An Insight by Molecular Sensory Science Approaches to Contributions and Variations of the Key Odorants in Shiitake Mushrooms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Hot-Air-Drying Processing
2.4. HS-SPME Analysis
2.5. DSE-SAFE Analysis
2.6. Gas Chromatography-Olfactometry (GC-O)
2.7. Detection Frequency Analysis (DFA)
2.8. Aroma Extract Dilution Analysis (AEDA)
2.9. Gas Chromatography-Mass Spectrometry (GC-MS)
2.10. Identification of Odorants
2.11. Quantitation of the Predominant Odorants
2.12. Calculation of Odor Activity Values (OAV)
2.13. Aroma Analysis
2.14. Aroma Recombination Experiments.
2.15. Statistical Analysis
3. Results and Discussion
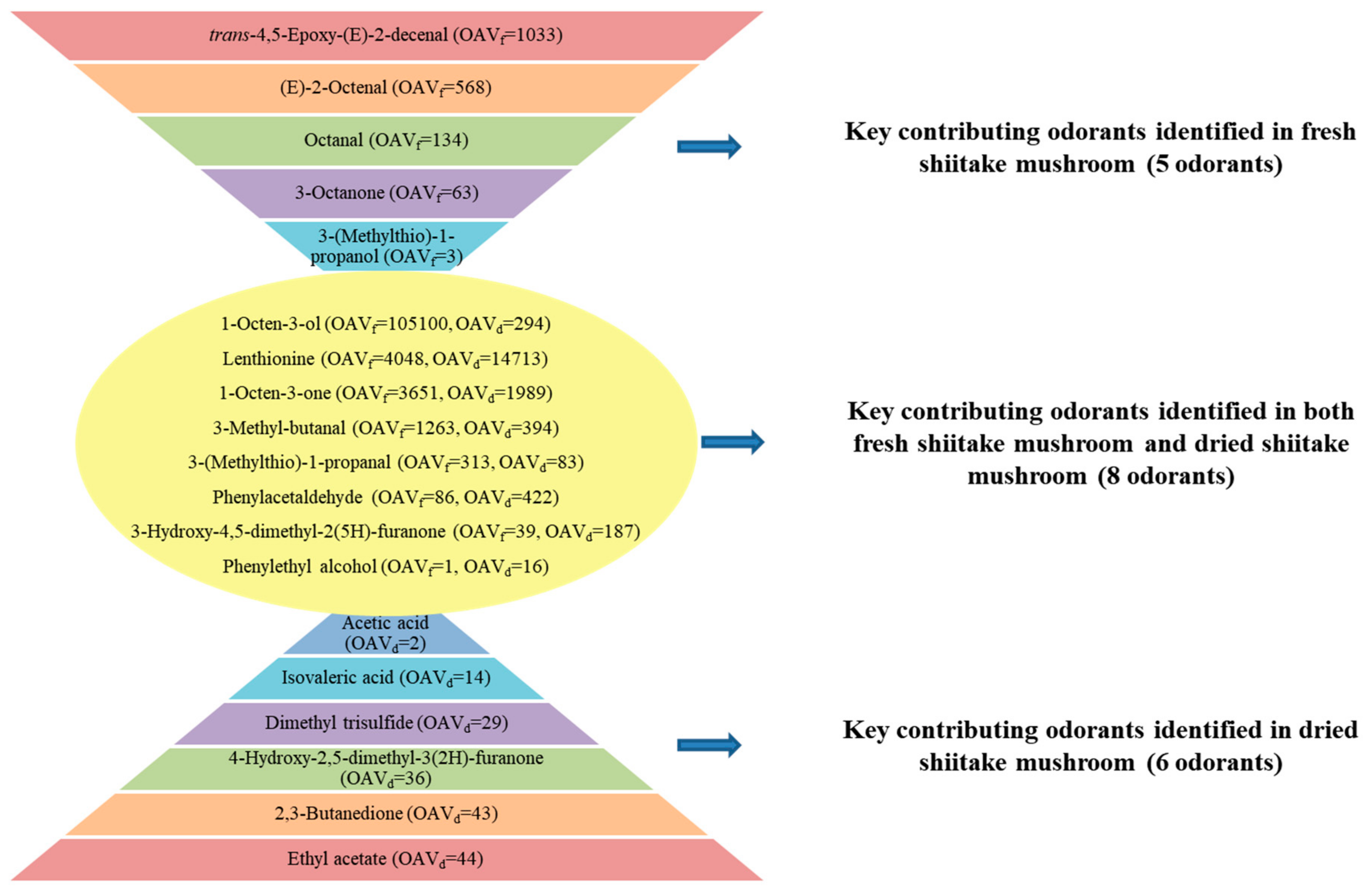
3.1. Odorants in Fresh Shiitake Mushrooms
3.1.1. Odorants Identified by HS-SPME
3.1.2. Odorants Identified by DSE-SAFE
3.2. Odorants in Dried Shiitake Mushrooms
3.2.1. Odorants Identified by HS-SPME
3.2.2. Odorants Identified by DSE-SAFE
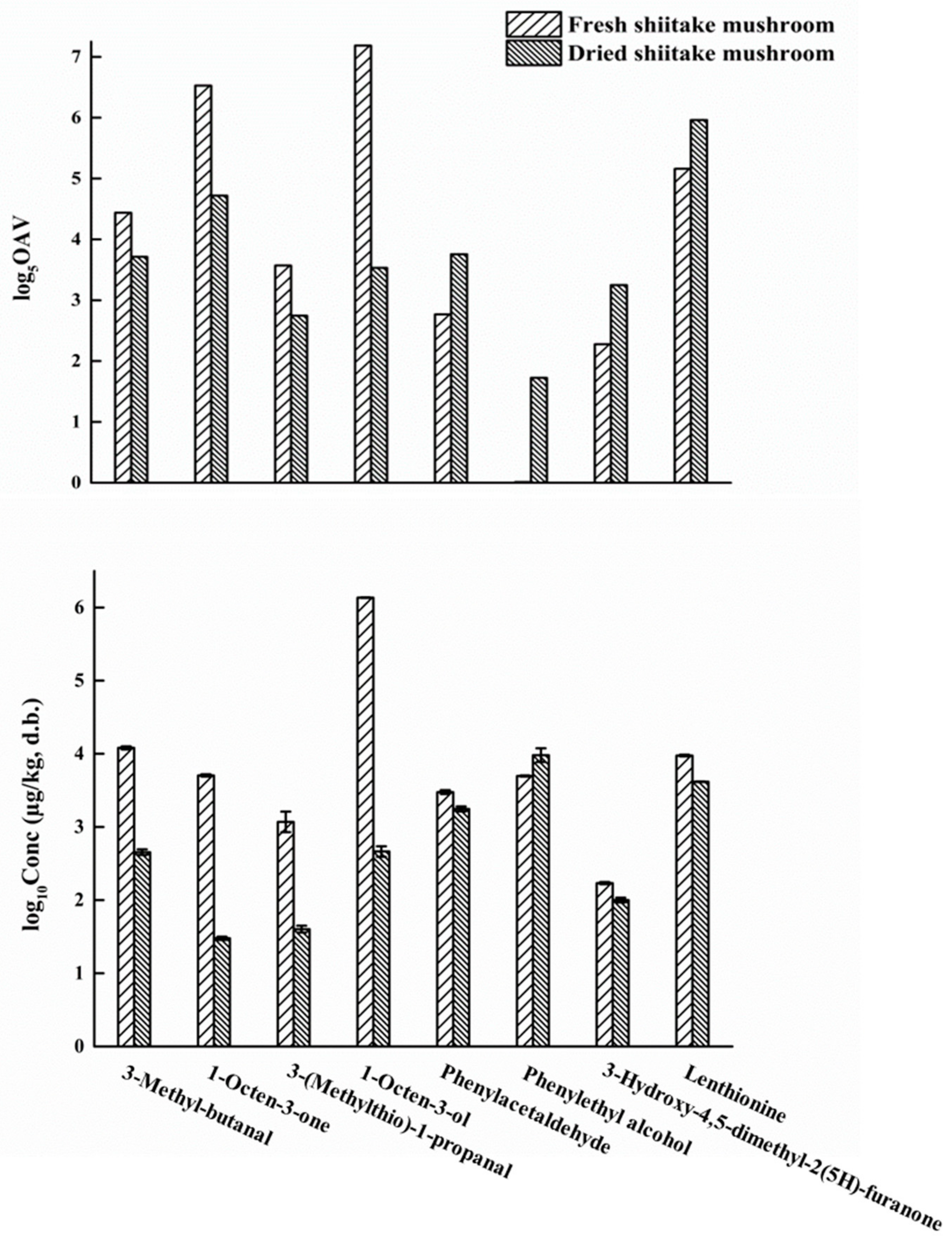
3.3. Concentrations and Odor-Activity Values (OAVs) of Positively Identified Predominant Odorants in Shiitake Mushrooms
| No. | Compound | Internal Standard | Target Ion a | IS Ion b | Response Factor | R2 c | Concentrations (µg/g) | Odor Threshold in Water (µg/g) | OAV d | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Dried | Fresh | Dried | ||||||||
| Esters | |||||||||||
| 2 | Ethyl acetate | 2-Methyl-3-heptanone | 61 | 57 | 5.8754 | 0.9940 | - | 218.34 ± 16.35 | 5e | - | 44 |
| Aldehydes | |||||||||||
| 3 | 3-Methyl-butanal | 2-Methyl-3-heptanone | 58 | 57 | 8.6580 | 0.9999 | 1.39 ± 0.07 | 0.43 ± 0.04 | 0.0011 f | 1263 | 394 |
| 7 | Octanal | 2-Methyl-3-heptanone | 84 | 57 | 8.6356 | 0.9995 | 0.45 ± 0.06 | - | 0.0034 g | 134 | - |
| 12 | (E)-2-Octenal | 2-Methyl-3-heptanone | 55 | 57 | 6.2578 | 0.9950 | 1.70 ± 0.09 | - | 0.003 e | 568 | - |
| 18 | Benzaldehyde | 2-Methyl-3-heptanone | 106 | 57 | 0.7579 | 0.9998 | 0.02 ± 0 | 0.83 ± 0.04 | 3.5 h | <1 | <1 |
| 23 | Phenylacetaldehyde | 2-Methyl-3-heptanone | 91 | 57 | 0.7399 | 0.9982 | 0.34 ± 0.02 | 1.69 ± 0.13 | 0.004 e | 86 | 422 |
| 32 | trans-4,5-Epoxy-(E)-2-decenal | 2-Methyl-3-heptanone | 81 | 57 | 29.1545 | 0.9984 | 0.13 ± 0.02 | - | 0.00013 g | 1033 | - |
| Ketones | |||||||||||
| 4 | 2,3-Butanedione | 2-Ethylbutyric acid | 86 | 88 | 1.0495 | 0.9947 | - | 0.04 ± 0 | 0.001 g | - | 43 |
| 6 | 3-Octanone | 2-Methyl-3-heptanone | 99 | 57 | 1.5314 | 1 | 1.34 ± 0.03 | 0.01 ± 0 | 0.0214 f | 63 | <1 |
| 8 | 1-Octen-3-one | 2-Methyl-3-heptanone | 55 | 57 | 1.2860 | 0.9997 | 0.58 ± 0.02 | 0.03 ± 0 | 0.000016 g | 36,547 | 1989 |
| Sulfur compounds | |||||||||||
| 5 | Dimethyl disulfide | 2-Methyl-3-heptanone | 94 | 57 | 0.8281 | 0.9967 | - | 1.42 ± 1.11 | 12 i | - | <1 |
| 11 | Dimethyl trisulfide | 2-Methyl-3-heptanone | 126 | 57 | 0.6147 | 0.9998 | - | 0.29 ± 0.01 | 0.01 i | - | 29 |
| 15 | 3-(Methyl-thio)-1-propanal | 2-Methyl-3-heptanone | 104 | 57 | 3.4317 | 0.9989 | 0.13 ± 0.04 | 0.04 ± 0 | 0.00043 g | 313 | 83 |
| 25 | 3-(Methyl-thio)-1-propanol | 2-Methyl-3-heptanone | 106 | 57 | 1.4308 | 0.9999 | 0.12 ± 0.02 | 0.01 ± 0 | 0.036 g | 3 | <1 |
| 41 | Lenthionine | 2-Methyl-3-heptanone | 142 | 57 | 0.2681 | 0.9998 | 1.09 ± 0.03 | 3.97 ± 0.04 | 0.00027 j | 4048 | 14,713 |
| Alcohols | |||||||||||
| 10 | 1-Hexanol | 2-Nonanol | 69 | 97 | 0.2128 | 0.9909 | 0.03 ± 0 | 0.36 ± 0.08 | 0.5 e | <1 | <1 |
| 16 | 1-Octen-3-ol | 2-Nonanol | 57 | 97 | 0.0991 | 0.9999 | 157.65 ± 1.17 | 0.44 ± 0.08 | 0.0015 f | 105,100 | 294 |
| 17 | 2-Ethyl-1-hexanol | 2-Nonanol | 57 | 97 | 0.0811 | 0.9999 | - | 0.14 ± 0.03 | 25.4822 f | - | <1 |
| 21 | (E)-2-Octen-1-ol | 2-Nonanol | 57 | 97 | 0.1390 | 0.9983 | 0.05 ± 0.01 | 0.18 ± 0.05 | 0.84 k | <1 | <1 |
| 30 | Phenylethyl alcohol | 2-Nonanol | 91 | 97 | 0.0416 | 0.9914 | 0.57 ± 0.01 | 9.20 ± 2.10 | 0.56423 f | 1 | 16 |
| Acids | |||||||||||
| 14 | Acetic acid | 2-Ethylbutyric acid | 60 | 88 | 1.2206 | 0.9971 | - | 177.54 ± 2.78 | 99 g | - | 2 |
| 24 | Isovaleric acid | 2-Ethylbutyric acid | 60 | 88 | 0.4357 | 0.9958 | 0.24 ± 0.01 | 7.06 ± 0.28 | 0.49 g | <1 | 14 |
| 34 | Octanoic acid | 2-Ethylbutyric acid | 60 | 88 | 0.8162 | 0.9937 | 0.52 ± 0.03 | - | 3 e | <1 | - |
| 37 | Benzoic acid | 2-Ethylbutyric acid | 105 | 88 | 0.7957 | 0.9958 | - | 5.28 ± 0.21 | 1 l | - | <1 |
| Furanones | |||||||||||
| 33 | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | 2-Ethylbutyric acid | 128 | 88 | 5.1975 | 0.9984 | - | 1.43 ± 0.18 | 0.04 g | - | 36 |
| 35 | 3-Hydroxy-4,5-dimethyl-2(5H)-furanone | 2-Ethylbutyric acid | 128 | 88 | 6.9541 | 0.9991 | 0.02 ± 0 | 0.09 ± 0.01 | 0.00049 g | 39 | 187 |
3.4. Aroma Recombination Verification
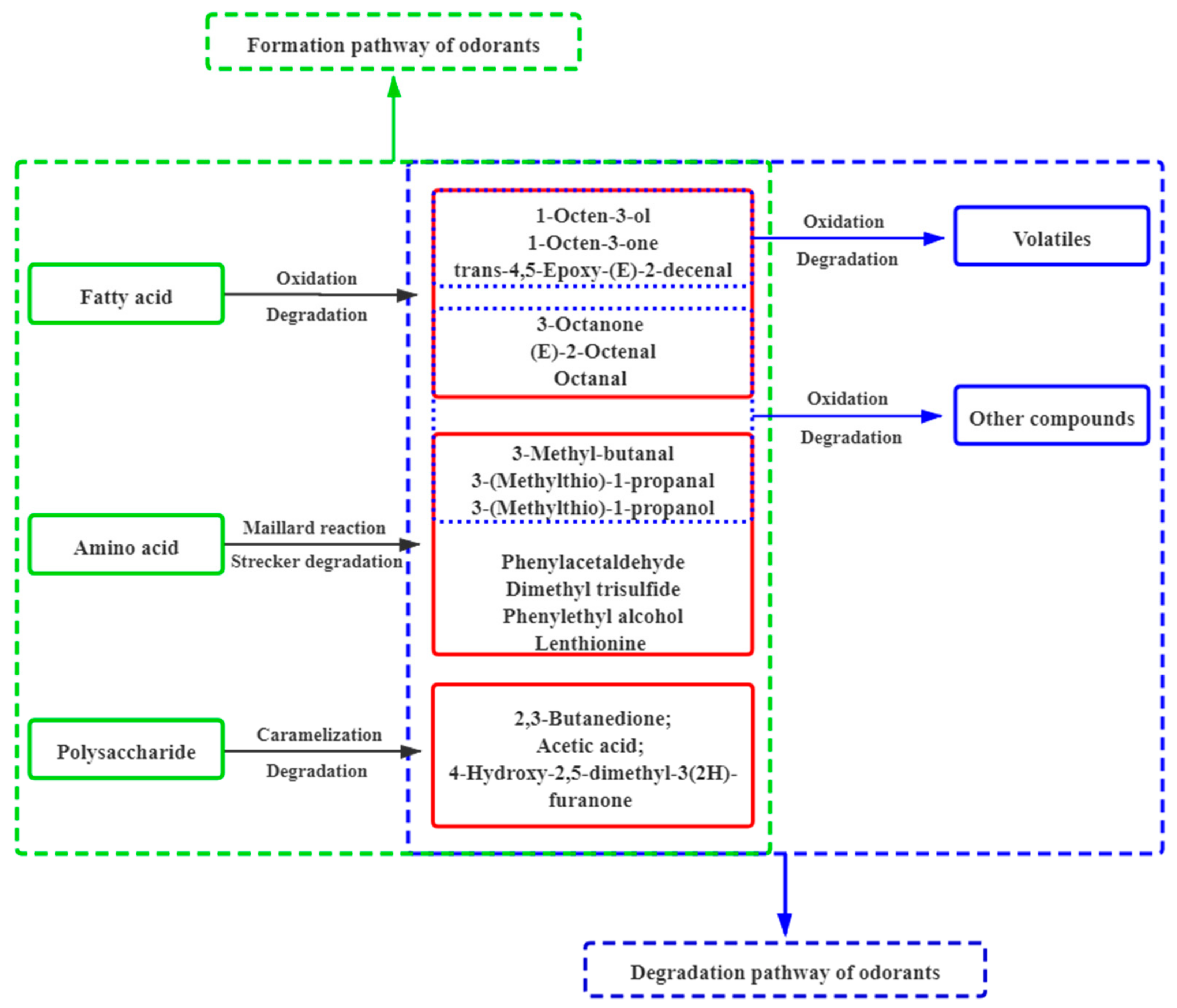
3.5. Variations and Aroma Chemistry of the Key Contributing Odorants in Shiitake Mushrooms after Hot-Air-Drying
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.-Y.; Huang, Y.; Wang, K.-X.; Dong, X.-P.; Yu, D.; Ge, L.-H.; Zhou, D.-Y.; Chen, C.Y. Microstructural characteristics of turbot (Scophthalmus maximus) muscle: Effect of salting and processing. Int. J. Food Prop. 2018, 21, 291–1302. [Google Scholar] [CrossRef] [Green Version]
- Parentelli, C.; Ares, G.; Corona, M.; Lareo, C.; Gámbaro, A.; Soubes, M.; Lema, P. Sensory and microbiological quality of shiitake mushrooms in modified-atmosphere packages. J. Sci. Food Agric. 2007, 87, 1645–1652. [Google Scholar] [CrossRef]
- Schmidberger, P.C.; Schieberle, P. Changes in the predominant aroma compounds of raw shiitake mushrooms (Lentinula edodes) induced by pan-frying as well as by rehydration of dry mushrooms. J. Agric. Food Chem. 2020, 68, 4493–4506. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Lee, J.H. Drying kinetics of whole and sliced shiitake mushrooms (Lentinus edodes). Food Sci. Biotechnol. 2011, 20, 419–427. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Chun, J.; Lee, H.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Reguła, J.; Siwulski, M. Dried shiitake (Lentinulla edodes) and oyster (Pleurotus ostreatus) mushrooms as a good source of nutrient. Acta Sci. Pol. Technol. Aliment. 2007, 6, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.; Racjan, M. The commercial exploitation of the white rot fungus Lentinula edodes (shiitake). Int. Biodeterior. Biodegrad. 1999, 43, 101–107. [Google Scholar] [CrossRef]
- Dermiki, M.; Phanphensophon, N.; Mottram, D.S.; Methven, L. Contributions of non-volatile and volatile compounds to the umami taste and overall flavour of shiitake mushroom extracts and their application as flavour enhancers in cooked minced meat. Food Chem. 2013, 141, 77–83. [Google Scholar] [CrossRef]
- Wu, F.; Tang, J.; Pei, F.; Wang, S.; Chen, G.; Hu, Q.; Zhao, L. The influence of four drying methods on nonvolatile taste components of White Hypsizygus marmoreus. Eur. Food Res. Technol. 2014, 240, 823–830. [Google Scholar] [CrossRef]
- Doymaz, I.; Pala, M. The effects of dipping pretreatments on air-drying rates of the seedless grapes. J. Food Eng. 2002, 52, 413–417. [Google Scholar] [CrossRef]
- Hiraide, M.; Miyazaki, Y.; Shibata, Y. The smell and odorous components of dried shiitake mushroom, Lentinula edodes I: Relationship between sensory evaluations and amounts of odorous components. J. Wood Sci. 2004, 50, 358–364. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Wu, C.-M.; Wang, Z. Volatile compounds in fresh and processed shiitake mushrooms (Lentinus edodes Sing.). Food Sci. Technol. Res. 2000, 6, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Gao, J.-X.; Xue, J.; Chen, D.; Lin, S.-Y.; Dong, X.-P.; Zhu, B.-W. Changes in aroma profile of shiitake mushroom (Lentinus edodes) during different stages of hot air drying. Foods 2020, 9, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieberle, P.; Hofmann, T. Mapping the combinatorial code of food flavors by means of molecular sensory science approach. In Food Flavors; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Xu, C.-H.; Chen, G.-S.; Xiong, Z.-H.; Fan, Y.-X.; Wang, X.-C.; Liu, Y. Applications of solid-phase microextraction in food analysis. TrAC Trends Anal. Chem. 2016, 80, 12–29. [Google Scholar] [CrossRef]
- Yong, G.-R.; Gebru, Y.A.; Kim, D.-W.; Kim, D.-H.; Han, H.-A.; Kim, Y.-H.; Kim, M.-K. Chemical composition and antioxidant activity of steam-distilled essential oil and glycosidically bound volatiles from maclura tricuspidata fruit. Foods 2019, 8, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantrell, M.S.; Seale, J.T.; Arispe, S.A.; McDougal, O.M. Determination of organosulfides from onion oil. Foods 2020, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Pyysalo, H. Identification of volatile compounds in seven edible fresh mushrooms. Acta Chem. Scand. 1976, 30b, 235–244. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef]
- Erten, E.S.; Cadwallader, K.R. Identification of predominant aroma components of raw, dry roasted and oil roasted almonds. Food Chem. 2017, 217, 244–253. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Delahunty, C.M.; Eyres, G.; Dufour, J.-P. Gas chromatography-olfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef] [PubMed]
- Maraval, I.; Mestres, C.; Pernin, K.; Ribeyre, F.; Boulanger, R.; Guichard, E.; Gunata, Z. Odor-Active Compounds in Cooked Rice Cultivars from Camargue (France) Analyzed by GC−O and GC−MS. J. Agric. Food Chem. 2008, 56, 5291–5298. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Fujiwara, K.; Mizutani, J.; Obata, Y. Volatile flavor components of caucas. J. Agric. Food Chem. 1971, 19, 992–994. [Google Scholar] [CrossRef]
- De-La-Fuente-Blanco, A.; Ferreira, V. Gas chromatography olfactometry (GC-O) for the (Semi) quantitative screening of wine aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef] [PubMed]
- Pollien, P.; Ott, A.; Montigon, F.; Baumgartner, M.; Muñoz-Box, A.R.; Chaintreau, A. Hyphenated headspace-gas chromatography-sniffing technique: Screening of impact odorants and quantitative aromagram comparisons. J. Agric. Food Chem. 1997, 45, 2630–2637. [Google Scholar] [CrossRef]
- Kerscher, R.; Grosch, W. Comparative evaluation of potent odorants of boiled beef by aroma extract dilution and concentration analysis. Z. Lebensm. Unters. Und Forsch. 1997, 204, 3–6. [Google Scholar] [CrossRef]
- Sanz, C.; Czerny, M.; Cid, C.; Schieberle, P. Comparison of potent odorants in a filtered coffee brew and in an instant coffee beverage by aroma extract dilution analysis (AEDA). Eur. Food Res. Technol. 2002, 214, 299–302. [Google Scholar] [CrossRef]
- Wang, M.; Ma, W.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
- Pu, D.; Duan, W.; Huang, Y.; Zhang, Y. Characterization of the key odorants contributing to retronasal olfaction during bread consumption. Food Chem. 2020, 318, 126520. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Guo, X.; Qin, Z.; Yao, Y.; Hu, X.; Wu, J. Identification of aroma-active compounds in jiashi muskmelon juice by GC-O-MS and OAV calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef]
- Zhang, W.; Lao, F.; Bi, S.; Pan, X.; Wu, J. Insights into the major aroma-active compounds in clear red raspberry juice (Rubus idaeus L. cv. Heritage) by molecular sensory science approaches. Food Chem. 2021, 336, 127721. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Cho, D.-B.; Seo, H.-Y.; Kim, K.-S. Analysis of the volatile flavor compounds produced during the growth stages of the shiitake mushrooms (Lentinus edodes). J. Food Sci. Nutr. 2003, 8, 306–314. [Google Scholar] [CrossRef]
- Politowicz, J.; Lech, K.; Lipan, L.; Figiel, A.; Carbonell-Barrachina, A.A. Volatile composition and sensory profile of shiitake mushrooms as affected by drying method. J. Sci. Food Agric. 2017, 98, 1511–1521. [Google Scholar] [CrossRef]
- Bozok, F.; Zarifikhosroshahi, M.; Kafkas, E.; Taşkin, H.; Buyukalaca, S. Comparison of volatile compounds of fresh boletus edulis and b. pinophilus in Marmara region of Turkey. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 192–195. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.F.; Moore, A.J.; Munafo, J.P. Key Odorants from the American Matsutake, Tricholoma magnivelare. J. Agric. Food Chem. 2020, 68, 9768–9775. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, P.; Lao, F.; Liu, J.; Liao, X.; Wu, J. Characterization of the major aroma-active compounds in Keitt mango juice: Comparison among fresh, pasteurization and high hydrostatic pressure processing juices. Food Chem. 2019, 289, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Buttery, R.G. Quantitative and sensory aspects of flavor of tomato and other vegetables and fruits. In Flavor Science: Sensible Principles and Techniques; Acree, T.E., Teranishi, R., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 259–286. [Google Scholar]
- Buttery, R.G.; Guadagni, D.G.; Ling, L.C.; Seifert, R.M.; Lipton, W. Additional volatile components of cabbage, broccoli, and cauliflower. J. Agric. Food Chem. 1976, 24, 829–832. [Google Scholar] [CrossRef]
- Eriksson, C.E.; Vallentin, K. Odor detectability of aldehydes and alcohols originating from lipid oxidation. Chem. Senses 1976, 2, 3–15. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, J.; Pei, F.; Mariga, A.M.; Ma, N.; Fang, Y.; Hu, Q. Effect of hot air drying on volatile compounds of Flammulina velutipes detected by HS-SPME–GC–MS and electronic nose. Food Chem. 2016, 196, 860–866. [Google Scholar] [CrossRef]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from darjeeling black tea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Misharina, T.A.; Muhutdinova, S.M.; Zharikova, G.G.; Terenina, M.B.; Krikunova, N.I.; Medvedeva, I.B. Formation of flavor of dry champignons (Agaricus bisporus L.). Appl. Biochem. Microbiol. 2010, 46, 108–113. [Google Scholar] [CrossRef]
- Hofmann, T.; Schieberle, P. Acetylformoin—An important progenitor of 4-hydroxy-2,5-dimethyl-3(2H)-furanone and 2-Acetyltetrahydropyridine during thermal food processing. In Proceedings of the 6th Wartburg Aroma Symposium; Rothe, M., Ed.; Eigenverlag: Bergholz-Rehbrucke, Germany, 2001; pp. 311–322. [Google Scholar]
- Yaylayan, V.A.; Keyhani, A. Origin of 2,3-pentanedione and 2,3-butanedione ind-glucose/l-alanine maillard model systems. J. Agric. Food Chem. 1999, 47, 3280–3284. [Google Scholar] [CrossRef]
- Mall, V.; Sellami, I.; Schieberle, P. New degradation pathways of the key aroma compound 1-penten-3-one during storage of not-from-concentrate orange juice. J. Agric. Food Chem. 2018, 66, 11083–11091. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ding, L.-L.; Hadiatullah, H.; Li, S.; Jiang, S. Characterization of the typical fragrant compounds in traditional Chinese-type soy sauce. Food Chem. 2020, 312, 126054. [Google Scholar] [CrossRef]
- Zeng, L.; Tan, H.; Liao, Y.; Jian, G.; Kang, M.; Dong, F.; Watanabe, N.; Yang, Z. Increasing temperature changes flux into multiple biosynthetic pathways for 2-phenylethanol in model systems of tea (Camellia sinensis) and other plants. J. Agric. Food Chem. 2019, 67, 10145–10154. [Google Scholar] [CrossRef] [PubMed]


| Odor Descriptors | Reference Odorants | Concentrations in Water (µg/mL) | |||||
|---|---|---|---|---|---|---|---|
| 0 a | 1 a | 2 a | 3 a | 4 a | 5 a | ||
| mushroom | 1-Octen-3-ol | 0 | 1.50 × 10−2 | 1.50 × 10−1 | 1.50 | 1.50 × 10 | 1.50 × 102 |
| grass | (E)-2-Octen-1-ol | 0 | 33.60 × 2−2 | 33.60 × 2−1 | 33.60 | 33.60 × 2 | 33.60 × 22 |
| metallic | trans-4,5-Epoxy-(E)-2-decenal | 0 | 0.13 × 10−2 | 0.13 × 10−1 | 0.13 | 0.13 × 10 | 0.13 × 102 |
| sulfury | Lenthionine | 0 | 0.27 × 10−2 | 0.27 × 10−1 | 0.27 | 0.27 × 10 | 0.27 × 102 |
| caramel | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | 0 | 10.00 × 5−2 | 10.00 × 5−1 | 10.00 | 10.00 × 5 | 10.00 × 52 |
| fatty | (E)-2-Octenal | 0 | 3.00 × 10−2 | 3.00 × 10−1 | 3.00 | 3.00 × 10 | 3.00 × 102 |
| cabbage | 3-(Methyl-thio)-1-propanol | 0 | 9.00 × 5−2 | 9.00 × 5−1 | 9.00 | 9.00 × 5 | 9.00 × 52 |
| chocolate | 3-Methyl-butanal | 0 | 1.10 × 10−2 | 1.10 × 10−1 | 1.10 | 1.10 × 10 | 1.10 × 102 |
| sweaty | Isovaleric acid | 0 | 44.10 × 3−2 | 44.10 × 3−1 | 44.10 | 44.10 × 3 | 44.10 × 32 |
| seasoning-like | 3-Hydroxy-4,5-dimethyl-2(5H)-furanone | 0 | 0.49 × 10−2 | 0.49 × 10−1 | 0.49 | 0.49 × 10 | 0.49 × 102 |
| cooked potato-like | 3-(Methyl-thio)-1-propanal | 0 | 0.43 × 10−2 | 0.43 × 10−1 | 0.43 | 0.43 × 10 | 0.43 × 102 |
| No. | Compound | CAS | Odor a | ID b | RI c | DF d | FD e | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Wax | HP-5 | Fresh | Dried | Fresh | Dried | |||||
| 1 | 2-Methyl-propanal | 78-84-2 | chocolate | RI,O,S | 743 | <700 | - | - | 27 | 3 |
| 2 | Ethyl acetate | 141-78-6 | pineapple | RI,MS,O,S | 887 | - | - | - | - | 27 |
| 3 | 3-Methyl-butanal | 590-86-3 | chocolate | RI,MS,O,S | 919 | <700 | 8 | 8 | 243 | 243 |
| 4 | 2,3-Butanedione | 431-03-8 | sweet, creamy | RI,MS,O | 980 | - | - | 2 | - | 3 |
| 5 | Dimethyl disulfide | 624-92-0 | cabbage | RI,MS,O,S | 1069 | 747 | - | - | - | 9 |
| 6 | 3-Octanone | 106-68-3 | mushroom | RI,MS,O,S | 1251 | 988 | 8 | 4 | 3 | 3 |
| 7 | Octanal | 124-13-0 | citrus-like | RI,MS,O,S | 1286 | 1006 | 6 | - | 3 | - |
| 8 | 1-Octen-3-one | 4312-99-6 | mushroom | RI,MS,O,S | 1289 | 965 | 8 | 8 | 81 | 3 |
| 9 | 2-Acetyl-1-pyrroline | 85213-22-5 | popcorn-like | RI,O | 1320 | 904 | 8 | 8 | 1 | 1 |
| 10 | 1-Hexanol | 111-27-3 | sweet, oily | RI,MS,O,S | 1351 | - | - | - | 3 | 27 |
| 11 | Dimethyl trisulfide | 3658-80-8 | sulfury | RI,MS,O,S | 1365 | 962 | - | 8 | - | 81 |
| 12 | (E)-2-Octenal | 2548-87-0 | fatty, nutty | RI,MS,O,S | 1410 | 1064 | - | - | 9 | - |
| 13 | Unknown | - | grass, beany | O | 1428 | - | 8 | 8 | 243 | - |
| 14 | Acetic acid | 64-19-7 | vinegar-like | RI,MS,O,S | 1432 | <700 | - | - | 1 | 81 |
| 15 | 3-(Methyl-thio)-1-propanal | 3268-49-3 | cooked potato-like | RI,MS,O,S | 1447 | - | 8 | 8 | 243 | 3 |
| 16 | 1-Octen-3-ol | 3391-86-4 | mushroom | RI,MS,O,S | 1456 | 977 | 8 | 8 | 81 | 1 |
| 17 | 2-Ethyl-1-hexanol | 104-76-7 | fresh, fatty | RI,MS,O,S | 1482 | 1031 | 2 | 8 | - | 9 |
| 18 | Benzaldehyde | 100-52-7 | almond, green | RI,MS,O,S | 1512 | 920 | 8 | 8 | 3 | 1 |
| 19 | (E)-2-Nonenal | 18829-56-6 | fatty, green | RI,MS,O,S | 1524 | 1156 | - | 6 | 1 | 1 |
| 20 | 1-Octanol | 111-87-5 | green, fresh | RI,MS,O,S | 1545 | 1070 | - | - | 1 | - |
| 21 | (E)-2-Octen-1-ol | 18409-17-1 | grass | RI,MS,O,S | 1614 | - | 8 | 8 | - | - |
| 22 | Butanoic acid | 107-92-6 | cheese, fecal | RI,MS,O,S | 1620 | 797 | 6 | 6 | - | 1 |
| 23 | Phenylacetaldehyde | 122-78-1 | floral | RI,MS,O,S | 1633 | 1041 | 8 | 8 | 729 | 81 |
| 24 | Isovaleric acid | 503-74-2 | sweaty | RI,MS,O,S | 1673 | 843 | - | 8 | 27 | 81 |
| 25 | 3-(Methyl-thio)-1-propanol | 505-10-2 | cabbage | RI,MS,O,S | 1709 | - | 8 | 6 | 3 | 27 |
| 26 | 1,2,4-Trithiolane | 289-16-7 | mushroom | RI,MS,O | 1721 | 1093 | 4 | 4 | 3 | 3 |
| 27 | Pentanoic acid | 109-52-4 | sweaty, rancid | RI,MS,O,S | 1744 | - | 4 | - | - | - |
| 28 | Dimethyl tetra-sulfide | 5756-24-1 | mushroom | RI,MS,O | - | 1205 | 4 | 6 | 243 | 729 |
| 29 | Unknown | - | rubber | O | 1787 | - | 3 | - | - | - |
| 30 | Phenylethyl alcohol | 60-12-8 | honey | RI,MS,O,S | 1897 | 1119 | - | 6 | 81 | 27 |
| 31 | Unknown | 699-10-5 | mushroom | MS,O | 1953 | - | 3 | - | 1 | 3 |
| 32 | trans-4,5-Epoxy-(E)-2-decenal | 134454-31-2 | metallic | RI,MS,O,S | 1994 | 1383 | 8 | - | 729 | - |
| 33 | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | 3658-77-3 | caramel | RI,MS,O,S | 2024 | 1063 | - | 8 | 1 | 729 |
| 34 | Octanoic acid | 124-07-2 | sweaty, fatty | RI,MS,O,S | 2087 | 1176 | 6 | - | 27 | 1 |
| 35 | 3-Hydroxy-4,5-dimethyl-2(5H)-furanone | 28664-35-9 | seasoning-like | RI,O,S | 2191 | 1084 | 6 | 6 | 81 | 81 |
| 36 | 1,2,4,5-Tetrathiane | 291-22-5 | sulfury, burnt | RI,MS,O | 2207 | 1335 | 8 | 8 | 729 | 729 |
| 37 | Benzoic acid | 65-85-0 | balsam-like | RI,MS,O,S | 2400 | - | - | - | - | 9 |
| 38 | 1, 2, 4, 6-Tetrathiepane | 292-45-5 | sulfury | RI,MS,O | 2445 | 1498 | 6 | 2 | 729 | 27 |
| 39 | Phenylacetic acid | 103-82-2 | floral | RI,MS,O | 2561 | 1241 | 4 | 8 | 81 | 27 |
| 40 | Unknown | - | fecal | O | 2574 | - | - | - | 27 | 27 |
| 41 | Lenthionine | 292-46-6 | sulfury, burnt | RI,MS,O | 2638 | 1619 | 8 | 8 | 729 | 729 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-L.; Lin, S.-Y.; Du, H.-T.; Qin, L.; Lei, L.-M.; Chen, D. An Insight by Molecular Sensory Science Approaches to Contributions and Variations of the Key Odorants in Shiitake Mushrooms. Foods 2021, 10, 622. https://doi.org/10.3390/foods10030622
Wang S-L, Lin S-Y, Du H-T, Qin L, Lei L-M, Chen D. An Insight by Molecular Sensory Science Approaches to Contributions and Variations of the Key Odorants in Shiitake Mushrooms. Foods. 2021; 10(3):622. https://doi.org/10.3390/foods10030622
Chicago/Turabian StyleWang, Si-Lu, Song-Yi Lin, Han-Ting Du, Lei Qin, Li-Ming Lei, and Dong Chen. 2021. "An Insight by Molecular Sensory Science Approaches to Contributions and Variations of the Key Odorants in Shiitake Mushrooms" Foods 10, no. 3: 622. https://doi.org/10.3390/foods10030622
APA StyleWang, S.-L., Lin, S.-Y., Du, H.-T., Qin, L., Lei, L.-M., & Chen, D. (2021). An Insight by Molecular Sensory Science Approaches to Contributions and Variations of the Key Odorants in Shiitake Mushrooms. Foods, 10(3), 622. https://doi.org/10.3390/foods10030622







