Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone Isolated from Natural Sources
Abstract
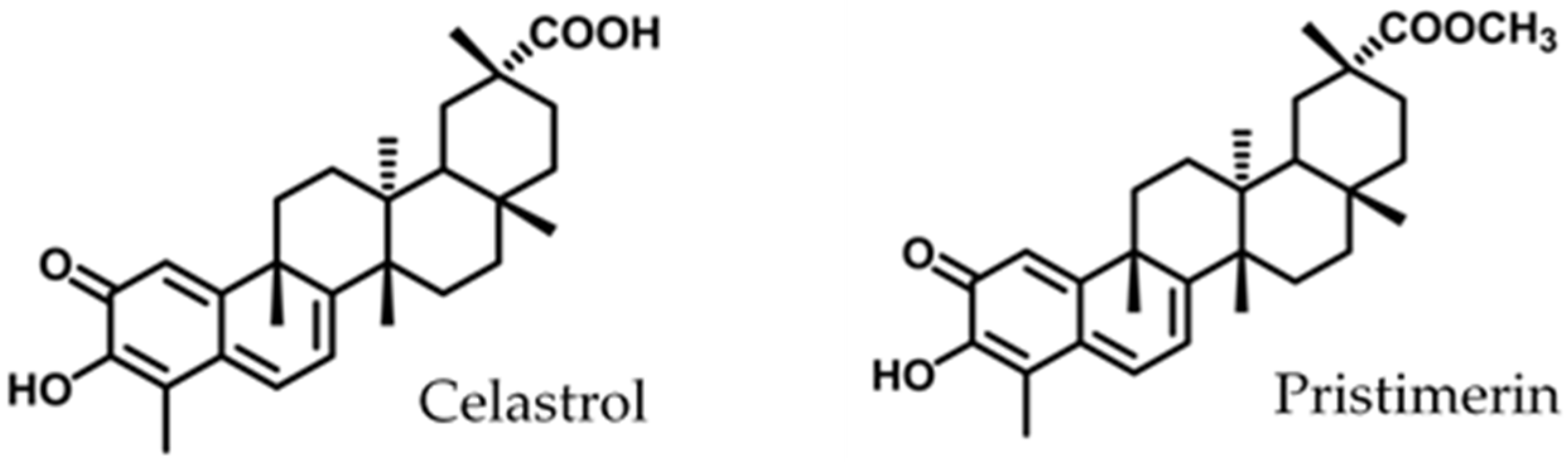
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Quinones and Others Antibacterial Compounds
2.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
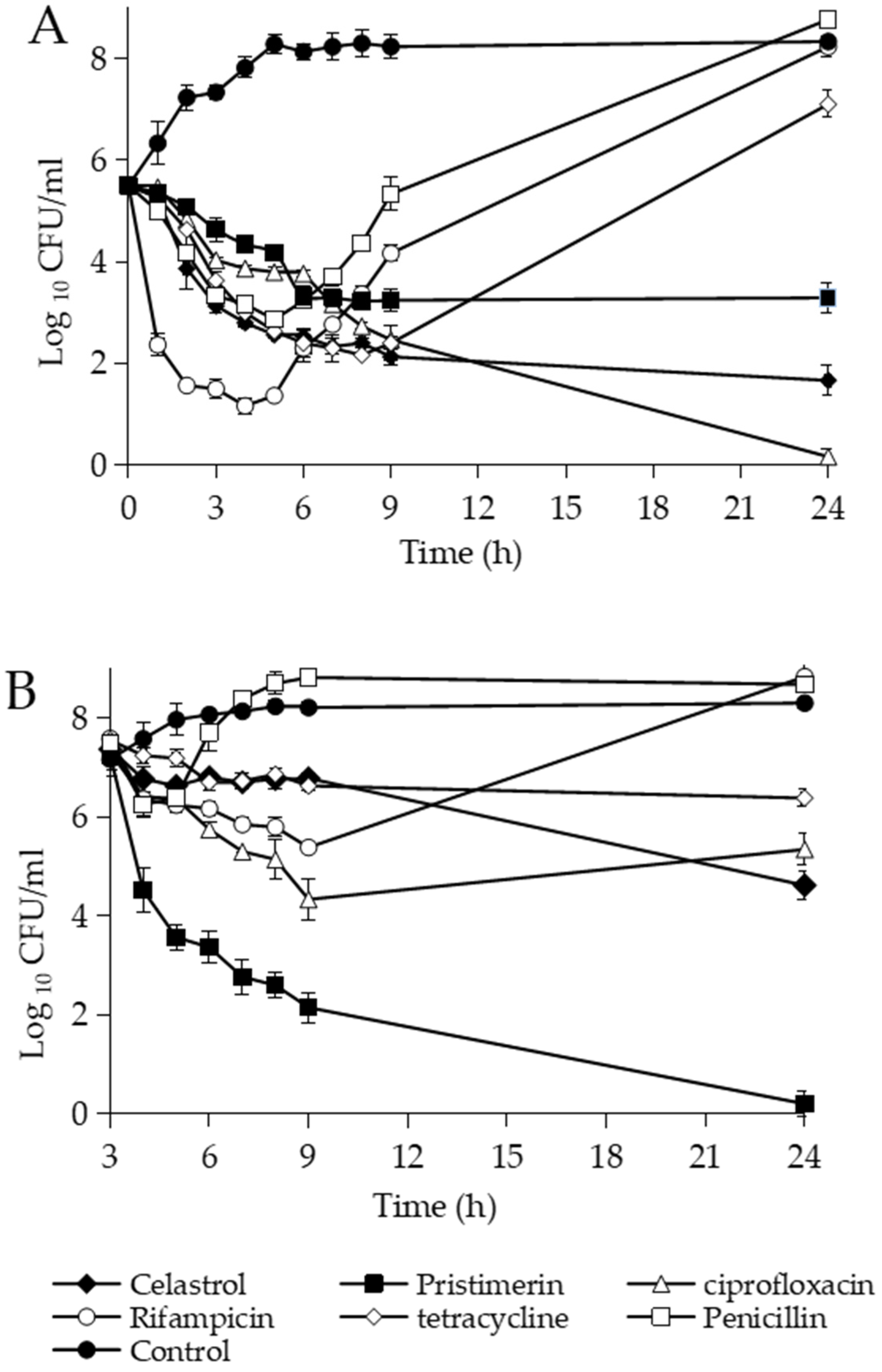
2.4. Bacterial Killing Assays
2.5. Inoculum Effect
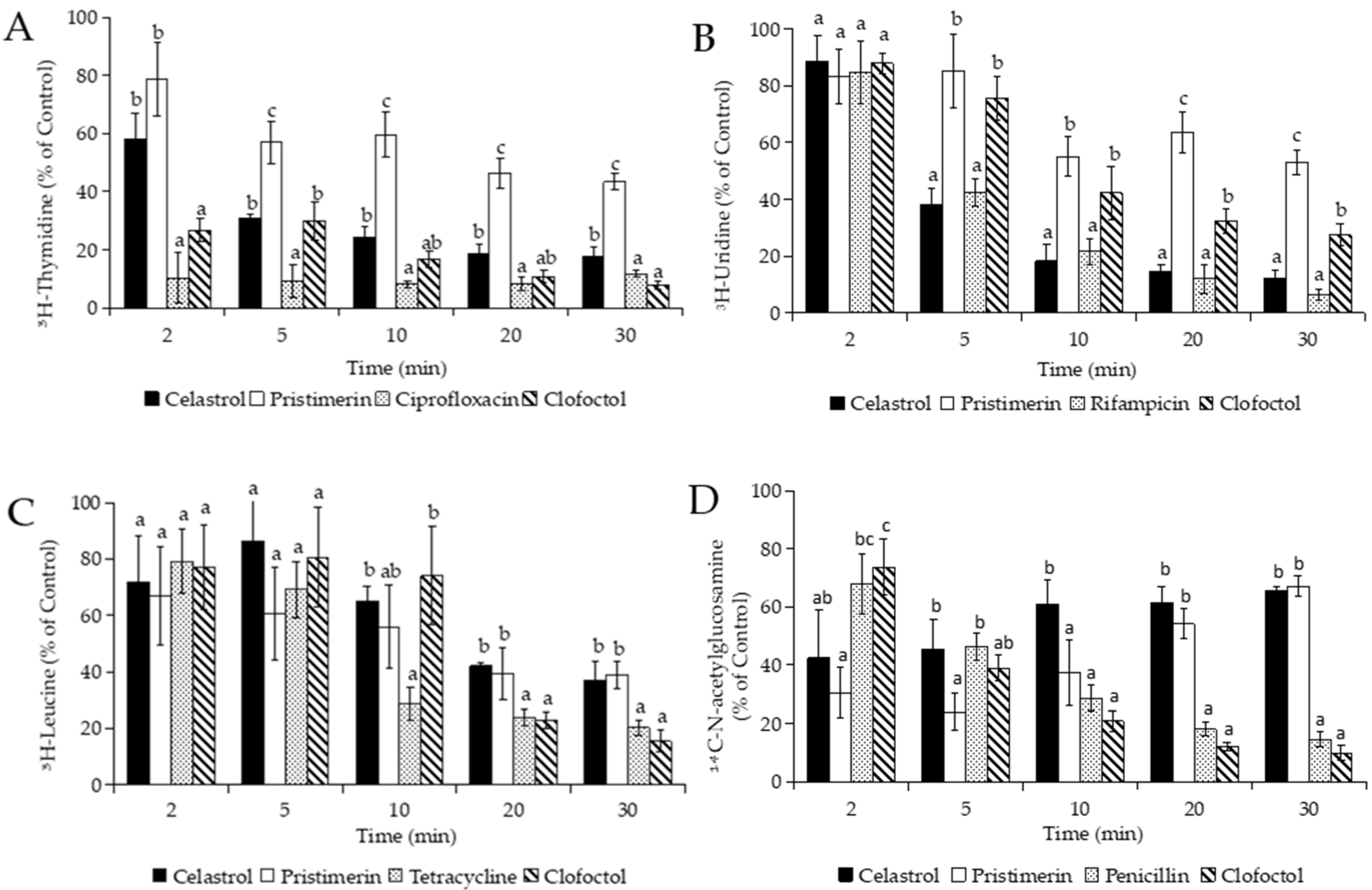
2.6. Measurement of Radioactive Precursor Incorporation
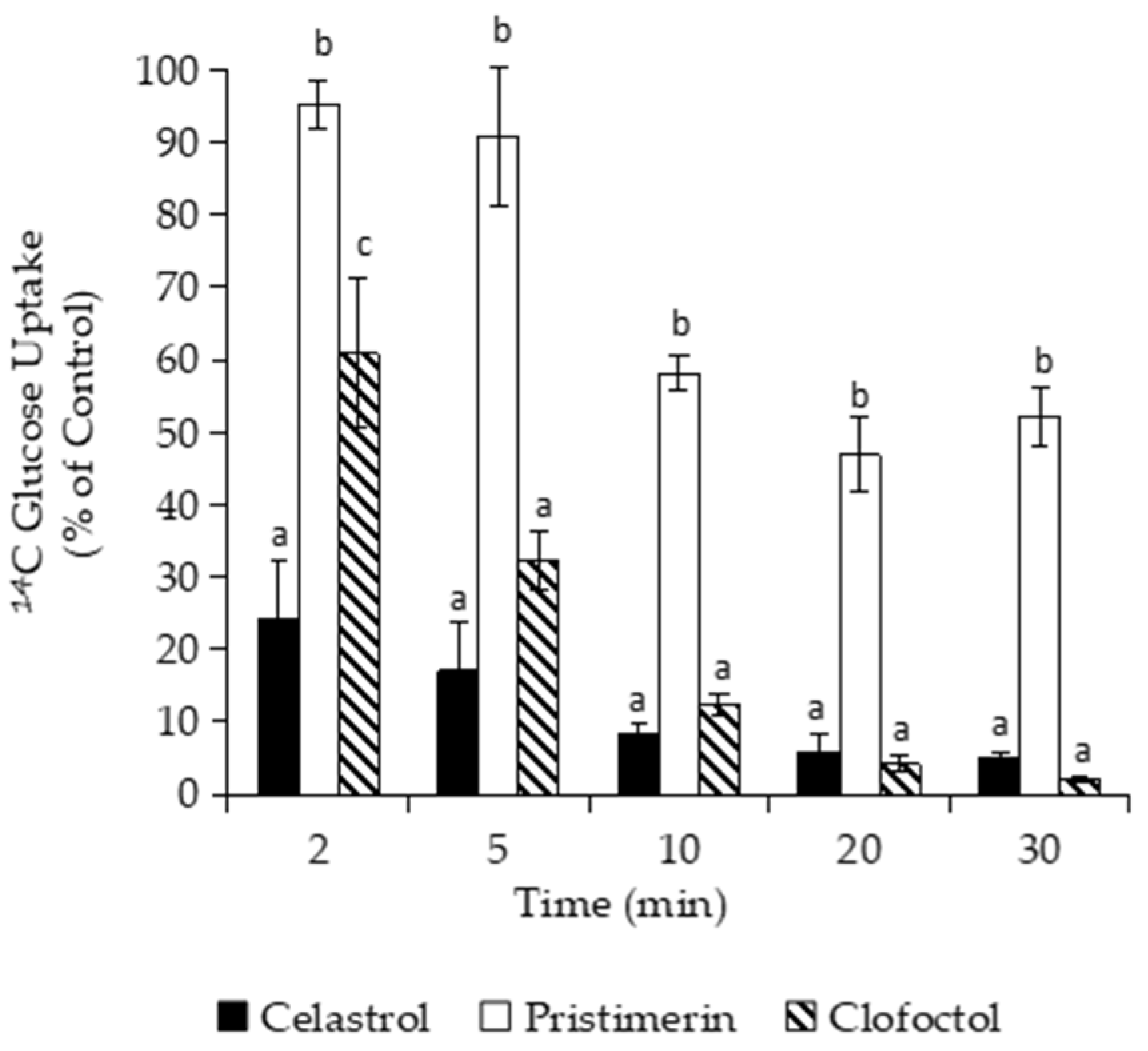
2.7. Measurement of Solutes Uptake
2.8. Inhibition of DNA Gyrase
2.9. Integrity of Cell Membrane
2.10. Transmission Electron Microscopy
2.11. Oxygen Consumption
2.12. Statistical Analysis
3. Results and Discussion
3.1. Antimicrobial Activity
3.2. Effects of Methylene Quinones against B. subtilis
3.3. Mechanism of Action
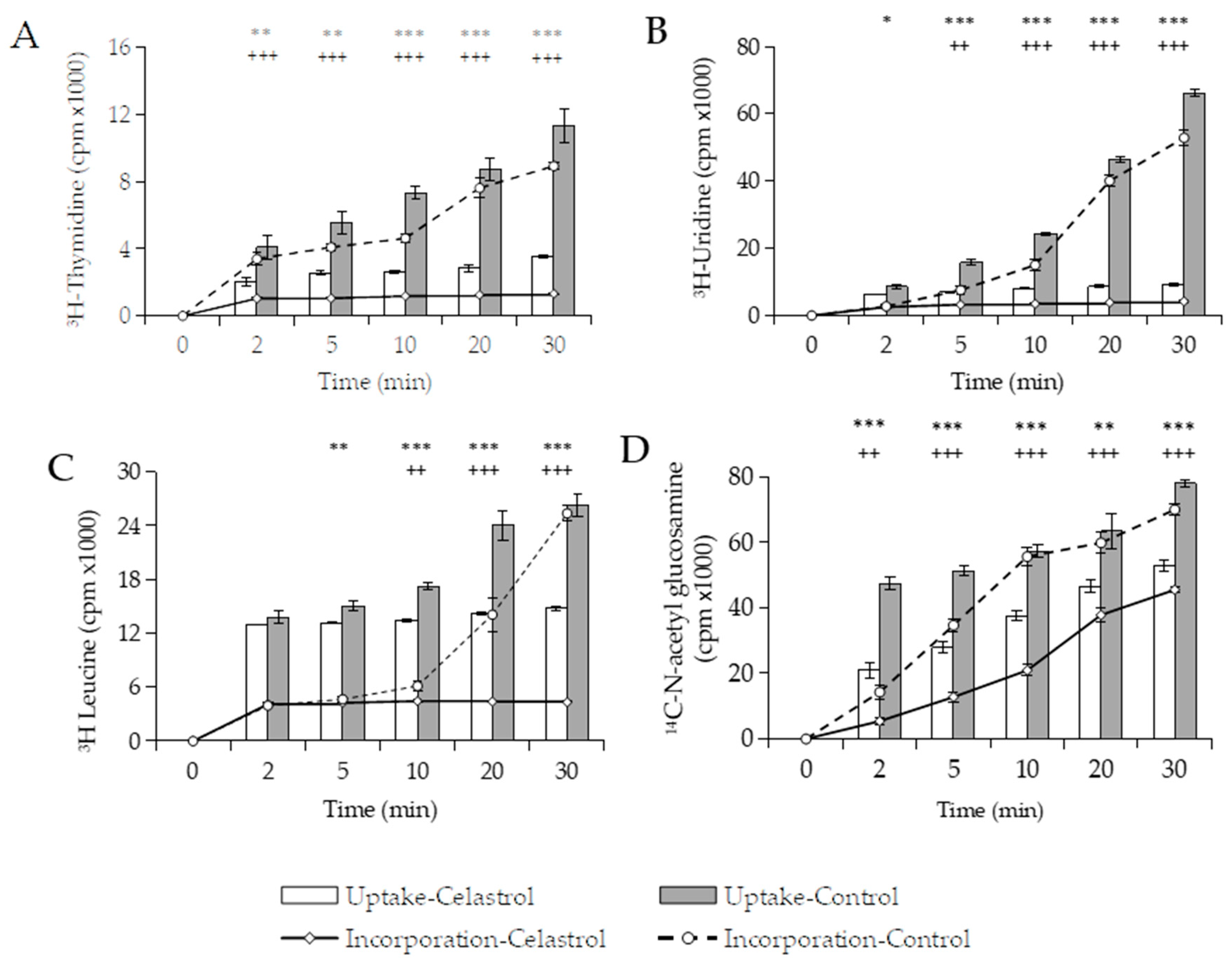
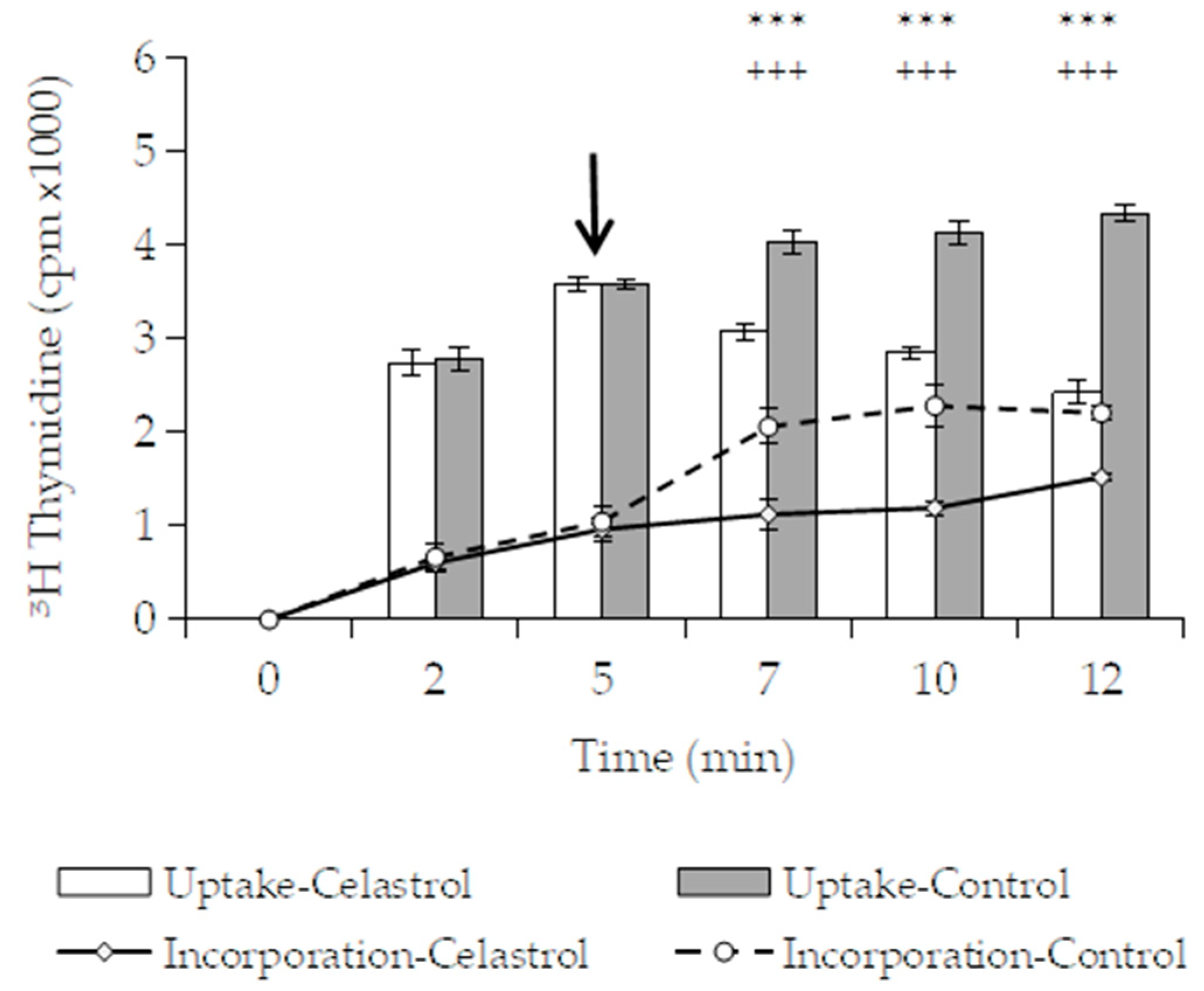
3.3.1. Effects of Macromolecular Synthesis and Initial Uptake of Solutes
3.3.2. Effect of Celastrol on the Integrity and Functions of the Cytoplasmic Membrane
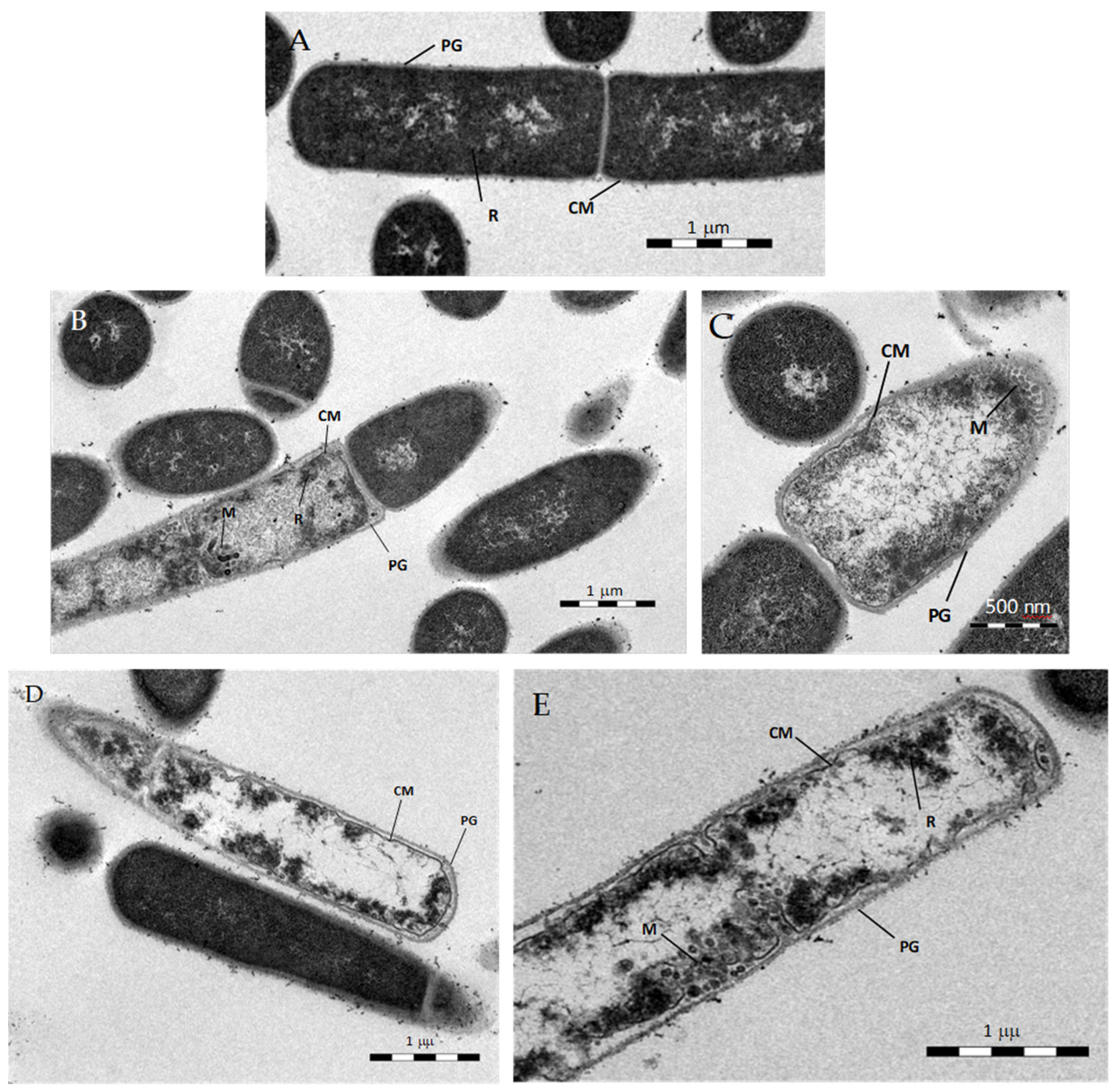
3.3.3. Transmission Electron Microscopy
3.3.4. Effect of Celastrol on Cellular Respiration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, A.G.; Bazzocchi, I.L.; Moujir, L.; Jiménez, I.A. Ethnobotanical uses of Celastraceae. Bioactive metabolites. In Studies in Natural Products Chemistry: Bioactive Natural Products (Part D); Atta-ur, R., Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2000; Volume 23, pp. 649–738. [Google Scholar]
- Simmons, M.P.; Cappa, J.J.; Archer, R.H.; Ford, A.J.; Eichstedt, D.; Clevinger, C.C. Phylogeny of the Celastreae (Celastraceae) and the relationships of Catha edulis (qat) inferred from morphological characters and nuclear and plastid genes. Mol. Phylogenet. Evol. 2008, 48, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L. Triterpenoid Quinonemethides and Related Compounds (celastroloids). In Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, G.W., Moore, R.E., Steglich, W., Tamm, C., Eds.; Springer: New York, NY, USA, 1996; Volume 67, pp. 1–123. [Google Scholar]
- Bashir, A.Y.; Hozeifa, M.H.; Zhang, L.-Y.; Jiang, Z.-Z. Anticancer Potential and Molecular Targets of Pristimerin: A Mini-Review. Curr. Cancer Drugs Targets 2017, 17, 100–108. [Google Scholar] [CrossRef]
- Hou, W.; Liu, B.; Xu, H. Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur. J. Med. Chem. 2020, 189, 112081. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; Shaaban, A.A.; Almaramhy, H.H.; Elkablawy, S.; Elkablawy, M.A. Pristimerin as a Novel Hepatoprotective Agent against Experimental Autoimmune Hepatitis. Front. Pharmacol. 2018, 9, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesha, S.H.; Moudgil, K.D. Celastrol and Its Role in Controlling Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 267–289. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Xu, Z.; Chen, L.; Luo, R.; Zhang, C.; Gao, F.; Zhang, J.; Fu, C. Celastrol: A Review of Useful Strategies Overcoming its Limitation in Anticancer Application. Front. Pharmacol. 2020, 11, 558741. [Google Scholar] [CrossRef] [PubMed]
- Cascão, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 2017, 4, 69. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Mukherjee, T.; Bishayee, A. Molecular targets of celastrol in cancer: Recent trends and advancements. Crit. Rev. Oncol. Hematol. 2018, 128, 70–81. [Google Scholar] [CrossRef]
- Lan, G.; Zhang, J.; Ye, W.; Yang, F.; Li, A.; He, W.; Zhang, W.D. Celastrol as a tool for the study of the biological events of metabolic diseases. Sci. China Chem. 2019, 62, 409–416. [Google Scholar] [CrossRef]
- Liu, J.; Lee, J.; Salazar Hernandez, M.A.; Mazitschek, R.; Ozcan, U. Treatment of Obesity with Celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfuhlmann, K.; Schriever, S.C.; Baumann, P.; Kabra, D.G.; Harrison, L.; Mazibuko-Mbeje, S.E.; Contreras, R.E.; Kyriakou, E.; Simonds, S.E.; Tiganis, T.; et al. Celastrol-Induced Weight Loss Is Driven by Hypophagia and Independent From UCP1. Diabetes 2018, 7, 2456–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moujir, L.; Gutiérrez-Navarro, A.M.; González, A.G.; Ravelo, A.G.; Luis, J.G. The relationship between structure and antimicrobial activity in quinones from the Celastraceae. Biochem. Syst. Ecol. 1990, 18, 25–28. [Google Scholar] [CrossRef]
- Sotanaphun, U.; Lipipun, V.; Suttisri, R.; Bavovada, R. Antimicrobial activity and stability of tingenone derivatives. Planta Med. 1999, 65, 450–452. [Google Scholar] [CrossRef]
- Moujir, L.; Gutiérrez-Navarro, A.M.; González, A.G.; Ravelo, A.G.; Luis, J.G. Mode of action of netzahualcoyone. Antimicrob. Agents Chemother. 1991, 35, 211–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De León, L.; Moujir, L. Activity and mechanism of the action of zeylasterone against B. subtilis. J. Appl. Microbiol. 2007, 104, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-G.; Lee, A.-Y.; Lee, S.-M.; Lim, K.-H.; Ha, E.-J.; Eom, Y.-B. Activity of novel inhibitors of Staphylococcus aureus biofilms. Folia Microbiol. 2017, 62, 157–167. [Google Scholar] [CrossRef]
- Ooi, N.; Eady, E.A.; Cove, J.H.; O’Neill, A.J. Redox-active compounds with a history of human use: Antistaphylococcal action and potential for repurposing as topical antibiofilm agents. J. Antimicrob. Chemother. 2015, 70, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Lee, D.; Eom, Y.-B. Anti-biofilm and Anti-Virulence Efficacy of Celastrol against Stenotrophomonas maltophilia. Int. J. Med. Sci. 2018, 15, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, M.; Sánchez, M.; Stupak, M.; García, M.; Rojo de Almeida, M.T.; Oberti, J.C.; Palermo, J.; Blustein, G. Antifouling Activity of Celastroids Isolated from Maytenus Species, Natural and Sustainable Alternatives for Marine Coatings. Ind. Eng. Chem. Res. 2014, 53, 7655–7659. [Google Scholar] [CrossRef]
- Luo, D.Q.; Wang, H.; Tian, X.; Shao, H.J.; Liu, J.K. Antifungal properties of pristimerin and celastrol isolated from Celastrus hypoleucus. Pest Manag. Sci. 2005, 61, 85–90. [Google Scholar] [CrossRef]
- Gullo, F.P.; Sardi, J.C.; Santos, V.A.; Sangalli-Leite, F.; Pitangui, N.S.; Rossi, S.A.; de Paula, E.; Silva, A.C.; Soares, L.A.; Silva, J.F.; et al. Antifungal activity of maytenin and pristimerin. Evid. Based Complement. Alternat. Med. 2012, 2012, 340787. [Google Scholar] [CrossRef]
- Haque, E.; Irfan, S.; Kamil, M.; Sheikh, S.; Hasan, A.; Ahmad, A.; Lakshmi, V.; Nazir, A.; Mir, S.S. Terpenoids with antifungal activity trigger mitochondrial dysfunction in Saccharomyces cerevisiae. Microbiology 2016, 85, 436–443. [Google Scholar] [CrossRef]
- Song, J.; Shang, N.; Baig, N.; Yao, J.; Shin, C.; Kim, B.K.; Li, Q.; Malwal, S.R.; Oldfield, E.; Feng, X.; et al. Aspergillus flavus squalene synthase as an antifungal target: Expression, activity, and inhibition. Biochem. Biophys. Res. Commun. 2019, 512, 517–523. [Google Scholar] [CrossRef]
- Seo, W.-D.; Lee, D.-Y.; Park, K.H.; Kim, J.-H. Downregulation of fungal cytochrome c peroxidase expression by antifungal quinonemethide triterpenoids. Appl. Biol. Chem. 2016, 59, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Li, C.; Jing, L.; Peng, X.; Wang, Q.; Jiang, N.; Xu, Q.; Zhao, G. Celastrol ameliorates Aspergillus fumigatus keratitis via inhibiting LOX-1. Int. Immunopharmacol. 2019, 70, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-K.; Hsu, S.-P.; Lin, C.-K.; Wu, Y.-H.; Lee, J.-C.; Young, K.-C. Celastrol inhibits hepatitis C virus replication by upregulating heme oxygenase-1 via the JNK MAPK/Nrf2 pathway in human hepatoma cells. Antiviral. Res. 2017, 146, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, G. Synthesis of celastrol derivatives as potential non-nucleoside hepatitis B virus inhibitors. Chem. Biol. Drug Des. 2020, 96, 1380–1386. [Google Scholar] [CrossRef]
- Kumar, T.D.K.; Murali, H.S.; Batra, H.V. Simultaneous detection of pathogenic B. cereus, S. aureus and L. monocytogenes by multiplex PCR. Indian J. Microbiol. 2009, 49, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Kubo, I.; Fujita, K.-I.; Nihei, K.-I.; Nihei, A. Antibacterial Activity of Akyl Gallates against Bacillus subtilis. J. Agric. Food Chem. 2004, 52, 1072–1076. [Google Scholar] [CrossRef]
- Gopal, N.; Hill, C.; Ross, P.R.; Beresford, T.P.; Fenelon, M.A.; Cotter, P.D. The Prevalence and Control of Bacillus and Related Spore-Forming Bacteria in the Dairy Industry. Front. Microbiol. 2015, 6, 1418. [Google Scholar] [CrossRef]
- Gaur, A.H.; Patrick, C.C.; McCullers, J.A.; Flynn, P.M.; Pearson, T.A.; Razzouk, B.I.; Thompson, S.J.; Shenep, J.L. Bacillus cereus Bacteremia and Meningitis in Immunocompromised Children. Clin. Infect. Dis. 2001, 32, 1456–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabscheck, G.; Silverman, L.; Ullrich, N.J. Bacillus cereus Cerebral Abscess during Induction Chemotherapy for Childhood Acute Leukemia. J. Pediatr. Hematol. Oncol. 2015, 37, 568–569. [Google Scholar] [CrossRef]
- Hansford, J.R.; Phillips, M.; Cole, C.; Francis, J.; Blyth, C.C.; Gottardo, N.G. Bacillus cereus bacteremia and multiple brain abscesses during acute lymphoblastic leukemia induction therapy. J. Pediatr. Hematol. Oncol. 2014, 36, e197–e201. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Nuñez, M.J.; Kennedy, M.L.; Jiménez, I.A.; Bazzocchi, I.L. Uragogin and blepharodin, unprecedented hetero-Diels-Alder adducts from Celastraceae species. Tetrahedron 2011, 67, 3030–3033. [Google Scholar] [CrossRef]
- Taddeo, V.A.; Castillo, U.C.; Martínez, M.L.; Menjivar, J.; Jimenéz, I.A.; Nunez, M.J.; Bazzochi, I.L. Development and validation of an HPLC-PDA method for biologically active quinonemethide triterpenoids isolated from Maytenus chiapensis. Medicines 2019, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS); National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing; 16th Informational Supplement; CLSI Document M7-A7; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006; Available online: https://clsi.org/ (accessed on 10 March 2021).
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests f or Bacteria that Grow Aerobically; Approved Standard, 9th ed.; CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Davis, B.D.; Mignoli, E.S. Mutants of Escherichia coli requiring methionine or vitamin B12. J. Bacteriol. 1950, 60, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Da Cruz Nizer, W.S.; Ferraz, A.C.; Moraes, T.F.S.; Lima, W.G.; Santos, J.P.; Duarte, L.P.; Ferreira, J.M.S.; de Brito Magalhäes, C.L.; Vieira-Filho, S.A.; dos Santos PereiraAndrade, A.C.; et al. Pristimerin isolated from Salacia crassifolia (Mart. Ex. Schult.) G. Don. (Celastraceae) roots as a potential antibacterial agent against Staphylococcus aureus. J. Ethnopharmacol. 2021, 266, 113423. [Google Scholar] [CrossRef] [PubMed]
- Moujir, L.; López, M.R.; Reyes, C.P.; Jiménez, I.A.; Bazzocchi, I.L. Structural requirements from antimicrobial activity of phenolic nor-triterpenes from Celastraceae species. Appl. Sci. 2019, 9, 2957. [Google Scholar] [CrossRef] [Green Version]
- He, Q.-W.; Feng, J.-H.; Hu, X.-L.; Long, H.; Huang, X.-F.; Jiang, Z.Z.; Zhang, X.-Q.; Ye, W.-C.; Wang, H. Synthesis and Biological Evaluation of Celastrol Derivatives as Potential Immunosuppressive Agents. J. Nat. Prod. 2020, 83, 2578–2586. [Google Scholar] [CrossRef]
- Yablonsly, F. Alteration of Membrane Permeability in Bacillus subtilis by Clofoctol. J. Gen. Microbiol 1983, 129, 1089–1195. [Google Scholar] [CrossRef] [Green Version]
- Oliva, B.; Miller, K.; Caggiano, N.; O’neill, A.J.; Cuny, G.D.; Hoemarm, M.Z.; Hauske, J.R.; Chopra, I. Biological properties of novel antistaphylococcal quinoline-indole agents. Antimicrob. Agents Chemother. 2003, 47, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-M.; Jhan, Y.-L.; Tsai, S.-J.; Chou, C.-H. The Pleiotropic Antibacterial Mechanisms of Ursolic Acid against Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2016, 21, 884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, W.-G.; Pogell, B.M. Mode of Action of Pamamycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 1981, 20, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De León, L.; López, M.R.; Moujir, L. Antibacterial properties of zeylasterone, a triterpenoid isolated from Maytenus blepharodes, against Staphylococcus aureus. Microbiol. Res. 2010, 165, 617–626. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N. The Potential of Systems Biology to Discover Antibacterial Mechanisms of Plant Phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunthof, C.J.; Van Schalkwijk, S.; Meijer, W.; Abee, T.; Hugenholtz, J. Fluorescent method for monitoring cheese starter permeabilization and lysis. Appl. Environ. Microbiol. 2001, 67, 4264–4271. [Google Scholar] [CrossRef] [Green Version]
- Laflamme, C.; Lavigne, S.; Ho, J.; Duchaine, C. Assessment of bacterial endospore viability with fluorescent dyes. J. Appl. Microbiol. 2004, 96, 684–692. [Google Scholar] [CrossRef]
- Lambert, P.A.; Hammond, S.M. Potassium fluxes, first indications of membrane damage in micro-organisms. Biochem. Biophys. Res. Commun. 1973, 18, 796–799. [Google Scholar] [CrossRef]
- Kalnenieks, U.; Balodite, E.; Rutkis, R. Metabolic Engineering of Bacterial Respiration: High vs. Low P/O and the Case of Zymomonas mobilis. Front. Bioeng. Biotechnol. 2019, 7, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagase, M.; Oto, J.; Sugiyama, S.; Yube, K.; Takaishi, Y.; Sakato, N. Apoptosis Induction in HL-60 Cells and Inhibition of Topoisomerase II by Triterpene Celastrol. Biosci. Biotechnol. Biochem. 2003, 67, 1883–1887. [Google Scholar] [CrossRef] [Green Version]
- Kolarič, A.; Anderluh, M.; Minovski, N. Two Decades of Successful SAR-Grounded Stories of the Novel Bacterial Topoisomerase Inhibitors (NBTIs). J. Med. Chem. 2020, 63, 5664–5674. [Google Scholar] [CrossRef] [PubMed]
- D’Atanasio, N.; Capezzone de Joannon, A.; Di Sante, L.; Mangano, G.; Ombrato, R.; Vitiello, M.; Bartella, C.; Magarò, G.; Prati, F.; Milanese, C.; et al. Antibacterial activity of novel dual bacterial DNA type II topoisomerase inhibitors. PLoS ONE 2020, 15, e0228509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Advances in the chemistry of natural and semisynthetic topoisomerase I/II inhibitors. In Studied Natural Products Chemistry; Atta-ur-Rahman, F., Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2017; Volume 54, pp. 21–86. [Google Scholar] [CrossRef]
- Setzer, W.N. Non-Intercalative Triterpenoid Inhibitors of Topoisomerase II: A Molecular Docking Study. Open Bioact. Compd. J. 2008, 1, 13–17. [Google Scholar] [CrossRef] [Green Version]

| Microorganisms | Celastrol | Pristimerin | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Bacillus subtilis | 0.156 | 2.5 | 1.25 | 10 |
| B. cereus | 0.625 | 2.5 | 10 | >40 |
| B. pumilus | 0.625 | 2.5 | 2 | 10 |
| B. megaterium | 1.25 | 5 | 20 | >40 |
| Staphylococcus aureus | 1.25 | >40 | >40 | >40 |
| S. epidermidis | 0.312 | 15 | 1.25 | >40 |
| S. saprophyticus | 2.5 | 10 | 10 | 10 |
| Mycobacterium smegmatis | 5 | >40 | >40 | >40 |
| Enterococcus faecalis | 1.25 | 40 | 20 | >40 |
| Triterpene | Dilution Factor | Mean Log10 CFU ± SD 1 | ||
|---|---|---|---|---|
| Initial Inoculum | Recovery at 3 h | Recovery at 6 h | ||
| Celastrol | 107 | 7.58 ± 0.11 | 7.60 ± 0.15 | 7.58 ± 0.05 |
| 106 | 6.41 ± 0.14 | 4.68 ± 0.27 | 4.08 ± 0.17 | |
| 105 | 5.54 ± 0.20 | 4.00 ± 0.21 | 3.15 ± 0.16 | |
| 104 | 4.67 ± 0.09 | 2.30 ± 0.25 | 1.27 ± 0.25 | |
| Pristimerin | 107 | 7.58 ± 0.12 | 7.51 ± 0.07 | 7.47 ± 0.05 |
| 106 | 6.35 ± 0.17 | 5.32 ± 0.20 | 4.71 ± 0.33 | |
| 105 | 5.32 ± 0.19 | 4.26 ± 0.16 | 3.27 ± 0.22 | |
| 104 | 4.75 ± 0.11 | 3.59 ± 0.12 | 1.56 ± 0.10 | |
| Bacteria | Time (min) | Oxygen Compsumption 1 | |||||
|---|---|---|---|---|---|---|---|
| Whole Cells (Glucose 1%) | Membrane Fraction (NADH 0.1 mM) | ||||||
| Control | NaCN | Celastrol | Control | NaCN | Celastrol | ||
| B. subtilis | 0 | 2.36 ± 0.10 a | 2.30 ± 0.07 a (2.4%) | 2.27 ± 0.12 a (3.9%) | 0.54 ± 0.05 a | 0.50 ± 0.06 a (6.9%) | 0.52 ± 0.08 a (4.0%) |
| 2 | 3.69 ± 0.17 a | 2.71 ± 0.05 b (26.5%) | 2.74 ± 0.15 b (25.7%) | 0.98 ± 0.11 a | 0.66 ± 0.00 b (31.7%) | 0.68 ± 0.01 b (30.6%) | |
| 4 | 5.42 ± 0.46 a | 2.77 ± 0.08 b (48.6%) | 3.11 ± 0.04 b (42.4%) | 1.27 ± 0.01 a | 0.79 ± 0.00 c (37.7%) | 0.82 ± 0.02 b (35.1%) | |
| 6 | 7.09 ± 0.21 a | 2.91 ± 0.03 c (58.9%) | 3.30 ± 0.02 b (53.5%) | 1.56 ± 0.05 a | 0.89 ± 0.01 b (43.0%) | 0.94 ± 0.03 b (39.4%) | |
| 8 | 8.53 ± 0.14 a | 2.97 ± 0.02 c (65.2%) | 3.42 ± 0.04 b (59.8%) | 1.80 ± 0.01 a | 0.94 ± 0.00 c (47.9%) | 1.01 ± 0.02 b (43.9%) | |
| E. coli | 0 | 2.16 ± 0.07 a | 2.04 ± 0.06 a,b (5.5%) | 2.00 ± 0.02 b (7.3%) | 2.22 ± 0.07 a | 2.10 ± 0.01 a,b (5.0%) | 2.07 ± 0.05 b (6.5%) |
| 2 | 3.35 ± 0.14 a | 2.16 ± 0.03 c (35.5%) | 3.08 ± 0.08 b (7.9%) | 3.07 ± 0.00 a | 2.20 ± 0.05 c (28.2%) | 2.41 ± 0.02 b (21.3%) | |
| 4 | 4.34 ± 0.24 a | 2.22 ± 0.03 b (48.8%) | 3.95 ± 0.14 a (9.0%) | 3.88 ± 0.01 a | 2.30 ± 0.03 c (40.6%) | 2.51 ± 0.01 b (35.3%) | |
| 6 | 5.80 ± 0.32 a | 2.32 ± 0.03 c (60.0%) | 4.84 ± 0.10 b (16.6%) | 4.74 ± 0.01 a | 2.34 ± 0.02 c (50.6%) | 2.61 ± 0.02 b (44.9%) | |
| 8 | 6.79 ± 0.19 a | 2.40 ± 0.05 c (64.6.4%) | 5.53 ± 0.09 b (18.5%) | 5.37 ± 0.00 a | 2.40 ± 0.08 c (55.3%) | 2.68 ± 0.01 b (50.0%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Montaño, N.; de León Guerra, L.; Moujir, L. Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone Isolated from Natural Sources. Foods 2021, 10, 591. https://doi.org/10.3390/foods10030591
Padilla-Montaño N, de León Guerra L, Moujir L. Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone Isolated from Natural Sources. Foods. 2021; 10(3):591. https://doi.org/10.3390/foods10030591
Chicago/Turabian StylePadilla-Montaño, Nayely, Leandro de León Guerra, and Laila Moujir. 2021. "Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone Isolated from Natural Sources" Foods 10, no. 3: 591. https://doi.org/10.3390/foods10030591
APA StylePadilla-Montaño, N., de León Guerra, L., & Moujir, L. (2021). Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone Isolated from Natural Sources. Foods, 10(3), 591. https://doi.org/10.3390/foods10030591








