |
| X1*X3 | −0.0002 | NS | −0.0001 | NS |
| X1*X4 | −0.0003 | NS | −0.0168 | 0.1285 ^ |
| X2*X3 | −0.0051 | 0.0327 | −0.0305 | 0.0443 |
| X2*X4 | 0.0174 | NS | −0.0090 | NS |
| X3*X4 | 0.2057 | 0.1299 ^ | 0.2027 | 0.0109 |
| X1*X1 | −0.0006 | 0.0879 | −0.0002 | NS |
| X2*X2 | −0.0062 | 0.0298 | −0.0406 | NS |
| X3*X3 | −0.0062 | <0.0001 | −0.0375 | 0.0001 |
| X4*X4 | −0.3910 | <0.0001 | 6.6207 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, P.; Zhang, Z.; Tsai, S.; Zhang, H.; Li, Y.; Yuan, F.; Wang, Q. Curcumin-Loaded Pickering Emulsion Formed by Ultrasound and Stabilized by Metal Organic Framework Optimization. Foods 2021, 10, 523. https://doi.org/10.3390/foods10030523
Ma P, Zhang Z, Tsai S, Zhang H, Li Y, Yuan F, Wang Q. Curcumin-Loaded Pickering Emulsion Formed by Ultrasound and Stabilized by Metal Organic Framework Optimization. Foods. 2021; 10(3):523. https://doi.org/10.3390/foods10030523
Chicago/Turabian StyleMa, Peihua, Zhi Zhang, Shawn Tsai, Hongchao Zhang, Yuan Li, Fang Yuan, and Qin Wang. 2021. "Curcumin-Loaded Pickering Emulsion Formed by Ultrasound and Stabilized by Metal Organic Framework Optimization" Foods 10, no. 3: 523. https://doi.org/10.3390/foods10030523





