Concentrating Model Solutions and Fruit Juices Using CO2 Hydrate Technology and Its Quantitative Effect on Phenols, Carotenoids, Vitamin C and Betanin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reactor System
2.3. Experimental Procedure
2.4. Sugar
2.5. Betanin
2.6. Total Phenolics
2.7. Total Carotenoids
2.8. Vitamin C
2.9. Statistical Data Evaluation
3. Results
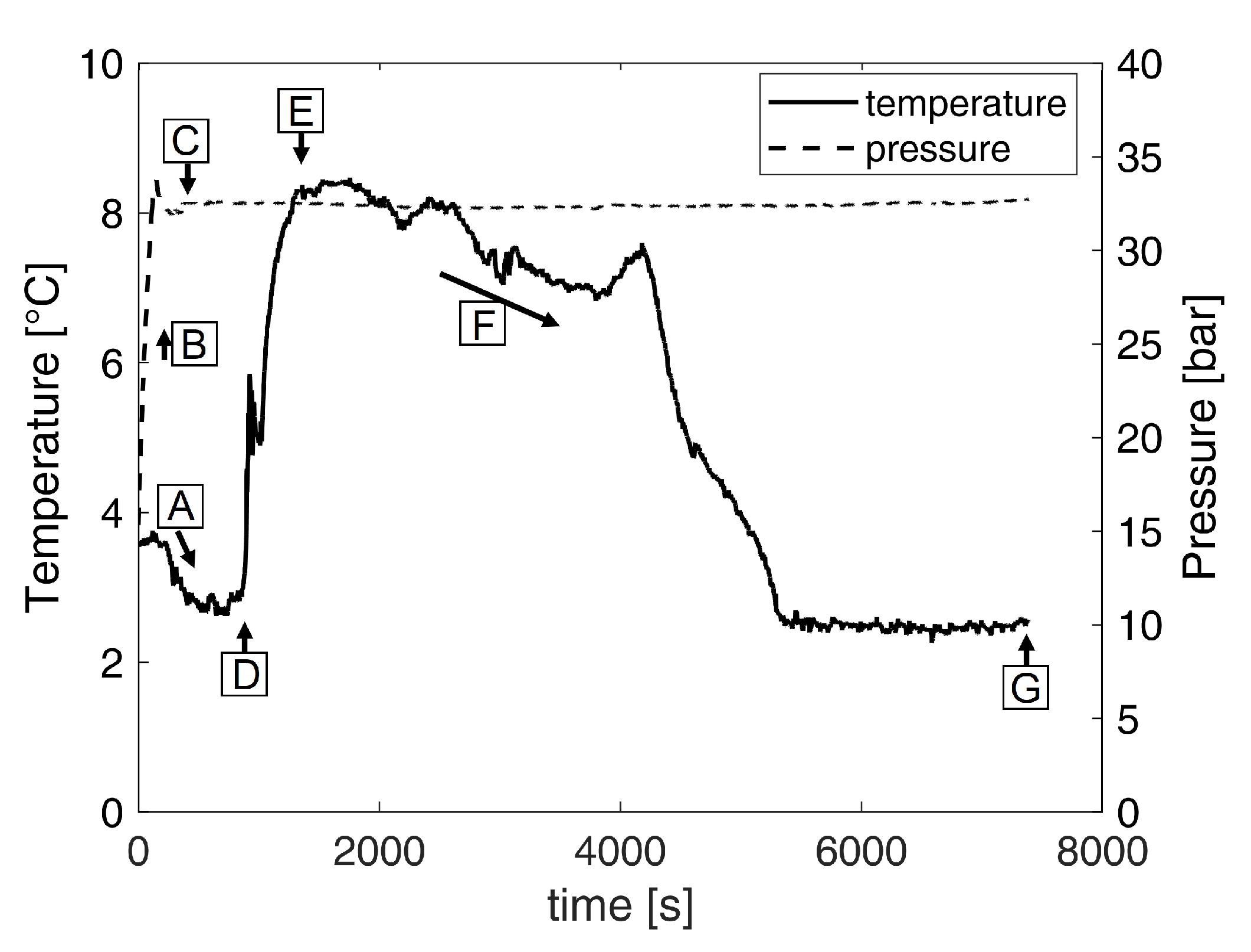
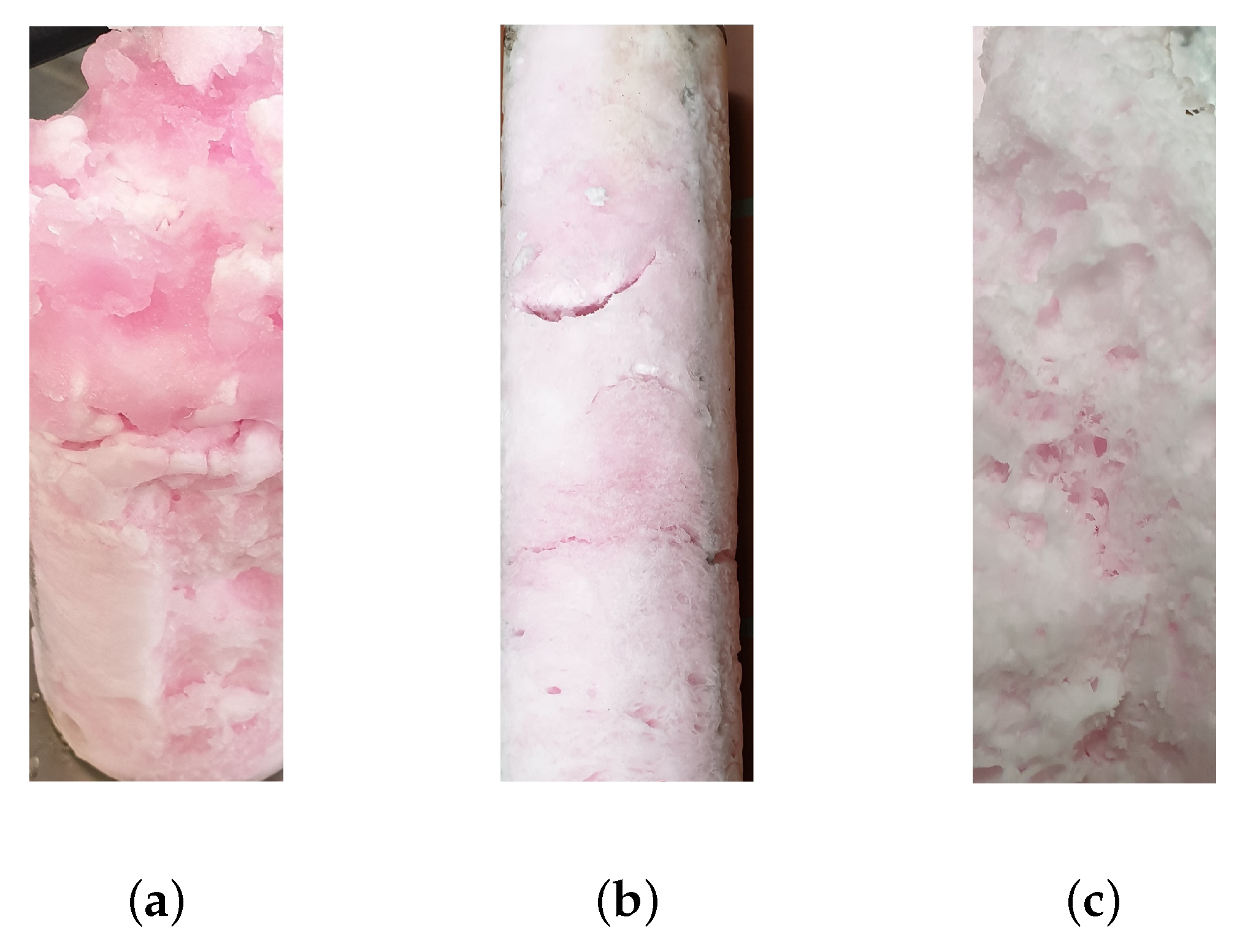
3.1. Hydrate Structure
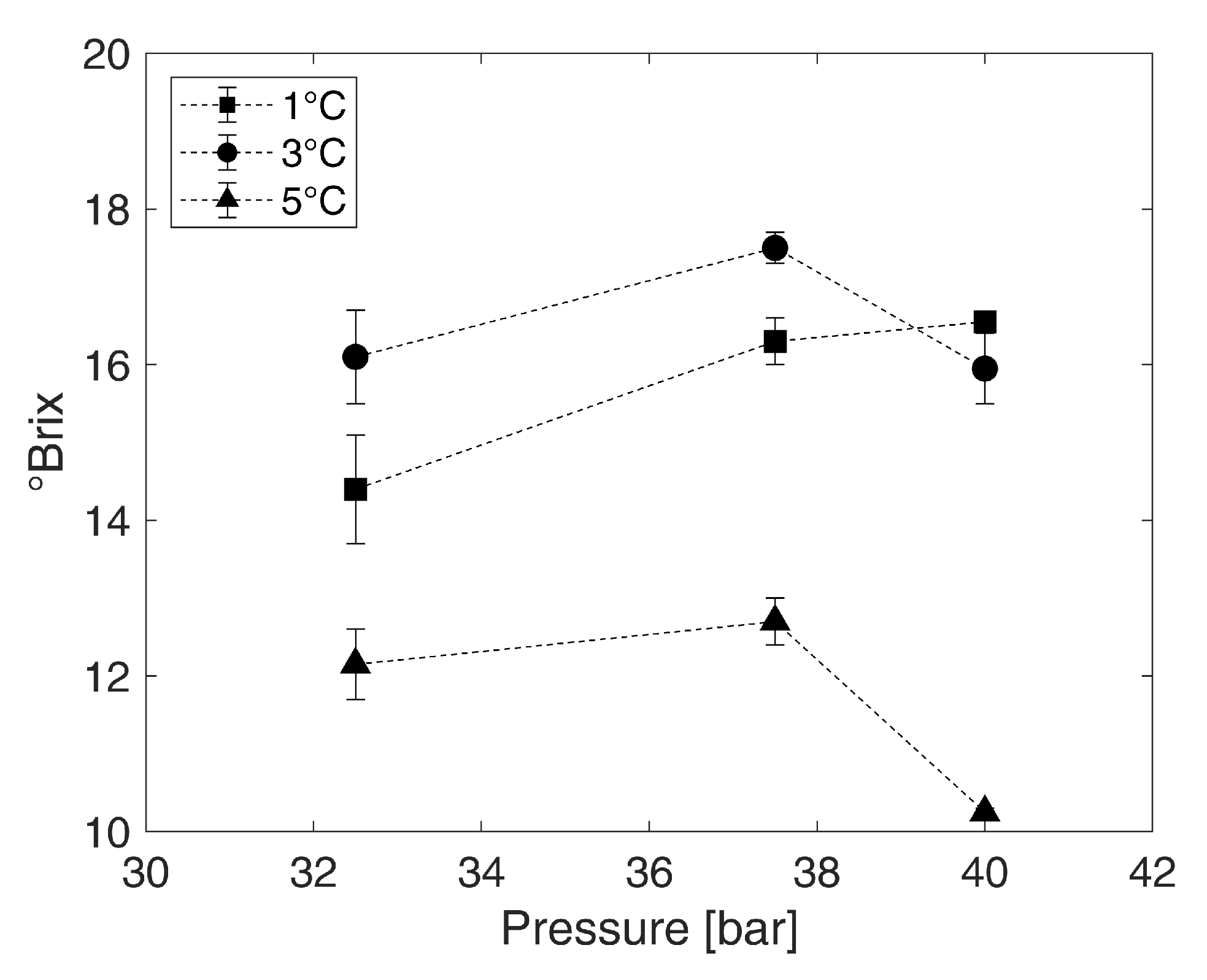
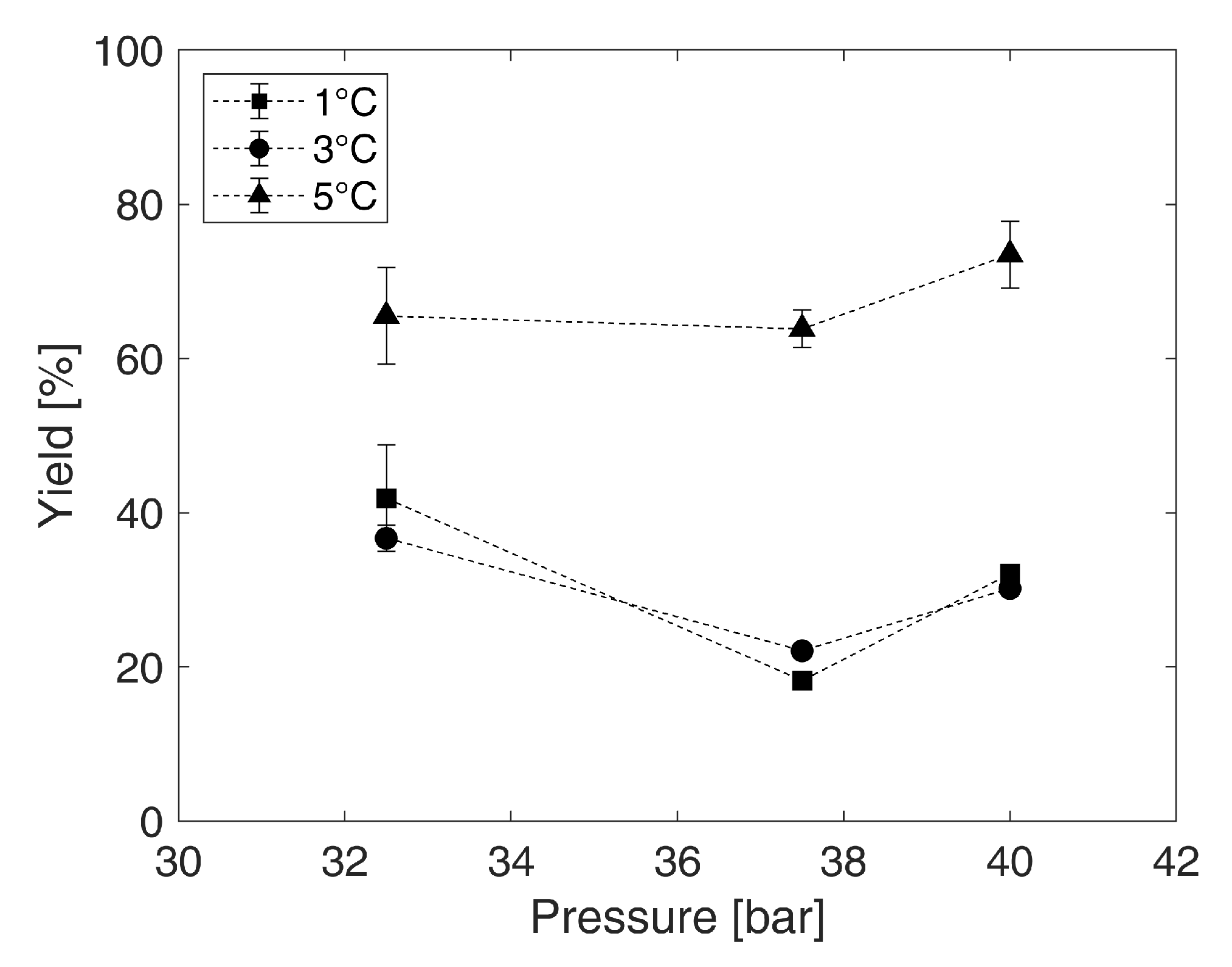
3.2. Identification of the Operating Point
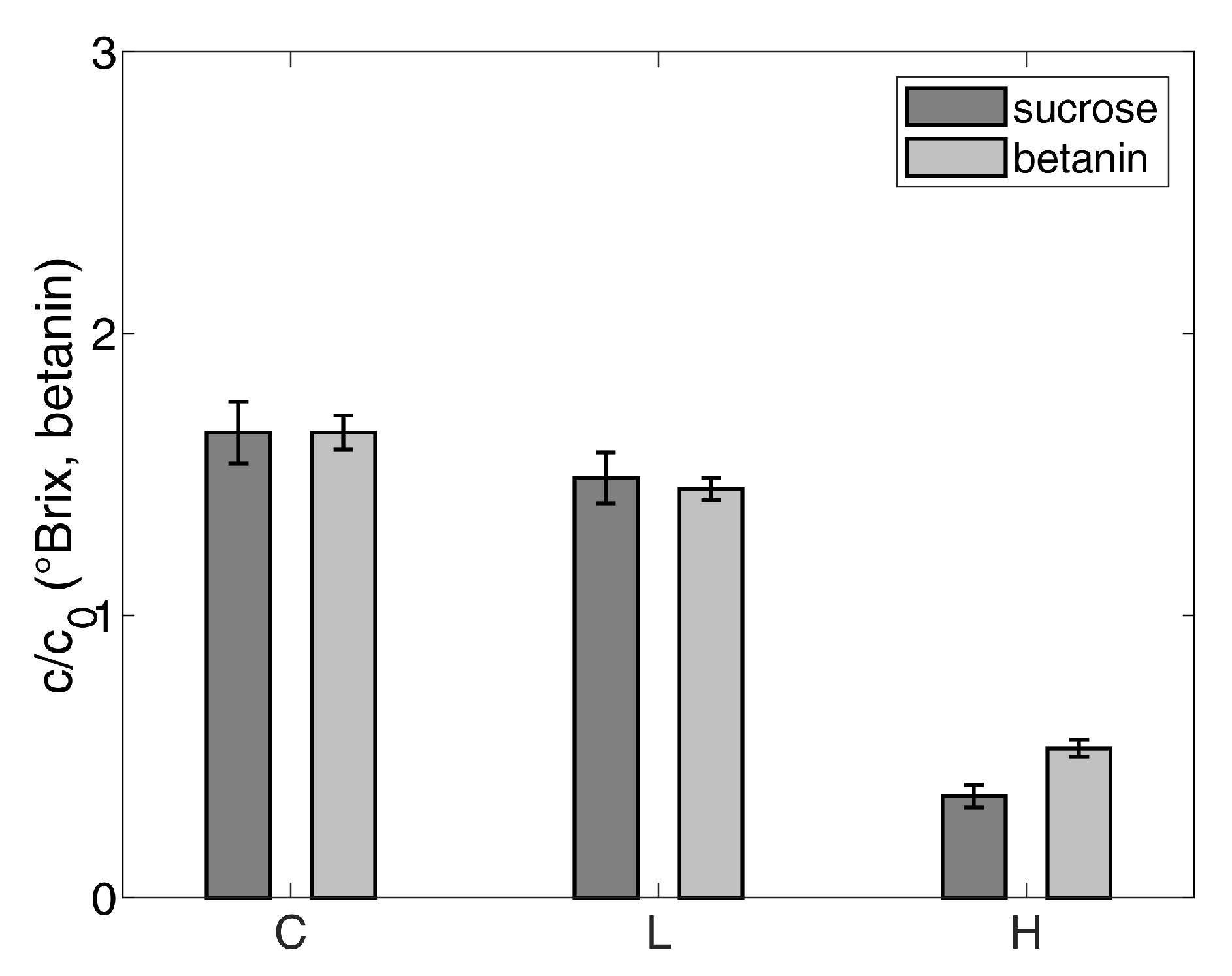
3.3. Effect of the Concentration Using Gas Hydrate Technology on Valuable Ingredients
4. Discussion
4.1. Identification of the Operating Point
4.2. Effect of the Concentration Using Gas Hydrate Technology on Valuable Ingredients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yahia, E.M. The Contribution of Fruit and Vegetable Consumption to Human Health. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; de la Rosa, L.A., Alvarez-Parrilla, E., González-Aguilar, G.A., Eds.; Blackwell Publishing: Ames, IA, USA, 2010; pp. 3–51. [Google Scholar]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.; Lim, S.; Andrews, K.G.; Engell, R.E.; Ezzati, M.; Mozaffarian, D. Global, Regional, and National Consumption of Sugar-Sweetened Beverages, Fruit Juices, and Milk: A Systematic Assessment of Beverage Intake in 187 Countries. PLoS ONE 2015, 10, e0124845. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, A.; Priyadarshini, A. Market Dimensions of the Fruit Juice Industry. In Fruit Juices: Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: London, UK, 2018; pp. 15–32. [Google Scholar]
- A.I.J.N. Worldwide Consumption of Fruit Juice and Fruit Nectar in 2017 and 2018, by Region (in Millions of Liters) [Graph]. Statista. 2019. Available online: https://www.statista.com/statistics/421179/worldwide-consumption-of-fruit-juice-and-fruit-nectar-by-region/ (accessed on 10 March 2021).
- A.I.J.N. Consumption of Fruit Juice and Fruit Nectar in Europe in 2017 and 2018, by Country (in Millions of Liters) [Graph]. Statista. 2019. Available online: https://www.statista.com/statistics/421405/fruit-juice-and-fruit-nectar-consumption-by-country-europe/ (accessed on 10 March 2021).
- Adnan, A.; Mushtaq, M.; ul Islam, T. Fruit Juice Concentrates. In Fruit Juices: Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: London, UK, 2018; pp. 217–240. [Google Scholar]
- Taylor, B. Fruit and juice processing. In Chemistry and Technology of Soft Drinks and Fruit Juices; Ashurst, P.R., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 31–64. [Google Scholar]
- Neves, M.F.; Trombin, V.G.; Marques, V.N.; Martinez, L.F. Global orange juice market: A 16-year summary and opportunities for creating value. Trop. Plant Pathol. 2020, 45, 166–174. [Google Scholar] [CrossRef]
- A.I.J.N. Konsum von Direktsaft und aus Konzentrat Gewonnenem Fruchtsaft in der Europäischen Union in den Jahren 2007 bis 2018 (in Millionen Liter) [Graph]. Statista. 2019. Available online: https://de.statista.com/statistik/daten/studie/388496/umfrage/konsum-von-direktsaft-und-aus-konzentrat-gewonnenem-fruchtsaft-in-der-eu/ (accessed on 10 March 2021).
- A.I.J.N. Konsum von Direktsaft und aus Konzentrat Gewonnenem Fruchtsaft in Deutschland in den Jahren 2007 bis 2014 (in Millionen Liter) [Graph]. Statista. 2015. Available online: https://de.statista.com/statistik/daten/studie/398797/umfrage/konsum-von-direktsaft-und-aus-konzentrat-gewonnenem-fruchtsaft-in-deutschland/ (accessed on 10 March 2021).
- Seidl, P.; Loekman, S.; Sardogan, M.; Voigt, E.; Claßen, T.; Ha, J.; Luzi, G.; Sevenich, R.; Agudo, J.R.; Rauh, C.; et al. Food technological potentials of CO2 gas hydrate technology for the concentration of selected juices. High Press. Res. 2019, 39, 344–356. [Google Scholar] [CrossRef]
- Claßen, T.; Seidl, P.; Loekman, S.; Gatternig, B.; Rauh, C.; Delgado, A. Review on the food technological potentials of gas hydrate technology. Curr. Opin. Food Sci. 2019, 29, 48–55. [Google Scholar] [CrossRef]
- Claßen, T.; Jaeger, M.; Loekman, S.; Gatternig, B.; Rauh, C.; Delgado, A. Concentration of apple juice using CO2 gas hydrate technology to higher sugar contents. Innov. Food Sci. Emerg. Technol. 2020, 65, 102458. [Google Scholar] [CrossRef]
- Aman, Z.M.; Koh, C.A. Interfacial phenomena in gas hydrate systems. Chem. Soc. Rev. 2016, 45, 1678–1690. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.D.; Linga, P.; Englezos, P.; Kim, Y.S.; Lee, M.S.; Do Kim, Y. Gas hydrate formation process for pre-combustion capture of carbon dioxide. Energy 2010, 35, 2729–2733. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Lee, J.D.; Ripmeester, J.; Englezos, P. A new apparatus to enhance the rate of gas hydrate formation: Application to capture of carbon dioxide. Int. J. Greenh. Gas Control 2010, 4, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate based gas separation (HBGS) process for carbon dioxide pre-combustion capture. Energy 2015, 85, 261–279. [Google Scholar] [CrossRef]
- Scondo, A.; Sinquin, A. Effect of Additives on CO2 Capture From Simulated Flue Gas By Hydrates Formation in Emulsion. In Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, UK, 17–21 July 2011. [Google Scholar]
- Kikkinides, E.S.; Yang, R.T.; Cho, S.H. Concentration and Recovery of CO2 from Flue Gas by Pressure Swing Adsorption. Ind. Eng. Chem. Res. 1993, 32, 2714–2720. [Google Scholar] [CrossRef]
- Peng, X.; Hu, Y.; Liu, Y.; Jin, C.; Lin, H. Separation of ionic liquids from dilute aqueous solutions using the method based on CO2 hydrates. J. Nat. Gas Chem. 2010, 19, 81–85. [Google Scholar] [CrossRef]
- Park, K.N.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.G.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B2+). Desalination 2011, 274, 91–96. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Unusual behavior of propane as a co-guest during hydrate formation in silica sand: Potential application to seawater desalination and carbon dioxide capture. Chem. Eng. Sci. 2014, 117, 342–351. [Google Scholar] [CrossRef]
- Kang, K.C.; Linga, P.; Park, K.N.; Choi, S.J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A Review of Clathrate Hydrate Based Desalination to Strengthen Energy-Water Nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2008. [Google Scholar]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.A. Towards a fundamental understanding of natural gas hydrates. Chem. Soc. Rev. 2002, 31, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, Y.; Liu, D.; Fan, L.; Tan, Z.; Zhang, Z.; Li, W.; Li, W. Experimental study of concentration of tomato juice by CO2 hydrate formation. Chem. Ind. Chem. Eng. Q. 2015, 21, 441–446. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Liu, D.; Fan, L.; Tan, Z. Concentrating orange juice through CO2 clathrate hydrate technology. Chem. Eng. Res. Des. 2015, 93, 773–778. [Google Scholar] [CrossRef]
- Purwanto, Y.A.; Oshita, S.; Seo, Y.; Kawagoe, Y. Concentration of liquid foods by the use of gas hydrate. J. Food Eng. 2001, 47, 133–138. [Google Scholar] [CrossRef]
- Safari, S.; Varaminian, F. Study the kinetics and thermodynamics conditions for CO2 hydrate formation in orange juice concentration. Innov. Food Sci. Emerg. Technol. 2019, 57, 102155. [Google Scholar] [CrossRef]
- Loekman, S.; Claßen, T.; Seidl, P.; Luzi, G.; Gatternig, B.; Rauh, C.; Delgado, A. Potential Application of Innovative Gas-Hydrate Technology in Fruit Juices Concentration Process. In Proceedings of the 2019 World Congress on Advances in Nano, Bio, Robotics, and Energy (ANBRE19), Jeju Island, Korea, 17–21 September 2019. [Google Scholar]
- Ghiasi, M.M.; Mohammadi, A.H.; Zendehboudi, S. Clathrate hydrate based approach for concentration of sugar aqueous solution, orange juice, and tomato juice: Phase equilibrium modeling using a thermodynamic framework. Fluid Phase Equilibria 2020, 512, 112460. [Google Scholar] [CrossRef]
- Chun, M.K.; Lee, H. Phase Equilibria of Carbon Dioxide Hydrate System in the Presence of Sucrose, Glucose, and Fructose. J. Chem. Eng. Data 1999, 44, 1081–1084. [Google Scholar] [CrossRef]
- Carroll, J. Natural Gas Hydrates: A Guide for Engineers; Gulf Professional Publishing: Cambridge, MA, USA, 2020. [Google Scholar]
- Sloan, E.D. Clathrate hydrates: The other common solid water phase. Ind. Eng. Chem. Res. 2000, 39, 3123–3129. [Google Scholar] [CrossRef]
- Jeffrey, G.A.; McMullan, R.K. The clathrate hydrates. In Progress in Inorganic Chemistry; Cotton, F.A., Ed.; Interscience Publishers: New York, NY, USA, 1967; pp. 43–108. [Google Scholar]
- Jeffrey, G.A. Hydrate inclusion compounds. J. Incl. Phenom. 1984, 1, 211–222. [Google Scholar] [CrossRef]
- Englezos, P.; Kalogerakis, N.; Dholabhai, P.; Bishnoi, P. Kinetics of formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1987, 42, 2647–2658. [Google Scholar] [CrossRef]
- Shindo, Y.; Lund, P.C.; Fujioka, Y.; Komiyama, H. Kinetics and mechanism of the formation of CO2 hydrate. Int. J. Chem. Kinet. 1993, 25, 777–782. [Google Scholar] [CrossRef]
- Brown, T.D.; Taylor, C.E.; Bernardo, M.P. Rapid Gas Hydrate Formation Processes: Will They Work? Energies 2010, 3, 1154–1175. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.T.; Zhu, J.H.; Fan, S.S.; Chen, G.J. Study on the kinetics of hydrate formation in a bubble column. Chem. Eng. Sci. 2007, 62, 1000–1009. [Google Scholar] [CrossRef]
- Linga, P.; Daraboina, N.; Ripmeester, J.A.; Englezos, P. Enhanced rate of gas hydrate formation in a fixed bed column filled with sand compared to a stirred vessel. Chem. Eng. Sci. 2012, 68, 617–623. [Google Scholar] [CrossRef]
- Xu, C.G.; Li, X.S.; Lv, Q.N.; Chen, Z.Y.; Cai, J. Hydrate-based CO2 (carbon dioxide) capture from IGCC (integrated gasification combined cycle) synthesis gas using bubble method with a set of visual equipment. Energy 2012, 44, 358–366. [Google Scholar] [CrossRef]
- Myre, D.; Macchi, A.; Servio, P. Synthesis of CO2 Hydrates in a Slurry Bubble Column. In Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, UK, 17–21 July 2011; pp. 1–8. [Google Scholar]
- Chernov, A.A.; Pil’nik, A.A.; Elistratov, D.S.; Mezentsev, I.V.; Meleshkin, A.V.; Bartashevich, M.V.; Vlasenko, M.G. New hydrate formation methods in a liquid-gas medium. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Andrés-Lacueva, C.; Medina-Remon, A.; Llorach, R.; Urpi-Sarda, M.; Khan, N.; Chiva-Blanch, G.; Zamora-Ros, R.; Rotches-Ribalta, M.; Lamuela-Raventós, R.M. Phenolic Compounds: Chemistry and Occurrence in Fruits and Vegetables. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; de la Rosa, L.A., Alvarez-Parrilla, E., González-Aguilar, G.A., Eds.; Blackwell Publishing: Ames, IA, USA, 2010; pp. 53–88. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Yahia, E.M.; de Jesús Ornelas-Paz, J. Chemistry, Stability, and Biological Actions of Carotenoids. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; de la Rosa, L.A., Alvarez-Parrilla, E., González-Aguilar, G.A., Eds.; Blackwell Publishing: Ames, IA, USA, 2010; pp. 177–222. [Google Scholar]
- DIN EN 12136:1997-12 Fruit and Vegetable Juices—Determination of Total Carotenoid Content and Individual Carotenoid Fractions; German Version EN 12136:1997. Available online: https://www.beuth.de/de/norm/din-en-12136/3572435 (accessed on 15 March 2021).
- Rückemann, H. Methoden zur Bestimmung von L-Ascorbinsäure mittels Hochleistungs-Flüssigchromatographie (HPLC). Z. Lebensm. Unters. Forsch. 1980, 171, 357–359. [Google Scholar] [CrossRef]
- Sood, S.P.; Sartori, L.E.; Wittmer, D.P.; Haney, W.G. High-pressure liquid chromatographic determination of ascorbic acid in selected foods and multivitamin products. Anal. Chem. 1976, 48, 796–798. [Google Scholar] [CrossRef] [PubMed]



| Medium | Analyzed Substances | Aim of Experiment | Hydrate Formation Repetitions |
|---|---|---|---|
| Model solution (water, sucrose) | Sucrose | Identification of working point. | 2 |
| Model solution (water, sucrose, betanin) | Sucrose Betanin | Evaluation of concentrate quality and preservation during hydrate formation. Visual assessment of separation quality by red/pink color of betanin. | 3 |
| Apple juice (clear) | Total phenolics Vitamin C | Evaluation of concentrate quality and preservation during hydrate formation. | 3 for total phenolics 2 for vitamin C |
| Apple juice (cloudy) | Vitamin C | Evaluation of concentrate quality and preservation during hydrate formation. | 2 |
| Orange juice | Total phenolics Total carotenoids Vitamin C | Evaluation of concentrate quality and preservation during hydrate formation. | 3 for total phenolics and total carotenoids 2 for vitamin C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolph, A.; El-Mohamad, A.; McHardy, C.; Rauh, C. Concentrating Model Solutions and Fruit Juices Using CO2 Hydrate Technology and Its Quantitative Effect on Phenols, Carotenoids, Vitamin C and Betanin. Foods 2021, 10, 626. https://doi.org/10.3390/foods10030626
Rudolph A, El-Mohamad A, McHardy C, Rauh C. Concentrating Model Solutions and Fruit Juices Using CO2 Hydrate Technology and Its Quantitative Effect on Phenols, Carotenoids, Vitamin C and Betanin. Foods. 2021; 10(3):626. https://doi.org/10.3390/foods10030626
Chicago/Turabian StyleRudolph, Alexander, Amna El-Mohamad, Christopher McHardy, and Cornelia Rauh. 2021. "Concentrating Model Solutions and Fruit Juices Using CO2 Hydrate Technology and Its Quantitative Effect on Phenols, Carotenoids, Vitamin C and Betanin" Foods 10, no. 3: 626. https://doi.org/10.3390/foods10030626






