Extending Shelf-Life and Quality of Minimally Processed Golden Delicious Apples with Three Bioactive Coatings Combined with Cinnamon Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Minimally Processed Apples
2.3. Bioactive Coating Formulations
2.4. Weight Loss Percentage
2.5. Firmness Analyses
2.6. Assay of Color (Brown Index, BI)
2.7. Total Phenolic Content
2.8. Total Acidity (TA) and Total Soluble Solids (TSS)
2.9. Respiration Rate
2.10. Microbial Analysis
2.11. Sensory Evaluation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss
3.2. Firmness Analysis
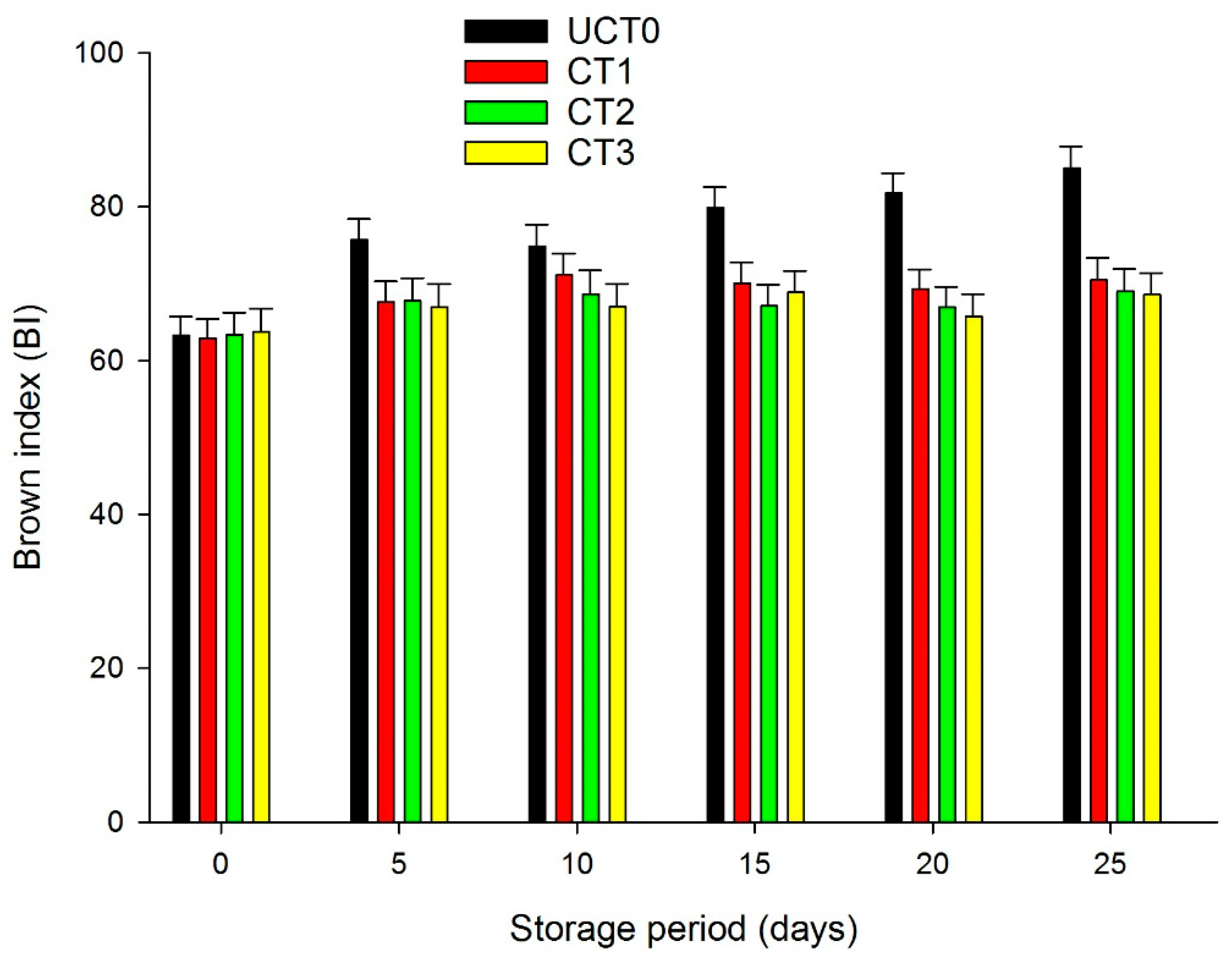
3.3. Color, Brown Index (BI)
3.4. Total Phenolic Content
3.5. Total Soluble Solids (TSS)
3.6. Titratable Acidity (TA)
3.7. Microbiology Analysis
3.8. Respiration Rate
3.9. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiumarelli, M.; Hubinger, M.D. Stability, solubility, mechanical and barrier properties of cassava starch—Carnauba wax edible coatings to preserve fresh-cut apples. Food Hydrocoll. 2012, 28, 59–67. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.; Faleiro, M.L.; Miguel, M.G.; Antunes, M.D. The effect of edible coatings on the nutritional quality of ‘Bravo de Esmolfe’ fresh-cut apple through shelf-life. LWT 2017, 75, 210–219. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.; Lee, C.; Choi, W. Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. LWT 2003, 36, 323–329. [Google Scholar] [CrossRef]
- Moreira, M.R.; Tomadoni, B.; Martín-Belloso, O.; Soliva-Fortuny, R. Preservation of fresh-cut apple quality attributes by pulsed light in combination with gellan gum-based prebiotic edible coatings. LWT 2015, 64, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Shahnawaz, M.; Wali, A.; Abbass, S. A study on the shelf life of minimally processed apple with edible coatings. Chem. J. 2013, 3, 20–22. [Google Scholar]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.; Vuong, Q.V. Starch-based edible coating formulation: Optimization and its application to improve the postharvest quality of “Cripps pink” apple under different temperature regimes. Food Packag. Shelf Life 2019, 22, 100409. [Google Scholar] [CrossRef]
- Sena, E.D.; Da Silva, P.S.O.; Batista, M.C.D.A.; Sargent, S.A.; Junior, L.F.G.; Pagani, A.A.C.; Carnelossi, M.A.G. Calcium application via hydrocooling and edible coating for the conservation and quality of cashew apples. Sci. Hortic. 2019, 256, 108531. [Google Scholar] [CrossRef]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality Control of Fresh-Cut Apples after Coating Application. Foods 2019, 8, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruelas-Chacon, X.; Aguilar-González, A.; Reyes-Vega, M.D.; Peralta-Rodríguez, R.D.; Corona-Flores, J.; Rebolloso-Padilla, O.N.; Aguilera-Carbo, A.F. Bioactive Protecting Coating of Guar Gum with Thyme Oil to Extend Shelf Life of Tilapia (Oreoschromis niloticus) Fillets. Polymers 2020, 12, 3019. [Google Scholar] [CrossRef] [PubMed]
- Saba, M.K.; Sogvar, O.B. Combination of carboxymethyl cellulose-based coatings with calcium and ascorbic acid impacts in browning and quality of fresh-cut apples. LWT 2016, 66, 165–171. [Google Scholar] [CrossRef]
- Qi, H.; Hu, W.; Jiang, A.; Tian, M.; Li, Y. Extending shelf-life of fresh-cut ´Fuji´ apples with chitosan-coatings. IFSET 2011, 12, 62–66. [Google Scholar] [CrossRef]
- Das, D.K.; Dutta, H.; Mahanta, C.L. Development of a rice starch-based coating with antioxidant and microbe-barrier properties and study of its effect on tomatoes stored at room temperature. LWT 2013, 50, 272–278. [Google Scholar] [CrossRef]
- Hamzah, H.M.; Osman, A.; Tan, C.P.; Ghazali, F.M. Carrageenan as an alternative coating for papaya (Carica papaya L. cv. Eksotika). Postharvest Biol. Technol. 2013, 75, 142–146. [Google Scholar] [CrossRef]
- Lin, L.-S.; Wang, B.-J.; Weng, Y.-M. Quality preservation of commercial fish balls with antimicrobial zein coatings. J. Food Qual. 2011, 34, 81–87. [Google Scholar] [CrossRef]
- Heyman, B.; De Vos, W.H.; Depypere, F.; Van der Meeren, P.; Dewettinck, K. Guar and xanthan gum differentially affect shear induced breakdown of native waxy maize starch. Food Hydrocoll. 2014, 35, 546–556. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Siripatrawan, U. Active food packaging from chitosan incorporated with plant polyphenols. In Novel Approaches of Nanotechnology in Food; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 465–507. [Google Scholar]
- Saurabh, C.K.; Gupta, S.; Variyar, P.S.; Sharma, A. Effect of addition of nanoclay, beeswax, tween-80 and glycerol on physicochemical properties of guar gum films. Ind. Crop. Prod. 2016, 89, 109–118. [Google Scholar] [CrossRef]
- Aydogdu, A.; Radke, C.J.; Bezci, S.; Kirtil, E. Characterization of curcumin incorporated guar gum/orange oil antimicrobial emulsion films. Int. J. Biol. Macromol. 2020, 148, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-S.; Song, H.-G.; Choi, I.; Lee, J.-S.; Han, J. Effects of mung bean starch/guar gum-based edible emulsion coatings on the staling and safety of rice cakes. Carbohydr. Polym. 2020, 247, 116696. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.; Chang, Y.; Lee, E.-S.; Choi, H.-D.; Han, J. Development of a starch/gum-based edible coating for rice cakes to retard retrogradation during storage. LWT 2018, 97, 516–522. [Google Scholar] [CrossRef]
- Saberi, B.; Thakur, R.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of physical and optical properties of biodegradable edible films based on pea starch and guar gum. Ind. Crop. Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Saberi, B.; Golding, J.B.; Marques, J.R.; Pristijono, P.; Chockchaisawasdee, S.; Scarlett, C.J.; Stathopoulos, C.E. Application of biocomposite edible coatings based on pea starch and guar gum on quality, storability and shelf life of ‘Valencia’ oranges. Postharvest Biol. Technol. 2018, 137, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.; Goto, G.; Mainardi, J.; Coelho, A.; Tadini, C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT 2013, 54, 346–352. [Google Scholar] [CrossRef]
- Lu, F.; Ding, Y.; Ye, X.; Liu, D. Cinnamon and nisin in alginate–calcium coating maintain quality of fresh northern snakehead fish fillets. LWT 2010, 43, 1331–1335. [Google Scholar] [CrossRef]
- Kechichian, V.; Ditchfield, C.; Veiga-Santos, P.; Tadini, C.C. Natural antimicrobial ingredients incorporated in biodegradable films based on cassava starch. LWT 2010, 43, 1088–1094. [Google Scholar] [CrossRef]
- SIAP. Avances de siembras y cosechas resumen por cultivo. Servicio de Información Agroalimentaria y Pesquera, Gobierno de México. 2019. Available online: http://infosiap.siap.gob.mx:8080/agricola_siap_gobmx/ResumenDelegacion.do (accessed on 4 November 2019).
- USDA. Annual Report Number MX2020-0069, Fresh Deciduous Fruit Annual. United States Department of Agriculture Foreign Agricultural Service. 2020. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Fresh%20Deciduous%20Fruit%20Annual_Mexico%20City_Mexico_11-01-2020 (accessed on 8 November 2020).
- De León-Zapata, M.A.; Sáenz-Galindo, A.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Jasso-Cantú, D.; Aguilar, C.N. Edible candelilla wax coating with fermented extract of tarbush improves the shelf life and quality of apples. Food Packag. Shelf Life 2015, 3, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Mitcham, E.J.; Crisosto, C.H.; Kader, A.A. Apple: ’Golden delicious’ color chart. UC Davis. 2007. Available online: http://postharvest.ucdavis.edu/Commodity_Resources/Fact_Sheets/Datastores/Fruit_English/?uid=3&ds=798 (accessed on 4 November 2019).
- Ruelas-Chacon, X.; Contreras-Esquivel, J.C.; Montañez, J.; Aguilera-Carbo, A.F.; Reyes-Vega, M.L.; Peralta-Rodriguez, R.D.; Sanchéz-Brambila, G. Guar Gum as an Edible Coating for Enhancing Shelf-Life and Improving Postharvest Quality of Roma Tomato (Solanum lycopersicum L.). J. Food Qual. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Borges, C.D.; Mendonça, C.R.B.; Nogueira, D.; Hartwig, E.S.; Rutz, J.K. Conservation of minimally processed apples using edible coatings made of turnip extract and xanthan gum. Braz. J. Food Technol. 2016, 19, 2015038. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.M.C.N.; Morais, A.M.B.B. Polyphenoloxidase activity and total phenolic content as related to browning of minimally processed Jonagored apple. J. Sci. Food Agric. 2001, 82, 120–126. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Song, H.-Y.; Jo, W.-S.; Song, N.-B.; Min, S.C.; Bin Song, K. Quality Change of Apple Slices Coated with Aloe vera Gel during Storage. J. Food Sci. 2013, 78, 1. [Google Scholar] [CrossRef]
- Sarengaowa; Hu, W.; Jiang, A.; Xiu, Z.; Feng, K. Effect of thyme oil-alginate-based coating on quality and microbial safety of fresh-cut apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Olivas, G.; Mattinson, D.; Barbosa-Cánovas, G. Alginate coatings for preservation of minimally processed ‘Gala’ apples. Postharvest Biol. Technol. 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Perez-Gago, M.; Serra, M.; del Río, M. Color change of fresh-cut apples coated with whey protein concentrate-based edible coatings. Postharvest Biol. Technol. 2006, 39, 84–92. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Łakoma, P.; Fortuna, T. Antioxidant Properties of Apple Slices Stored in Starch-based Films. Int. J. Food Prop. 2016, 20, 1117–1128. [Google Scholar] [CrossRef] [Green Version]
- Romani, V.P.; Hernández, C.P.; Martins, V.G. Pink pepper phenolic compounds incorporation in starch/protein blends and its potential to inhibit apple browning. Food Packag. Shelf Life 2018, 15, 151–158. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Zhu, Y.; Han, W.; Xuan, H.; Ge, L. The preservation effect of ascorbic acid and calcium chloride modified chitosan coating on fresh-cut apples at room temperature. Colloids Surf. A Physicochem. Eng. Asp. 2016, 502, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Zhelyazkov, S.; Zsivanovits, G.; Brashyanova, B.; Zsivanovits, M.M. Shelf-life extension of fresh-cut apple cubes with chitosan coating. Bulg. J. Agric. Sci. 2014, 20, 536–540. [Google Scholar]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Aloe vera based edible coatings improve the quality of minimally processed ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Yaman, O.; Bayoιndιrlι, L. Effects of an Edible Coating and Cold Storage on Shelf-life and Quality of Cherries. LWT Food Sci. Technol. 2002, 35, 146–150. [Google Scholar] [CrossRef]
- Chauhan, O.; Raju, P.; Singh, A.; Bawa, A. Shellac and aloe-gel-based surface coatings for maintaining keeping quality of apple slices. Food Chem. 2011, 126, 961–966. [Google Scholar] [CrossRef]
- ISO 4833:2003(E). International Standard. Microbiology of food and animal feeding stuff-Colony-count technique at 30 °C. Available online: https://www.pau.edu/msrlibrary/iso/pdf/iso_4833_2003_ed3_en_34524_3_cpdf.pdf (accessed on 11 November 2019).
- RM615-2003-Digesa.minsa.gob.pe. Norma sanitaria que establece los criterios microbiológicos de calidad sanitaria e inocuidad para los alimentos y bebidas de consumo humano. Available online: http://www.digesa.minsa.gob.pe/norma_consulta/Proy_RM615-2003.pdf (accessed on 11 November 2019).
- ISO 21527-1:2008(E). International Standard. Microbiology of Food and Animal Feeding Stuff-Enumeration of Yeast and Moulds-Part 1. Available online: http://www.smartjd.org/pdf/181/11285379.pdf (accessed on 11 November 2019).
- Supapvanich, S.; Mitrsang, P.; Srinorkham, P.; Boonyaritthongchai, P.; Wongs-Aree, C. Effects of fresh Aloe vera gel coating on browning alleviation of fresh cut wax apple (Syzygium samarangenese) fruit cv. Taaptimjaan. J. Food Sci. Technol. 2016, 53, 2844–2850. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Microorganisms | Storage Period (days) | Treatments | |||
|---|---|---|---|---|---|
| UCT0 | CT1 | CT2 | CT3 | ||
| Mesophilic bacteria (log CFU/g) | 0 | 1.60 d A | 1.58 d A | 1.56 d A | 1.62 d A |
| 5 | 2.19 c A | 1.72 c B | 1.81 c B | 1.79 c B | |
| 10 | 2.32 b c A | 1.83 b c B | 1.95 b c B | 2.02 b c B | |
| 15 | 2.49 b c A | 1.88 b c B | 2.01 b c B | 2.00 b c B | |
| 20 | 2.62 a b A | 1.92 a b B | 2.10 a b B | 2.09 a B | |
| 25 | 3.25 a A | 2.00 a B | 2.20 a B | 2.10 a B | |
| Yeast and molds (log CFU/g) | 0 | 1.15 d A | 1.12 d A | 1.14 d A | 1.13 d A |
| 5 | 1.77 c A | 1.46 c C | 1.57 c B | 1.54 c B | |
| 10 | 1.90 b A | 1.58 b C | 1.71 b c B | 1.77 b B | |
| 15 | 2.10 a b A | 1.62 b C | 1.77 b c B | 1.75 b B | |
| 20 | 2.20 a A | 1.66 a b C | 1.86 b B | 1.84 a B | |
| 25 | 2.17 a A | 1.74 a C | 1.96 a B | 1.85 a B | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solís-Contreras, G.A.; Rodríguez-Guillermo, M.C.; de la Luz Reyes-Vega, M.; Aguilar, C.N.; Rebolloso-Padilla, O.N.; Corona-Flores, J.; de Abril Alexandra Soriano-Melgar, L.; Ruelas-Chacon, X. Extending Shelf-Life and Quality of Minimally Processed Golden Delicious Apples with Three Bioactive Coatings Combined with Cinnamon Essential Oil. Foods 2021, 10, 597. https://doi.org/10.3390/foods10030597
Solís-Contreras GA, Rodríguez-Guillermo MC, de la Luz Reyes-Vega M, Aguilar CN, Rebolloso-Padilla ON, Corona-Flores J, de Abril Alexandra Soriano-Melgar L, Ruelas-Chacon X. Extending Shelf-Life and Quality of Minimally Processed Golden Delicious Apples with Three Bioactive Coatings Combined with Cinnamon Essential Oil. Foods. 2021; 10(3):597. https://doi.org/10.3390/foods10030597
Chicago/Turabian StyleSolís-Contreras, Gisselle Anahí, María Consuelo Rodríguez-Guillermo, María de la Luz Reyes-Vega, Cristobal N. Aguilar, Oscar Noé Rebolloso-Padilla, José Corona-Flores, Lluvia de Abril Alexandra Soriano-Melgar, and Xochitl Ruelas-Chacon. 2021. "Extending Shelf-Life and Quality of Minimally Processed Golden Delicious Apples with Three Bioactive Coatings Combined with Cinnamon Essential Oil" Foods 10, no. 3: 597. https://doi.org/10.3390/foods10030597









