Analysis of Biofilm Formation on the Surface of Organic Mung Bean Seeds, Sprouts and in the Germination Environment
Abstract
:1. Introduction
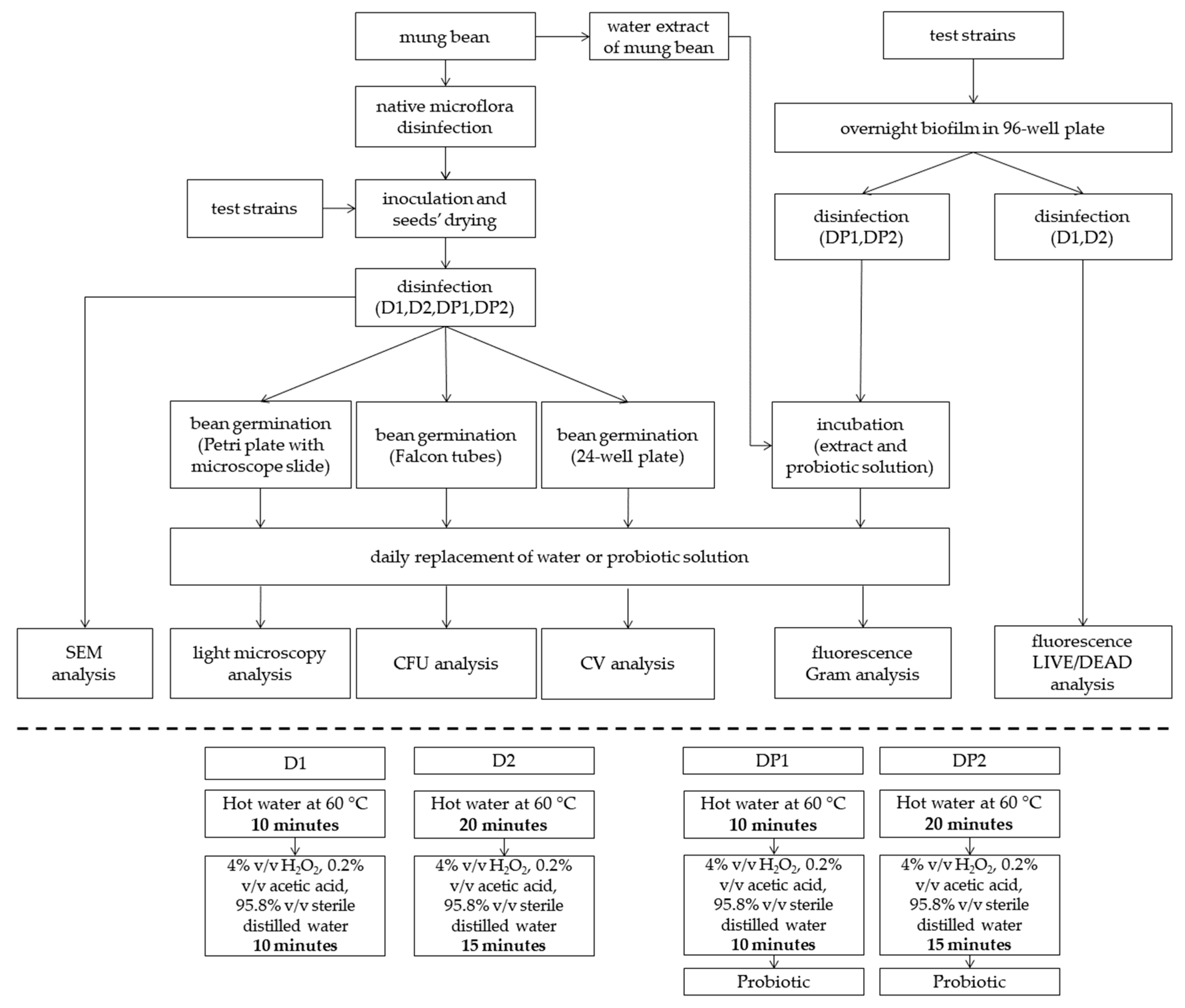
2. Materials and Methods
2.1. Mung Bean
2.2. Bacterial Cultures
2.3. Beans Inoculation
2.4. Sanitizers and Treatments
2.5. Analysis of the Biofilm
2.5.1. Colony Formation Units
2.5.2. Crystal Violet Method
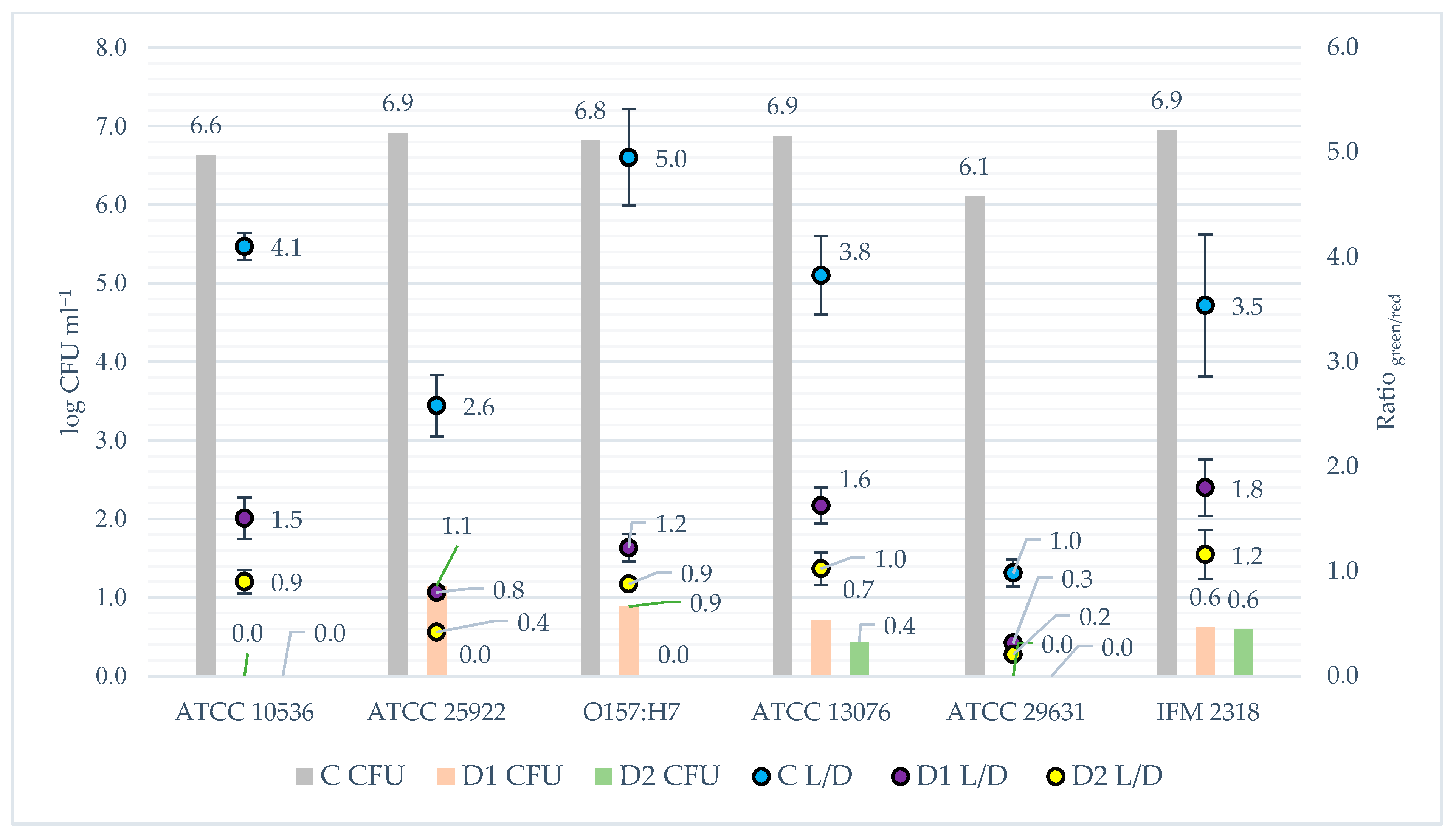
2.5.3. Fluorescence Microplate LIVE/DEAD Method
2.5.4. Fluorescence Microplate Gram Method
2.5.5. Light Microscopy
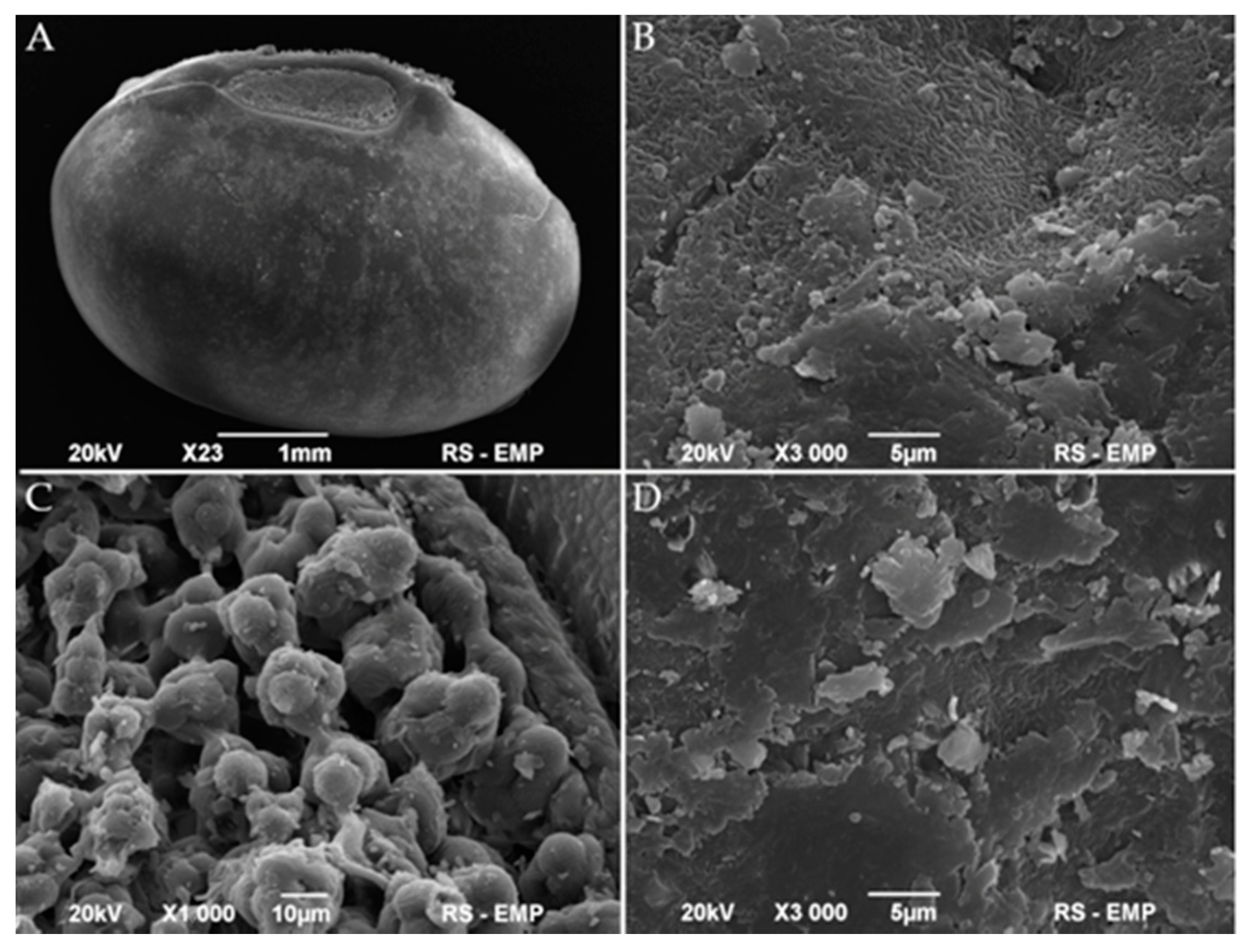
2.5.6. Scanning Electron Microscopy (SEM)
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Iacumin, L.; Comi, G. Microbial Quality of Raw and Ready-to-Eat Mung Bean Sprouts Produced in Italy. Food Microbiol. 2019, 82, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Mustar, S.; Nazaimoon, W.M.W. The Effect of Sanitizers on the Native Microflora of Mung Bean Seeds (Vigna Radiata). J. Food Technol. 2010, 8, 234–238. [Google Scholar] [CrossRef]
- Gabriel, A.A. Microbial Quality of Chlorine Soaked Mung Bean Seeds and Sprouts. Food Sci. Technol. Res. 2005, 11, 95–100. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Z.; Li, Y.; Shang, Y.; Zhang, G.; Cao, W. Reduction of Escherichia Coli O157:H7 and Salmonella Enteritidis on Mung Bean Seeds and Sprouts by Slightly Acidic Electrolyzed Water. Food Control 2011, 22, 792–796. [Google Scholar] [CrossRef]
- Government of Canada. Canada Health Policy on Managing Health Risk Associated with the Consumption of Sprouted Seeds and Beans. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/policies/policy-managing-health-risk-associated-consumption-sprouted-seeds-beans.html (accessed on 12 November 2020).
- Bayer, C.; Bernard, H.; Prager, R.; Rabsch, W.; Hiller, P.; Malorny, B.; Pfefferkorn, B.; Frank, C.; de Jong, A.; Friesema, I.; et al. An Outbreak of Salmonella Newport Associated with Mung Bean Sprouts in Germany and the Netherlands, October to November 2011. Eurosurveillance 2014, 19, 20665. [Google Scholar] [CrossRef]
- Xiao, Z.; Nou, X.; Luo, Y.; Wang, Q. Comparison of the Growth of Escherichia Coli O157: H7 and O104: H4 during Sprouting and Microgreen Production from Contaminated Radish Seeds. Food Microbiol. 2014, 44, 60–63. [Google Scholar] [CrossRef]
- Peles, F.; Zoltan, G.; Bácskai, T.; Szabó, Z.; Murvai, M.; Kovács, B. Microbiological Quality of Organic Wheat Grains and Sprouts. An. Univers Orad Facl Prtcia Medi 2012, 18, 53–60. [Google Scholar]
- Monahan, C. FY 2014–2016 Microbiological Sampling Assignment Summary Report: Sprouts; FDA: Silver Spring, MD, USA, 2017; pp. 1–28.
- Boqvist, S.; Söderqvist, K.; Vågsholm, I. Food Safety Challenges and One Health within Europe. Acta Vet. Scand. 2018, 60, 1. [Google Scholar] [CrossRef]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; der Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A.; et al. Epidemic Profile of Shiga-Toxin–Producing Escherichia Coli O104:H4 Outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. Shiga Toxin-Producing E. Coli (STEC) O104:H4 2011 Outbreaks in Europe: Taking Stock. EFSA J. 2011, 9, 2390. [Google Scholar] [CrossRef]
- Nutrition, C. Outbreak Investigation of E. Coli O103: Clover Sprouts (February 2020); FDA: Silver Spring, MD, USA, 2020.
- Dikici, A.; Koluman, A.; Calicioglu, M. Comparison of Effects of Mild Heat Combined with Lactic Acid on Shiga Toxin Producing Escherichia Coli O157:H7, O103, O111, O145 and O26 Inoculated to Spinach and Soybean Sprout. Food Control 2015, 50, 184–189. [Google Scholar] [CrossRef]
- Praeger, U.; Herppich, W.B.; Hassenberg, K. Aqueous Chlorine Dioxide Treatment of Horticultural Produce: Effects on Microbial Safety and Produce Quality–A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A. Guideline for Disinfection and Sterilization in Healthcare Facilities; CDC: Atlanta, GA, USA, 2008; p. 163.
- Kitis, M. Disinfection of Wastewater with Peracetic Acid: A Review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.D.; Nan, Y.; Stanford, K.; Holley, R.; McAllister, T.; Narváez-Bravo, C. SalmoFreshTM Effectiveness in Controlling Salmonella on Romaine Lettuce, Mung Bean Sprouts and Seeds. Int. J. Food Microbiol. 2019, 305, 108250. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of Plasma-Activated Water on Microbial Quality and Physicochemical Characteristics of Mung Bean Sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Świeca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Bochnak, J.; Złotek, U.; Baraniak, B. Lactobacillus Plantarum 299V Improves the Microbiological Quality of Legume Sprouts and Effectively Survives in These Carriers during Cold Storage and in Vitro Digestion. PLoS ONE 2018, 13, e0207793. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Fu, T.-J.; Smith, M.A. Microbial Contamination in Sprouts: How Effective Is Seed Disinfection Treatment? J. Food Sci. 2013, 78, R495–R501. [Google Scholar] [CrossRef] [Green Version]
- Nagar, V.; Godambe, L.P.; Shashidhar, R. Development of Microbiologically Safe Mung Bean Sprouts Using Combination Treatment of Sodium Hypochlorite and Gamma Radiation. Int. J. Food Sci. Technol. 2016, 51, 595–601. [Google Scholar] [CrossRef]
- Neo, S.Y. Efficacy of Chlorine and Peroxyacetic Acid on Reduction of Natural Microflora, Escherichia Coli O157:H7, Listeria Monocyotgenes and Salmonella Spp. on Mung Bean Sprouts. Food Microbiol. 2013, 6, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Muriana, P.M. Efficacy of Commercial Sanitizers Used in Food Processing Facilities for Inactivation of Listeria Monocytogenes, E. Coli O157:H7, and Salmonella Biofilms. Foods 2019, 8, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and Microgreens: Trends, Opportunities, and Horizons for Novel Research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Liu, D.; Walcott, R.; Mis Solval, K.; Chen, J. Influence of Bacterial Competitors on Salmonella Enterica and Enterohemorrhagic Escherichia Coli Growth in Microbiological Media and Attachment to Vegetable Seeds. Foods 2021, 10, 285. [Google Scholar] [CrossRef]
- Han, R.; Klu, Y.A.K.; Chen, J. Attachment and Biofilm Formation by Selected Strains of Salmonella enterica and Entrohemorrhagic Escherichia coli of Fresh Produce Origin. J. Food Sci. 2017, 82, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Fett, W.F.; Cooke, P.H. Scanning Electron Microscopy of Native Biofilms on Mung Bean Sprouts. Can. J. Microbiol. 2003, 49, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, H.P.; Wang, H.H.; Agle, M.E. Biofilms in the Food Environment; Blackwell Publishing Ltd.: Oxford, UK, 2007; ISBN 978-0-8138-2058-3. [Google Scholar]
- Trząskowska, M.; Dai, Y.; Delaquis, P.; Wang, S. Pathogen Reduction on Mung Bean Reduction of Escherichia Coli O157:H7, Salmonella Enterica and Listeria Monocytogenes on Mung Bean Using Combined Thermal and Chemical Treatments with Acetic Acid and Hydrogen Peroxide. Food Microbiol. 2018, 76, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kadurugamuwa, J.L.; Sin, L.; Albert, E.; Yu, J.; Francis, K.; DeBoer, M.; Rubin, M.; Bellinger-Kawahara, C.; Parr, T.R.; Contag, P.R. Direct Continuous Method for Monitoring Biofilm Infection in a Mouse Model. Infect. Immun. 2003, 71, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Lianou, A.; Koutsoumanis, K.P. Strain Variability of the Biofilm-Forming Ability of Salmonella Enterica under Various Environmental Conditions. Int. J. Food Microbiol. 2012, 160, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Doijad, S.P.; Barbuddhe, S.B.; Garg, S.; Poharkar, K.V.; Kalorey, D.R.; Kurkure, N.V.; Rawool, D.B.; Chakraborty, T. Biofilm-Forming Abilities of Listeria Monocytogenes Serotypes Isolated from Different Sources. PLoS ONE 2015, 10, e0137046. [Google Scholar] [CrossRef] [Green Version]
- Oates, A.; Bowling, F.L.; Boulton, A.J.M.; Bowler, P.G.; Metcalf, D.G.; McBain, A.J. The Visualization of Biofilms in Chronic Diabetic Foot Wounds Using Routine Diagnostic Microscopy Methods. J. Diabetes Res. 2014, 2014, 153586. [Google Scholar] [CrossRef]
- Fransisca, L.; Park, H.K.; Feng, H. E. Coli O157:H7 Population Reduction from Alfalfa Seeds with Malic Acid and Thiamine Dilauryl Sulfate and Quality Evaluation of the Resulting Sprouts. J. Food Sci. 2012, 77, M121–M126. [Google Scholar] [CrossRef]
- Li, H.; Gänzle, M. Some Like It Hot: Heat Resistance of Escherichia Coli in Food. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Guo, D.; Yang, J.; Tortorello, M.L.; Zhang, W. Survival and Heat Resistance of Salmonella Enterica and Escherichia Coli O157:H7 in Peanut Butter. Appl. Environ. Microbiol. 2011, 77, 8434–8438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Zhang, G.; Gerner-Smidt, P.; Mantripragada, V.; Ezeoke, I.; Doyle, M.P. Thermal Inactivation of Salmonella in Peanut Butter. J. Food Prot. 2009, 72, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Otta, S.K.; Karunasagar, I.; Karunasagar, I. Biofilm Formation by Salmonella Spp. on Food Contact Surfaces and Their Sensitivity to Sanitizers. Int. J. Food Microbiol. 2001, 64, 367–372. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial Adhesion and Biofilms on Surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Kim, N.-N.; Kim, W.J.; Kang, S.-S. Anti-Biofilm Effect of Crude Bacteriocin Derived from Lactobacillus Brevis DF01 on Escherichia Coli and Salmonella Typhimurium. Food Control 2019, 98, 274–280. [Google Scholar] [CrossRef]
- Ramachandran, G.; Aheto, K.; Shirtliff, M.E.; Tennant, S.M. Poor Biofilm-Forming Ability and Long-Term Survival of Invasive Salmonella Typhimurium ST313. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [Green Version]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving Structure to the Biofilm Matrix: An Overview of Individual Strategies and Emerging Common Themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-Review. Res. Rev. J. Eng. Technol. 2017, 6, 4. [Google Scholar]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining Conditions for Biofilm Inhibition and Eradication Assays for Gram-Positive Clinical Reference Strains. BMC Microbiol. 2018, 18, 173. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007; European Union: Brussels, Belgium, 2018; Volume 150. [Google Scholar]
- Swieca, M.; Gawlik-Dziki, U.; Jakubczyk, A.; Bochnak, J.; Sikora, M.; Suliburska, J. Nutritional Quality of Fresh and Stored Legumes Sprouts—Effect of Lactobacillus Plantarum 299v Enrichment. Food Chem. 2019, 288, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ołdak, A.; Zielińska, D.; Rzepkowska, A.; Kołożyn-Krajewska, D. Comparison of Antibacterial Activity of Lactobacillus Plantarum Strains Isolated from Two Different Kinds of Regional Cheeses from Poland: Oscypek and Korycinski Cheese. BioMed Res. Int. 2017, 2017, 6820369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Lathrop, A. Effects of Lactobacillus Plantarum, Pediococcus Acidilactici, and Pediococcus Pentosaceus on the Growth of Listeria Monocytogenes and Salmonella on Alfalfa Sprouts. J. Food Prot. 2019, 82, 522–527. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic Acid Bacteria and Natural Antimicrobials to Improve the Safety and Shelf-Life of Minimally Processed Sliced Apples and Lamb’s Lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Wakeling, C.; Bach, S.; Orban, S.; Delaquis, P. Disinfection of Alfalfa and Radish Sprouting Seed Using Oxidizing Agents and Treatments Compliant with Organic Food Production Principles. J. Food Prot. 2019, 83, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Warriner, K.; Spaniolas, S.; Dickinson, M.; Wright, C.; Waites, W.M. Internalization of Bioluminescent Escherichia Coli and Salmonella Montevideo in Growing Bean Sprouts. J. Appl. Microbiol. 2003, 95, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Rhee, M.S. Environment-Friendly Mild Heat and Relative Humidity Treatment Protects Sprout Seeds (Radish, Mung Bean, Mustard, and Alfalfa) against Various Foodborne Pathogens. Food Control 2018, 93, 17–22. [Google Scholar] [CrossRef]
- Zhou, R.; Li, J.; Zhou, R.; Zhang, X.; Yang, S. Atmospheric-Pressure Plasma Treated Water for Seed Germination and Seedling Growth of Mung Bean and Its Sterilization Effect on Mung Bean Sprouts. Innov. Food Sci. Emerg. Technol. 2019, 53, 36–44. [Google Scholar] [CrossRef]
- Danhorn, T.; Fuqua, C. Biofilm Formation by Plant-Associated Bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef]
- Fuqua, C.; Matthysse, A.G. Methods for studying bacterial biofilms associated with plants. In Methods in Enzymology; Doyle, R.J., Ed.; Microbial Growth in Biofilms—Part B: Special Environments and Physicochemical Aspects; Academic Press: Cambridge, MA, USA, 2001; Volume 337, pp. 3–18. [Google Scholar]
- Solomon, E.B.; Matthews, K.R. Interaction of Live and Dead Escherichia Coli O157:H7 and Fluorescent Microspheres with Lettuce Tissue Suggests Bacterial Processes Do Not Mediate Adherence. Lett. Appl. Microbiol. 2006, 42, 88–93. [Google Scholar] [CrossRef] [PubMed]



| Sanitation Method 1 | Time [Days] | E. coli ATCC 10536 | L. plantarum 299v 3 | E. coli ATCC 25922 | L. plantarum 299v 4 | E. coli O157:H7 | L. plantarum 299v 5 |
|---|---|---|---|---|---|---|---|
| C | 0 | 6.63 ± 0.37 a | n/a 2 | 6.92 ± 0.49 a | n/a | 6.82 ± 0.30 a | n/a |
| 2 | 8.54 ± 0.13 b | n/a | 8.04 ± 0.17 a | n/a | 7.99 ± 0.32 abc | n/a | |
| 4 | 8.46 ± 0.09 b | n/a | 8.51 ± 0.35 a | n/a | 8.12 ± 0.12 bc | n/a | |
| 6 | 8.00 ± 0.07 b | n/a | 8.39 ± 0.06 a | n/a | 7.98 ± 0.18 abc | n/a | |
| D1 | 0 | 0.00 ± 0.00 a | n/a | 1.72 ± 0.45 a | n/a | 0.88 ± 0.83 a | n/a |
| 2 | 5.67 ± 0.01 b | n/a | 7.64 ± 0.08 b | n/a | 7.44 ± 0.20 b | n/a | |
| 4 | 7.81 ± 0.51 cd | n/a | 8.49 ± 0.04 b | n/a | 7.58 ± 0.07 b | n/a | |
| 6 | 7.24 ± 0.06 d | n/a | 8.68 ± 0.17 b | n/a | 7.73 ± 0.05 b | n/a | |
| D2 | 0 | 0.00 ± 0.00 a | n/a | 0.00 ± 0.00 a | n/a | 0.00 ± 0.00 a | n/a |
| 2 | 0.00 ± 0.00 a | n/a | 3.59 ± 0.13 b | n/a | 5.48 ± 0.01 b | n/a | |
| 4 | 6.02 ± 0.06 b | n/a | 8.55 ± 0.04 c | n/a | 7.34 ± 0.09 c | n/a | |
| 6 | 8.34 ± 0.08 c | n/a | 8.54 ± 0.09 c | n/a | 7.99 ± 0.16 c | n/a | |
| DP1 | 0 | 0.00 ± 0.00 a | n/a | 1.15 ± 1.04 a | n/a | 0.88 ± 0.83 a | n/a |
| 1 | 3.93 ± 0.54 b | 6.35 ± 0.32 a | 3.72 ± 0.65 b | 6.56 ± 0.10 a | 6.10 ± 0.24 b | 6.65 ± 0.16 a | |
| 2 | 6.65 ± 0.10 c | 8.37 ± 0.04 b | 8.17 ± 0.15 c | 7.94 ± 0.24 b | 7.57 ± 0.08 c | 8.34 ± 0.06 b | |
| 4 | 7.60 ± 0.03 d | 8.70 ± 0.02 b | 8.26 ± 0.09 c | 8.58 ± 0.38 b | 7.46 ± 0.05 c | 8.81 ± 0.24 bc | |
| 6 | 7.69 ± 0.10 d | 8.61 ± 0.06 b | 8.48 ± 0.10 c | 8.42 ± 0.10 b | 7.35 ± 0.03 c | 7.57 ± 0.88 a | |
| DP2 | 0 | 0.00 ± 0.00 a | n/a | 0.00 ± 0.00 a | n/a | 0.00 ± 0.00 a | n/a |
| 1 | 0.00 ± 0.00 a | 6.74 ± 0.16 a | 0.00 ± 0.00 a | 6.57 ± 0.34 a | 2.11 ± 1.84 b | 6.52 ± 0.12 a | |
| 2 | 6.51 ± 0.04 b | 8.48 ± 0.47 b | 2.18 *a | 7.62 ± 0.09 b | 6.13 ± 0.05 c | 8.04 ± 0.03 b | |
| 4 | 5.49 ± 0.08 b | 8.43 ± 0.34 b | 5.55 ± 0.11 b | 8.23 ± 0.11 bc | 3.77 ± 0.30 b | 8.23 ± 0.16 b | |
| 6 | 7.12 ± 0.51 c | 7.67 ± 0.05 c | 7.73 ± 0.81 c | 8.49 ± 0.20 c | 6.81 ± 1.06 c | 8.40 ± 0.10 b |
| Sanitation Method 1 | Time [Days] | S. enteritidis ATCC 13076 | L. plantarum 299v 3 | S. enteritidis ATCC 29631 | L. plantarum 299v 4 | S. hofit IFM 2318 | L. plantarum 299v 5 |
|---|---|---|---|---|---|---|---|
| C | 0 | 6.88 ± 0.34 a | n/a 2 | 6.11 ± 0.11 ac | n/a | 6.95 ± 0.34 ac | n/a |
| 2 | 8.55 ± 0.02 bc | n/a | 8.35 ± 0.00 bcd | n/a | 8.58 ± 0.08 bcd | n/a | |
| 4 | 7.72 ± 0.56 ac | n/a | 8.32 ± 0.14 abcd | n/a | 8.46 ± 0.05 abcd | n/a | |
| 6 | 8.84 ± 0.22 bc | n/a | 8.56 ± 0.36 bcd | n/a | 8.81 ± 0.18 bcd | n/a | |
| D1 | 0 | 2.14 *a | n/a | 0.00 ± 0.00 a | n/a | 1.87 *a | n/a |
| 2 | 7.40 ± 0.14 b | n/a | 4.12 ± 0.08 bc | n/a | 7.42 ± 0.06 b | n/a | |
| 4 | 8.20 ± 0.32 b | n/a | 3.18 ± 0.00 c | n/a | 8.81 ± 0.06 b | n/a | |
| 6 | 8.19 ± 0.02 b | n/a | 2.91 ± 0.37 c | n/a | 8.30 ± 0.22 b | n/a | |
| D2 | 0 | 1.30 *a | n/a | 0.00 ± 0.00 a | n/a | 1.78 *a | n/a |
| 2 | 2.85 ± 0.19 b | n/a | 0.00 ± 0.00 a | n/a | 7.44 ± 0.04 b | n/a | |
| 4 | 8.29 ± 0.06 c | n/a | 0.00 ± 0.00 a | n/a | 7.85 ± 0.19 b | n/a | |
| 6 | 8.55 ± 0.16 c | n/a | 0.00 ± 0.00 a | n/a | 8.14 ± 0.06 b | n/a | |
| DP1 | 0 | 2.15 *a | n/a | 0.00 ± 0.00 a | n/a | 1.88 *a | n/a |
| 1 | 5.83 ± 0.05 b | 6.76 ± 0.09 a | 0.00 ± 0.00 a | 6.37 ± 0.16 a | 2.90 ± 0.05 b | 6.57 ± 0.20 a | |
| 2 | 6.46 ± 0.13 c | 7.61 ± 0.04 b | 2.62 ± 0.54 bc | 7.98 ± 0.04 b | 8.28 ± 0.05 c | 8.13 ± 0.05 b | |
| 4 | 8.27 ± 0.14 d | 8.67 ± 0.44 c | 3.67 ± 0.17 bc | 8.48 ± 0.23 c | 8.62 ± 0.07 c | 8.58 ± 0.33 bc | |
| 6 | 8.66 ± 0.09 d | 8.73 ± 0.06 c | 2.74 *ab | 8.53 ± 0.18 c | 8.34 ± 0.17 c | 8.64 ± 0.03 c | |
| DP2 | 0 | 1.30 *a | n/a | 0.00 ± 0.00 a | n/a | 1.78 *a | n/a |
| 1 | 2.65 *a | 6.37 ± 0.17 a | 2.18 *a | 6.52 ± 0.21 a | 0.00 ± 0.00 a | 6.49 ± 0.02 a | |
| 2 | 7.83 ± 0.11 b | 8.41 ± 0.22 b | 0.00 ± 0.00 a | 7.98 ± 0.09 b | 5.18 ± 0.00 b | 8.17 ± 0.04 b | |
| 4 | 8.18 ± 0.09 b | 8.19 ± 0.25 b | 0.00 ± 0.00 a | 8.40 ± 0.09 b | 7.35 ± 0.03 c | 8.37 ± 0.16 b | |
| 6 | 7.97 ± 0.24 b | 8.38 ± 0.44 b | 0.00 ± 0.00 a | 8.25 ± 0.07 b | 8.21 ± 0.16 c | 8.50 ± 0.05 b |
| Sanitation Method | Time [Days] | E. coli ATCC 10536 | E. coli ATCC 25922 | E. coli O157:H7 | S. enteritidis ATCC 13076 | S. enteritidis ATCC 29631 | S. hofit IFM 2318 |
|---|---|---|---|---|---|---|---|
| CP | 1 | 2.18 ± 0.33 a | 3.45 ± 0.31 a | 3.49 ± 0.26 a | 1.94 ± 0.16 a | 1.53 ± 0.19 a | 3.67 ± 0.11 a |
| 2 | 1.40 ± 0.13 b | 1.47 ± 0.22 bd | 3.13 ± 0.11 ac | 2.65 ± 0.28 b | 2.00 ± 0.36 a | 2.93 ± 0.19 bcd | |
| 4 | 2.99 ± 0.20 c | 1.73 ± 0.12 bde | 2.44 ± 0.06 bc | 2.53 ± 0.09 b | 1.87 ± 0.08 a | 2.59 ± 0.17 bc | |
| 6 | 3.75 ± 0.17 d | 2.10 ± 0.44 cde | 2.92 ± 0.22 bc | 2.91 ± 0.22 b | 2.67 ± 0.13 b | 3.24 ± 0.05 bd | |
| DP1 | 1 | 1.45 ± 0.04 ac | 1.29 ± 0.09 a | 1.83 ± 0.17 a | 1.45 ± 0.12 a | 1.26 ± 0.19 ac | 1.72 ± 0.08 a |
| 2 | 1.22 ± 0.04 a | 1.04 ± 0.04 a | 1.82 ± 0.28 a | 1.43 ± 0.20 a | 1.13 ± 0.13 ac | 1.45 ± 0.19 a | |
| 4 | 1.83 ± 0.06 ac | 1.43 ± 0.23 a | 1.84 ± 0.24 a | 2.00 ± 0.07 b | 1.52 ± 0.27 acd | 1.84 ± 0.14 a | |
| 6 | 2.32 ± 0.26 b | 2.24 ± 0.14 b | 2.26 ± 0.25 b | 2.37 ± 0.12 b | 1.94 ± 0.04 bcd | 2.33 ± 0.14 b | |
| DP2 | 1 | 1.49 ± 0.11 acd | 1.42 ± 0.14 a | 1.42 ± 0.11 a | 1.44 ± 0.15 a | 1.22 ± 0.07 a | 1.44 ± 0.11 a |
| 2 | 1.35 ± 0.04 ac | 1.08 ± 0.02 a | 1.18 ± 0.11 a | 1.25 ± 0.04 a | 1.41 ± 0.12 a | 1.41 ± 0.11 a | |
| 4 | 1.94 ± 0.22 ad | 1.44 ± 0.09 a | 2.08 ± 0.10 b | 2.09 ± 0.04 b | 1.62 ± 0.20 a | 2.09 ± 0.22 b | |
| 6 | 2.75 ± 0.29 b | 2.56 ± 0.03 b | 2.87 ± 0.18 c | 2.28 ± 0.25 b | 2.16 ± 0.22 b | 2.50 ± 0.22 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, M.; Trząskowska, M. Analysis of Biofilm Formation on the Surface of Organic Mung Bean Seeds, Sprouts and in the Germination Environment. Foods 2021, 10, 542. https://doi.org/10.3390/foods10030542
Kruk M, Trząskowska M. Analysis of Biofilm Formation on the Surface of Organic Mung Bean Seeds, Sprouts and in the Germination Environment. Foods. 2021; 10(3):542. https://doi.org/10.3390/foods10030542
Chicago/Turabian StyleKruk, Marcin, and Monika Trząskowska. 2021. "Analysis of Biofilm Formation on the Surface of Organic Mung Bean Seeds, Sprouts and in the Germination Environment" Foods 10, no. 3: 542. https://doi.org/10.3390/foods10030542
APA StyleKruk, M., & Trząskowska, M. (2021). Analysis of Biofilm Formation on the Surface of Organic Mung Bean Seeds, Sprouts and in the Germination Environment. Foods, 10(3), 542. https://doi.org/10.3390/foods10030542






