Cow’s Milk Processing—Friend or Foe in Food Allergy?
Abstract
:1. Introduction
2. Brief Overview on Immune-Mediated Diseases Caused by Milk Proteins
2.1. IgE- and Cell-Mediated Allergies
2.2. IgE-Mediated Milk Allergy
2.2.1. Prevalence
2.2.2. Treatmen
2.2.3. Prevention
3. Milk and Its Components
4. Cow’s Milk Allergens
4.1. Whey
4.1.1. Beta-Lactoglobulin (Bos d 5)
4.1.2. Alpha-Lactalbumin (Bos d 4)
4.1.3. Serum Albumin (Bos d 6)
4.2. Caseins (Bos d 8; Bos d 9–12)
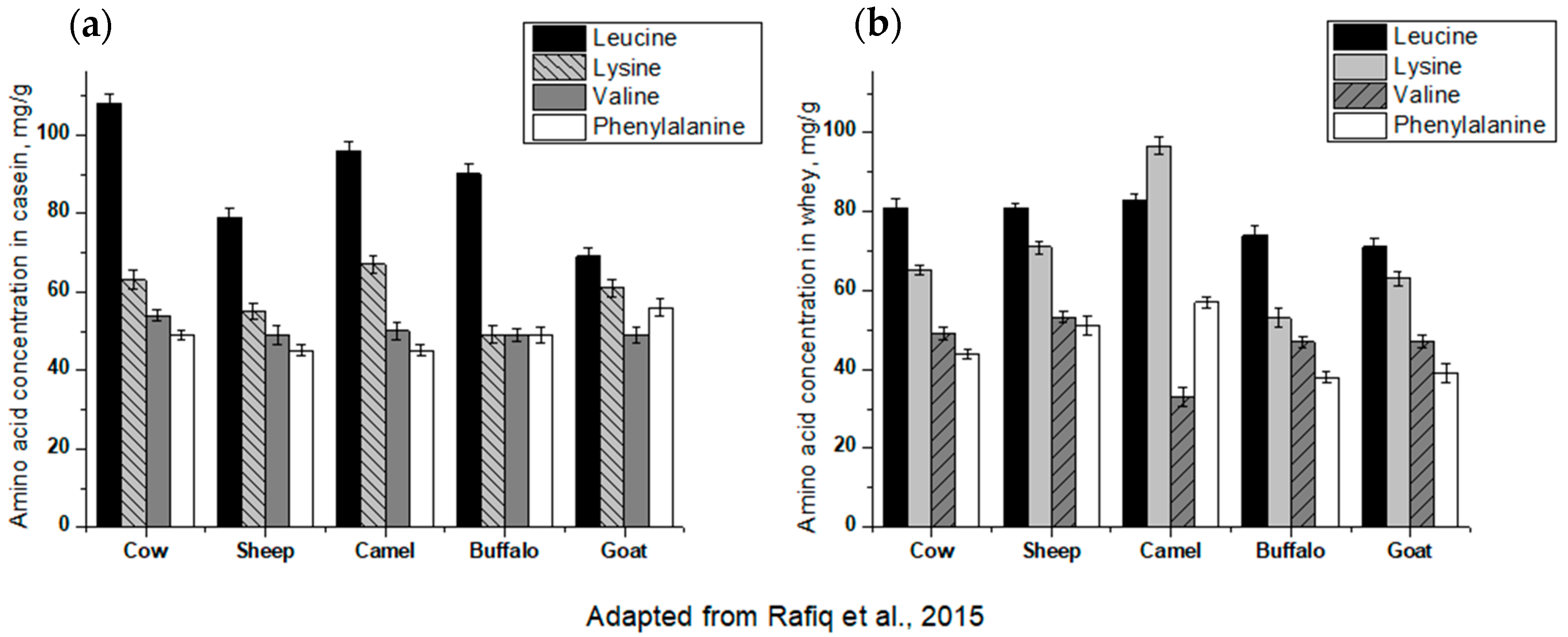
4.3. IgE Cross-Reactivity of Milk Proteins from Different Species
5. Food Processing: Applied Techniques and Effect on the Allergenicity of Individual Cow’s Milk Proteins
5.1. Thermal Processing
5.1.1. Pasteurization
5.1.2. Sterilization
5.1.3. Ultrahigh Temperature (UHT) Processing
5.1.4. Baking Milk
5.1.5. Spray Drying
5.2. Nonthermal Processing
5.2.1. Homogenization
5.2.2. High Pressure Homogenization (HPH)
5.2.3. Ultrasonic Treatment
5.2.4. Enzymatic Processes
5.2.5. Fermentation
5.3. Novel Techniques
5.3.1. Irradiation
5.3.2. Microwave Treatment
5.3.3. Cold Plasma Treatment
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bleasdale, M.; Richter, K.K.; Janzen, A.; Brown, S.; Scott, A.; Zech, J.; Wilkin, S.; Wang, K.; Schiffels, S.; Desideri, J.; et al. Ancient proteins provide evidence of dairy consumption in eastern Africa. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.A.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Caubet, J.-C.; Szajewska, H.; Shamir, R.; Nowak-Węgrzyn, A. Non-IgE-mediated gastrointestinal food allergies in children. Pediatr. Allergy Immunol. 2017, 28, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Mehr, S.; Kakakios, A.; Frith, K.; Kemp, A.S. Food Protein-Induced Enterocolitis Syndrome: 16-Year Experience. Pediatrics 2009, 123, e459–e464. [Google Scholar] [CrossRef]
- Caubet, J.C.; Ford, L.S.; Sickles, L.; Järvinen, K.M.; Sicherer, S.H.; Sampson, H.A.; Nowak-Węgrzyn, A. Clinical features and resolution of food protein–induced enterocolitis syndrome: 10-year experience. J. Allergy Clin. Immunol. 2014, 134, 382–389.e4. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Toyran, M.; Civelek, E.; Misirlioglu, E.; Kirsaclioglu, C.; Kocabas, C.N. Characteristics and Prognosis of Allergic Proctocolitis in Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 69–73. [Google Scholar] [CrossRef]
- Calvani, M.; Anania, C.; Cuomo, B.; D’Auria, E.; Decimo, F.; Indirli, G.; Marseglia, G.; Mastrorilli, V.; Sartorio, M.; Santoro, A.; et al. Non–IgE- or Mixed IgE/Non–IgE-Mediated Gastrointestinal Food Allergies in the First Years of Life: Old and New Tools for Diagnosis. Nutrients 2021, 13, 226. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; the EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Sicherer, S.H. Cow’s milk allergy prevention. Ann. Allergy Asthma Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, R.; Della Porta, M.; Manoni, M.; Iotti, S.; Pinotti, L.; Maier, J.A. Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Heliyon 2020, 6, 05390. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Dairy-Based Functional Beverages. Milk-Based Beverages 2019, 67–93. [Google Scholar] [CrossRef]
- Wal, J.-M. Cow’s milk proteins/allergens. Ann. Allergy Asthma Immunol. 2002, 89, 3–10. [Google Scholar] [CrossRef]
- Monaci, L.; Tregoat, V.; Van Hengel, A.J.; Anklam, E. Milk allergens, their characteristics and their detection in food: A review. Eur. Food Res. Technol. 2006, 223, 149–179. [Google Scholar] [CrossRef]
- Rafiq, S.; Huma, N.; Pasha, I.; Sameen, A.; Mukhtar, O.; Khan, M.I. Chemical Composition, Nitrogen Fractions and Amino Acids Profile of Milk from Different Animal Species. Asian-Australas. J. Anim. Sci. 2016, 29, 1022–1028. [Google Scholar] [CrossRef]
- Borková, M.; Snášelová, J. Possibilities of different animal milk detection in milk and dairy products—A review. Czech J. Food Sci. 2005, 23, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Guo, M. Chemistry and Biological Properties of Human Milk. Curr. Nutr. Food Sci. 2008, 4, 305–320. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s milk allergy: From allergens to new forms of diagnosis, therapy and prevention. Methods 2014, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Deeth, H.; Bansal, N. Whey Proteins. Whey Proteins 2019, 1–50. [Google Scholar] [CrossRef]
- Sánchez, L.; Pérez, M.D.; Parrón, J.A. HPP in dairy products: Impact on quality and applications. In Present and Future of High Pressure Processing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–272. [Google Scholar]
- Krunic, T.; Rakin, M.; Bulatovic, M.; Zaric, D. The Contribution of Bioactive Peptides of Whey to Quality of Food Products. Food Process. Increased Qual. Consum. 2018, 251–285. [Google Scholar] [CrossRef]
- Witteman, A.M.; Van Leeuwen, J.; Van Der Zee, J.S.; Aalberse, R.C. Food Allergens in House Dust. Int. Arch. Allergy Immunol. 1995, 107, 566–568. [Google Scholar] [CrossRef]
- Qin, B.Y.; Jameson, G.B.; Bewley, M.C.; Baker, E.N.; Creamer, L.K. Functional implications of structural differences between variants A and B of bovine β-lactoglobulin. Protein Sci. 2008, 8, 75–83. [Google Scholar] [CrossRef]
- Sawyer, L. β-Lactoglobulin; Springer International Publishing: Berlin/Heidelberg, Germany, 2012; pp. 211–259. [Google Scholar]
- Chatchatee, P.; Jarvinen, K.M.; Bardina, L.; Vila, L.; Beyer, K.; Sampson, H.A. Identification of IgE and IgG binding epitopes on β- and κ-casein in cow’s milk allergic patients. Clin. Exp. Allergy 2001, 31, 1256–1262. [Google Scholar] [CrossRef]
- Izadi, Z.; Divsalar, A.; Saboury, A.A.; Sawyer, L. β-lactoglobulin-pectin Nanoparticle-based Oral Drug Delivery System for Potential Treatment of Colon Cancer. Chem. Biol. Drug Des. 2016, 88, 209–216. [Google Scholar] [CrossRef]
- Ball, G.; Shelton, M.J.; Walsh, B.J.; Hill, D.J.; Hosking, C.S.; Howden, M.E.H. A major continuous allergenic epitope of bovine beta-lactoglobulin recognized by human IgE binding. Clin. Exp. Allergy 1994, 24, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Ghalandari, B.; Divsalar, A.; Eslami-Moghadam, M.; Saboury, A.A.; Haertlé, T.; Amanlou, M.; Parivar, K. Probing of the Interaction Between β-Lactoglobulin and the Anticancer Drug Oxaliplatin. Appl. Biochem. Biotechnol. 2014, 175, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Caira, S.; Pizzano, R.; Picariello, G.; Pinto, G.; Cuollo, M.; Chianese, L.; Addeo, L.C.A.F. Allergenicity of Milk Proteins. Milk Protein 2012. [Google Scholar] [CrossRef] [Green Version]
- Permyakov, E.A.; Berliner, L.J. α-Lactalbumin: Structure and function. FEBS Lett. 2000, 473, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Heine, W.E.; Klein, P.D.; Reeds, P.J. The Importance of oc-Lactalbumin in Infant Nutrition1. Available online: https://academic.oup.com/jn/article-abstract/121/3/277/4754512 (accessed on 27 January 2021).
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Ostrovsky, A.V.; Kalinichenko, L.P.; Emelyanenko, V.I.; Klimanov, A.V.; Permyakov, E.A. Environment of tryptophan residues in various conformational states of α-lactalbumin studied by time-resolved and steady-state fluorescence spectrosc. Biophys. Chem. 1988, 30, 105–112. [Google Scholar] [CrossRef]
- Goulding, D.; Fox, P.; O’Mahony, J. Milk proteins: An overview. Milk Proteins 2020, 21–98. [Google Scholar] [CrossRef]
- Thorn, D.C.; Ecroyd, H.; Carver, J.A. The two-faced nature of milk casein proteins: Amyloid fibril formation and chaperone-like activity. Aust. J. Dairy Technol. 2009, 6, 34–40. [Google Scholar]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods. J. Dairy Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein Proteins: Structural and Functional Aspects. In Milk Proteins—From Structure to Biological Properties and Health Aspects; InTech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef] [Green Version]
- Dalgleish, D.G.; Corredig, M. The Structure of the Casein Micelle of Milk and Its Changes During Processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Li, X.; Hu, J.; Liu, X.; Zhang, S.; Tang, C.; Zhou, P. Effects of casein micellar structure on the stability of milk protein-based conjugated linoleic acid microcapsules. Food Chem. 2018, 269, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.; Jimenez-Flores, R.; Bleck, G.; Brown, E.; Butler, J.; Creamer, L.; Hicks, C.; Hollar, C.; Ng-Kwai-Hang, K.; Swaisgood, H. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef] [Green Version]
- Chianese, L.; Quarto, M.; Pizzolongo, F.; Calabrese, M.G.; Caira, S.; Mauriello, R.; De Pascale, S.; Addeo, F. Occurrence of genetic polymorphism at the αs1-casein locus in Mediterranean water buffalo milk. Int. Dairy J. 2009, 19, 181–189. [Google Scholar] [CrossRef]
- Archila, L.D.; Khan, F.S.; Bhatnagar, N.; Robinson, D.; Farrington, M.L.; Kwok, W.W. αS1-Casein elucidate major T-cell responses in cow’s milk allergy. J. Allergy Clin. Immunol. 2017, 140, 854–857.e6. [Google Scholar] [CrossRef] [Green Version]
- Cocco, R.R.; Järvinen, K.-M.; Sampson, H.A.; Beyer, K. Mutational analysis of major, sequential IgE-binding epitopes in αs1-casein, a major cow’s milk allergen. J. Allergy Clin. Immunol. 2003, 112, 433–437. [Google Scholar] [CrossRef]
- Farrell, H. Milk Proteins | Casein Nomenclature, Structure, and Association. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 765–771. [Google Scholar]
- De Gaudry, D.K.; Lohner, S.; Schmucker, C.; Kapp, P.; Motschall, E.; Hörrlein, S.; Röger, C.; Meerpohl, J.J. Milk A1 β-casein and health-related outcomes in humans: A systematic review. Nutr. Rev. 2019, 77, 278–306. [Google Scholar] [CrossRef]
- Miranda, G.; Anglade, P.; Mahé, M.F.; Erhardt, G. Biochemical characterization of the bovine genetic K-casein C and E variants. Anim. Genet. 2009, 24, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Bonfatti, V.; Chiarot, G.; Carnier, P. Glycosylation of κ-casein: Genetic and nongenetic variation and effects on rennet coagulation properties of milk. J. Dairy Sci. 2014, 97, 1961–1969. [Google Scholar] [CrossRef]
- Gandhi, S.; Roy, I. Doxorubicin-loaded casein nanoparticles for drug delivery: Preparation, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2019, 121, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Restani, P.; Gaiaschi, A.; Plebani, A.; Beretta, B.; Cavagni, G.; Fiocchi, A.; Poiesi, C.; Velonà, T.; Ugazio, A.G.; Galli, C.L. Cross-reactivity between milk proteins from different animal species. Clin. Exp. Allergy 1999, 29, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Ehlayel, M.S.; Abu Hazeima, K.; Al-Mesaifri, F.; Bener, A. Camel milk: An alternative for cow’s milk allergy in children. Allergy Asthma Proc. 2011, 32, 255–258. [Google Scholar] [CrossRef]
- Bellioni-Businco, B.; Paganelli, R.; Lucenti, P.; Giampietro, P.G.; Perbornc, H.; Businco, L. Allergenicity of goat’s milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 1999, 103, 1191–1194. [Google Scholar] [CrossRef]
- Businco, L.; Giampietro, P.G.; Lucenti, P.; Lucaroni, F.; Pini, C.; Di Felice, G.; Iacovacci, P.; Curadi, C.; Orlandi, M. Allergenicity of mare’s milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2000, 105, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Griep, E.R.; Cheng, Y.; Moraru, C.I. Efficient removal of spores from skim milk using cold microfiltration: Spore size and surface property considerations. J. Dairy Sci. 2018, 101, 9703–9713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak-Wegrzyn, A.; Fiocchi, A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 234–237. [Google Scholar] [CrossRef]
- Morisawa, Y.; Kitamura, A.; Ujihara, T.; Zushi, N.; Kuzume, K.; Shimanouchi, Y.; Tamura, S.; Wakiguchi, H.; Saito, H.; Matsumoto, K. Effect of heat treatment and enzymatic digestion on the B cell epitopes of cow’s milk proteins. Clin. Exp. Allergy 2009, 39, 918–925. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, J.; Yao, M.; Jiang, M.; Luo, Y. Effects of heat treatment on the antigenicity of four milk proteins in milk protein concentrates. Food Agric. Immunol. 2015, 27, 401–413. [Google Scholar] [CrossRef]
- Azdad, O.; Mejrhit, N.; El Kabbaoui, M.; Chda, A.; Ouahidi, I.; Tazi, A.; Bencheikh, R.; Aarab, L. Effect of heating and enzymatic hydrolysis on casein cow milk sensitivity in Moroccan population. Food Agric. Immunol. 2017, 29, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Bu, G.; Luo, Y.; Zheng, Z.; Zheng, H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric. Immunol. 2009, 20, 195–206. [Google Scholar] [CrossRef]
- Bloom, K.A.; Huang, F.R.; Bencharitiwong, R.; Bardina, L.; Ross, A.; Sampson, H.A.; Nowak-Węgrzyn, A. Effect of heat treatment on milk and egg proteins allergenicity. Pediatr. Allergy Immunol. 2014, 25, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Abbring, S.; Kusche, D.; Roos, T.C.; Diks, M.A.P.; Hols, G.; Garssen, J.; Baars, T.; Esch, B.C.A.M.V. Milk processing increases the allergenicity of cow’s milk-Preclinical evidence supported by a human proof-of-concept provocation pilot. Clin. Exp. Allergy 2019, 49, 1013–1025. [Google Scholar] [CrossRef] [Green Version]
- Graversen, K.B.; Ballegaard, A.R.; Kræmer, L.H.; Hornslet, S.E.; Sørensen, L.V.; Christoffersen, H.F.; Jacobsen, L.N.; Untersmayr, E.; Smit, J.J.; Bøgh, K.L. Cow’s milk allergy prevention and treatment by heat-treated whey—A study in Brown Norway rats. Clin. Exp. Allergy 2020, 50, 708–721. [Google Scholar] [CrossRef]
- Deeth, H.; Datta, N. Ultra-high temperature treatment (UHT) | Heating Systems. Encycl. Dairy Sci. 2002, 2642–2652. [Google Scholar] [CrossRef]
- Shadur, B.; Fong, A.; Altavilla, B.; Saad, R.A.; Wainstein, B.K. Skin testing with ultra-heat-treated (UHT) cow’s milk in children with cow’s milk allergy. Ann. Allergy Asthma Immunol. 2020, 124, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Sopo, S.M.; Greco, M.; Monaco, S.; Bianchi, A.; Cuomo, B.; Liotti, L.; Iacono, I. Matrix effect on baked milk tolerance in children with IgE cow milk allergy. Allergol. Immunopathol. 2016, 44, 517–523. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Bloom, K.A.; Sicherer, S.H.; Shreffler, W.G.; Noone, S.; Wanich, N.; Sampson, H.A. Tolerance to extensively heated milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 342–347.e2. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Nachshon, L.; Appel, M.Y.; Elizur, A.; Levy, M.B.; Eisenberg, E.; Sampson, H.A.; Katz, Y. Efficacy of baked milk oral immunotherapy in baked milk–reactive allergic patients. J. Allergy Clin. Immunol. 2015, 136, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Węgrzyn, A.; Lawson, K.; Masilamani, M.; Kattan, J.; Bahnson, H.; Sampson, H.A. Increased Tolerance to Less Extensively Heat-Denatured (Baked) Milk Products in Milk-Allergic Children. J. Allergy Clin. Immunol. Pract. 2018, 6, 486–495.e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Zhang, L.; Hettinga, K.; Zhou, P. Characterizing the changes of bovine milk serum proteins after simulated industrial processing. LWT 2020, 133, 110101. [Google Scholar] [CrossRef]
- Yang, F.; Zou, L.; Wu, Y.; Wu, Z.; Yang, A.; Chen, H.; Li, X. Structure and allergenicity assessments of bovine β-lactoglobulin treated by sonication-assisted irradiation. J. Dairy Sci. 2020, 103, 4109–4120. [Google Scholar] [CrossRef] [PubMed]
- Zenker, H.E.; Ewaz, A.; Deng, Y.; Savelkoul, H.F.J.; Van Neerven, R.J.; De Jong, N.W.; Wichers, H.J.; Hettinga, K.A.; Teodorowicz, M. Differential Effects of Dry vs. Wet Heating of β-Lactoglobulin on Formation of sRAGE Binding Ligands and sIgE Epitope Recognition. Nutrients 2019, 11, 1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, O.M.; Hau, J.; Kollerup, J. Effect of homogenization and pasteurization on the allergenicity of bovine milk analysed by a murine anaphylactic shock model. Clin. Exp. Allergy 1987, 17, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Høst, A.; Samuelsson, E.-G. Allergic reactions to raw, pasteurized, and homogenized/pasteurized cow milk: A comparison. Allergy 1988, 43, 113–118. [Google Scholar] [CrossRef]
- Michalski, M.-C. On the supposed influence of milk homogenization on the risk of CVD, diabetes and allergy. Br. J. Nutr. 2007, 97, 598–610. [Google Scholar] [CrossRef]
- Michalski, M.-C.; Januel, C. Does homogenization affect the human health properties of cow’s milk? Trends Food Sci. Technol. 2006, 17, 423–437. [Google Scholar] [CrossRef]
- Mulder, H.; Walstra, P. The milk fat globule. In Emulsion Science as Applied to Milk Products and Comparable Foods; Commonwealth Agricultural Bureau: Bucks, UK, 1974; pp. 101–128. [Google Scholar]
- Paajanen, L.; Tuure, T.; Poussa, T.; Korpela, R. No difference in symptoms during challenges with homogenized and unhomogenized cow’s milk in subjects with subjective hypersensitivity to homogenized milk. J. Dairy Res. 2003, 70, 175–179. [Google Scholar] [CrossRef]
- Paschke, A.; Besler, M. Stability of bovine allergens during food processing. Ann. Allergy Asthma Immunol. 2002, 89, 16–20. [Google Scholar] [CrossRef]
- Han, T.; Wang, M.; Wang, Y.; Tang, L. Effects of high-pressure homogenization and ultrasonic treatment on the structure and characteristics of casein. LWT 2020, 130, 109560. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, C.; Li, T.; Sun, D.; Gao, H.; Gao, Z.; Mu, Z. Effect of ultrasound on the structure and functional properties of transglutaminase-crosslinked whey protein isolate exposed to prior heat treatment. Int. Dairy J. 2019, 88, 79–88. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Q.; Wang, Y.; Fu, L. Effect of Ultrasound Treatment on Allergenicity Reduction of Milk Casein via Colloid Formation. J. Agric. Food Chem. 2020, 68, 4678–4686. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-X.; Tu, Z.-C.; Yang, W.; Wang, H.; Zhang, L.; Ma, D.; Huang, T.; Liu, J.; Li, X. Investigation into allergenicity reduction and glycation sites of glycated β-lactoglobulin with ultrasound pretreatment by high-resolution mass spectrometry. Food Chem. 2018, 252, 99–107. [Google Scholar] [CrossRef]
- Santos, H.; Capelo, J. Trends in ultrasonic-based equipment for analytical sample treatment. Talanta 2007, 73, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.-C.; Li, A.-L.; Yang, L.-J.; Zhao, R.; Fan, W.-G.; Huo, G.-C. Comparison of immunomodulating properties of Beta-lactoglobulin and its hydrolysates. Iran. J. Allergy Asthma Immunol. 2014, 13, 26–32. [Google Scholar]
- Ena, J.M.; Beresteijn, E.C.H.; Robben, A.J.P.M.; Schmidt, D.G. Whey Protein Antigenicity Reduction by Fungal Proteinases and a Pepsin/Pancreatin Combination. J. Food Sci. 1995, 60, 104–110. [Google Scholar] [CrossRef]
- Sélo, I.; Clément, G.; Bernard, H.; Chatel, J.; Créminon, C.; Peltre, G.; Wal, J. Allergy to bovine β-lactoglobulin: Specificity of human IgE to tryptic peptides. Clin. Exp. Allergy 1999, 29, 1055–1063. [Google Scholar] [CrossRef]
- Puerta, A.; Diez-Masa, J.C.; De Frutos, M. Immunochromatographic determination of β-lactoglobulin and its antigenic peptides in hypoallergenic formulas. Int. Dairy J. 2006, 16, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, F.J.; Peñas, E.; Baeza, M.L.; Gomez, R. Effects of combined microwave and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins. Int. Dairy J. 2008, 18, 918–922. [Google Scholar] [CrossRef] [Green Version]
- El Mecherfi, K.E.; Curet, S.; Lupi, R.; Larré, C.; Rouaud, O.; Choiset, Y.; Rabesona, H.; Haertlé, T. Combined microwave processing and enzymatic proteolysis of bovine whey proteins: The impact on bovine β-lactoglobulin allergenicity. J. Food Sci. Technol. 2019, 56, 177–186. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Chen, F.; Liu, K.; Zhu, T. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy Sci. Technol. 2013, 93, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Villas-Boas, M.B.; Vieira, K.P.; Trevizan, G.; Zollner, R.D.L.; Netto, F.M. The effect of transglutaminase-induced polymerization in the presence of cysteine on β-lactoglobulin antigenicity. Int. Dairy J. 2010, 20, 386–392. [Google Scholar] [CrossRef]
- Stojadinovic, M.; Pieters, R.; Smit, J.; Velickovic, T.C. Cross-Linking of -Lactoglobulin Enhances Allergic Sensitization Through Changes in Cellular Uptake and Processing. Toxicol. Sci. 2014, 140, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Fernando, A.L. Control of Milk Allergenicity. J. Dairy Vet. Sci. 2017, 2. [Google Scholar] [CrossRef]
- Yao, M.; Xu, Q.; Luo, Y.; Shi, J.; Li, Z. Study on reducing antigenic response and IgE-binding inhibitions of four milk proteins of Lactobacillus casei 1134. J. Sci. Food Agric. 2014, 95, 1303–1312. [Google Scholar] [CrossRef]
- Shi, J.; Luo, Y.; Xiao, Y.; Li, Z.; Xu, Q.; Yao, M. Effects of fermentation by Lactobacillus casei on the antigenicity and allergenicity of four bovine milk proteins. Int. Dairy J. 2014, 35, 75–80. [Google Scholar] [CrossRef]
- Kazemi, R.; Taheri-Kafrani, A.; Motahari, A.; Kordesedehi, R. Allergenicity reduction of bovine milk β-lactoglobulin by proteolytic activity of lactococcus lactis BMC12C and BMC19H isolated from Iranian dairy products. Int. J. Biol. Macromol. 2018, 112, 876–881. [Google Scholar] [CrossRef]
- Alessandri, C.; Sforza, S.; Palazzo, P.; Lambertini, F.; Paolella, S.; Zennaro, D.; Rafaiani, C.; Ferrara, R.; Bernardi, M.L.; Santoro, M.; et al. Tolerability of a Fully Maturated Cheese in Cow’s Milk Allergic Children: Biochemical, Immunochemical, and Clinical Aspects. PLoS ONE 2012, 7, e40945. [Google Scholar] [CrossRef] [Green Version]
- Monaco, S.; Russo, G.; Romano, A.; Liotti, L.; Verga, M.; Sopo, S.M. Yogurt is tolerated by the majority of children with IgE-mediated cow’s milk allergy. Allergol. Immunopathol. 2019, 47, 322–327. [Google Scholar] [CrossRef] [PubMed]
- De La Hoz, L.; Netto, F.M. Structural modifications of β-lactoglobulin subjected to gamma radiation. Int. Dairy J. 2008, 18, 1126–1132. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, J.-H.; Yook, H.-S.; Kang, K.-O.; Lee, S.-Y.; Hwang, H.-J.; Byun, M.-W. Effects of Gamma Radiation on the Allergenic and Antigenic Properties of Milk Proteins. J. Food Prot. 2001, 64, 272–276. [Google Scholar] [CrossRef]
- Miteva, D.; Solak, A.; Dyankova, S.; Nacheva, I.; Dimov, K. Assessment of allergenicity of irradiated dairy products in a Balb/c mice model. Pharmacia 2020, 67, 129–133. [Google Scholar] [CrossRef]
- Banik, S.; Bandyopadhyay, S.; Ganguly, S. Bioeffects of microwave—A brief review. Bioresour. Technol. 2003, 87, 155–159. [Google Scholar] [CrossRef]
- Gomaa, A.I.; Nsonzi, F.; Sedman, J.; Ismail, A.A. Enhanced Unfolding of Bovine β-Lactoglobulin Structure Using Microwave Treatment: A Multi-Spectroscopic Study. Food Biophys. 2016, 11, 370–379. [Google Scholar] [CrossRef]
- Gomaa, A.; Sedman, J.; Ismail, A. An investigation of the effect of microwave treatment on the structure and unfolding pathways of β-lactoglobulin using FTIR spectroscopy with the application of two-dimensional correlation spectroscopy (2D-COS). Vib. Spectrosc. 2013, 65, 101–109. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.W.; Lu, P.; Rulikowska, A.; Boehm, D.; O’Neill, G.; Bourke, P. The effect of atmospheric cold plasma treatment on the antigenic properties of bovine milk casein and whey proteins. Food Chem. 2021, 342, 128283. [Google Scholar] [CrossRef] [PubMed]

| Milk Proteins | Conc. (g/L) | Molecular Mass (kDa) | Biological Function | Amino Acid No. | Allergenic Activity * | |
|---|---|---|---|---|---|---|
| Whey proteins (20% ≈ 7 g/L) | α-lactalbumin Bos d 4 | 1.2–1.5 | 14.2 | Contributes to lactose synthesis | 123 | Major |
| β-lactoglobulin Bos d 5 | 3–4 | 18.3 | Binds to numerous hydrophobic and amphiphilic ligands (defined biological function still unclear) | 162 | Major | |
| BSA Bos d 6 | 0.4 | 67 | Binds to fatty acids, flavors, metal ions | 583 | Minor | |
| Immunoglobulins Bos d 7 | 0.47 | 76.2 | Antibacterial and antiviral activities | Minor | ||
| Caseins (80% ≈ 29 g/L) Bos d 8 | αs1-casein Bos d 9 | 12–14 | 23.6 | Calcium binding | 199 | Major |
| αs2-casein Bos d 10 | 3.75–4 | 25.2 | Calcium binding | 207 | Minor | |
| β-casein Bos d 11 | 10.5–12 | 24.0 | Calcium binding | 209 | Major | |
| κ-casein Bos d 12 | 3.75–4 | 19.0 | Stabilization and coagulation of milk | 169 | Minor | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geiselhart, S.; Podzhilkova, A.; Hoffmann-Sommergruber, K. Cow’s Milk Processing—Friend or Foe in Food Allergy? Foods 2021, 10, 572. https://doi.org/10.3390/foods10030572
Geiselhart S, Podzhilkova A, Hoffmann-Sommergruber K. Cow’s Milk Processing—Friend or Foe in Food Allergy? Foods. 2021; 10(3):572. https://doi.org/10.3390/foods10030572
Chicago/Turabian StyleGeiselhart, Sabine, Aleksandra Podzhilkova, and Karin Hoffmann-Sommergruber. 2021. "Cow’s Milk Processing—Friend or Foe in Food Allergy?" Foods 10, no. 3: 572. https://doi.org/10.3390/foods10030572
APA StyleGeiselhart, S., Podzhilkova, A., & Hoffmann-Sommergruber, K. (2021). Cow’s Milk Processing—Friend or Foe in Food Allergy? Foods, 10(3), 572. https://doi.org/10.3390/foods10030572





