1. Introduction
In 2019, the world’s hen egg production exceeded 1.6 billion eggs, 28.7% higher production than a decade before [
1]. Such a remarkable increase brought about a parallel increase in the concerns for animal welfare and environment in the European Union. Contextually, more than 50% of hens were reared in cage-free systems, while 18% of hens were reared in alternative production systems (free-range and organic) in Europe in 2019 [
2].
This increasing interest in products obtained under non-industrial production systems allows the development of sustainable farming practices [
3]. These sustainable farming practices may involve the use of native breeds adapted to the local environment, with great rusticity and resistance to meteorological situations and diseases, as well as great ability to search for food in the wild [
4,
5]. Consequently, it is through the conservation of animal genetic resources, that economic sustainability in the rural areas is promoted [
6,
7].
The Utrerana avian breed is one example of rustic Spanish hen, located in Andalusia (Southern Spain), which is officially considered to be endangered as stated in the Royal Decree—Law 45/2019 from the 8 February 2019. Its four varieties, namely, White, Franciscan, Black, and Partridge, are classified depending on the color of its feathers and tarsi [
8]. It was initially oriented toward egg production, however, the introduction of rather productive commercial hybrid genotypes in Europe caused the displacement of the Utrerana hen breed to a secondary position [
9]. As a result, the breed census reached a critical situation, which was parallel to a decrease in its productive indices derived from the patent lack of productive selection [
10]. Although the number of individuals has multiplied in the last years, only 1548 animals were registered in the studbook of the breed during 2019 [
11].
The enhancement of local products can be a strategy for the conservation of autochthonous genotypes, by avoiding the loss of connection between products, the local breeds from which they derive, and the area in which these were produced, as has been described for industrial products [
12]. In line with this situation, the definition of the breed’s productive role became compulsory to maximize the breed’s potential to satisfy current commercial demands. The characterization of Utrerana’s egg as the main product of the breed was configured in the context of a set of strategies that sought the obtention of competitive sustainable products in the framework of the recent emerging diseases and climate change [
13].
The acceptability of the eggs by consumers is mainly affected by the characteristics that describe their quality. Egg quality depends on several parameters, which are related to the eggshell, the albumen, and the yolk. Quality traits can be classified into external and internal quality, depending on whether the egg has to be broken to be scored (internal quality) or not (external quality) [
14,
15]. Egg weight, eggshell strength, albumen quality, and yolk color intensity are among the most important egg traits of commercial interest [
14,
16,
17,
18]. Eggs are commercially classified into four classes depending on their total egg weight: S (<53 g), M (53–63 g), L (63–73 g), and XL (>73 g).
Egg quality traits have been reported to be multifactorially dependent mainly on the laying hen’s age and nutritional factors [
16,
19,
20,
21]. However, there are some relevant pieces of evidence for the influence of the genotype on some of these egg quality traits on the relative proportion of yolk and albumen, albumen quality, or chemical composition [
14,
22,
23,
24,
25,
26].
Utrerana’s egg not only constitutes a differentiated product in terms of internal and external quality–related traits [
14] and chemical composition [
23]. Additionally, its sensory characteristics have been reported to differ from the eggs of commercial lines [
27].
Consequently, the present study aimed to determine the contributions of external and internal quality parameters to the eggs produced by each of the four varieties of Utrerana hens and a control flock of a commercial laying lineage. Canonical discriminant analysis was used to design a statistical tool that permits determining whether specific eggs may correctly fit the features of the different commercial size categories (S, M, L, and XL), which may support the standardization of the Utrerana varieties’ eggs as products and may address and support their suitability to cover particular sections of the market for egg consumption.
4. Discussion
Involving autochthonous breeds in animal production systems may promote the evolution of sustainable ways of producing. Native breeds can be used in search of productive improvement by taking advantage of genotypes adapted to the climatology and orography, as well as to the technical, productive, and cultural conditions of the area. On the other hand, commercial chains are increasingly requesting more products derived from non-industrial processes. This context makes it necessary to characterize the eggs of the Utrerana avian breed according to their commercial size while defining how the different quality-related parameters affect the differentiation between eggs across the different varieties and breeds studied. The results obtained in the present study may suggest how to approach the different strategies to make an endangered breed profitable, thus ensuring its conservation by establishing production models to which it is adapted.
The selection of the individuals in the sample was performed considering that the hybrid commercial cycle and both genotypes reach 50% of laying (egg production during a laying cycle). Contextually, the typical production cycle in commercial layers (Leghorn hens among others) lasts about 72 weeks [
44]. However, this cycle may extend until 156 weeks in around a third of the Utrerana population [
45]. Additionally, according to Kuo et al. [
46], the age at sexual maturity is estimated by age in weeks when 50% egg production is reached. In this regard, the same authors suggested the age when 50% egg production in White Leghorn is reached to be around 21 weeks. By contrast, the information reported by Orozco Piñán [
45] suggested the average age of Utrerana hens at the moment of the first laying was 25 weeks. Furthermore, the breeding criterion of both breeds may differ, as while White Leghorn hens breeders have traditionally selected animals for precocity [
47], Utrerana breeders have not sought this trait as a priority rather benefiting from the natural lay cycle of the breed [
45]. Zita et al. [
48] suggested that egg quality characteristics are affected by the interaction of genotype (breed and strain) and hen’s age, rather than exerting their effects independently.
Multicollinearity analyses revealed high correlations between major diameter and minor diameter and egg shape index, since both measurements comprise the formula for its calculation. The same happens with the formula of Haugh units, which includes the variables of egg weight and albumen height, which consequently were eliminated due to multicollinearity problems. Moreover, egg weight can be calculated by separately summing albumen weight, yolk weight, and eggshell weight variables, which may be the logical source for the redundancies detected.
Degree of lightness (L*) and chromaticity coordinates (a* and b*) comprise the L*a*b* color space [
49]. In this context, coordinates of shell a* and shell b* are difficult to interpret and can be correlated in white-shelled eggs, such as those of the Utrerana avian breed [
20]. As suggested by other authors [
50], a* and b* parameters measure chromaticity. More specifically, redness–greenness and yellowness–blueness, respectively. Positive values of a* are linked to increased amounts of redness in eggshell color, whereas negative values of a* relate to increased amounts of greenness in the eggshell color.
Similarly, the representativity of yellow and blue components in eggs of any color are represented by positive and negative values of b*, respectively. In this context, Odabaşi et al. [
50], suggested that the lighter the shell color (higher L*), the lesser the redness of the color of the eggshell is as well. This was in line with the results reported by Aygun [
51], who reported shell L* could be considered as a discriminative color criterion as the lesser the amount of shell L*, the darker the eggshell color turns to be.
The visual defects in yolk and albumen are produced by meat and blood spots. The presence of these visual defects is regarded as an undesirable feature in eggs that causes rejection by consumers [
52].
These undesirable findings may stem from the synthesis of the different parts of the egg during ovulation due to the rupture of an ovarian follicle at a different position from the stigma [
53]. In these situations, variations in the chromaticity coordinates of the yolk color could appear, thus, may be one of the sources of multicollinearity problems between the presence of visual defects and yolk a* and yolk b*.
Albumen weight, eggshell weight, and yolk weight variables reported the best discriminating properties (
Table 7). These three quality-related traits compose the egg weight, which is the main criterion on which the commercial classification of eggs relies. At the same time, albumen represents about 55–65% of the egg weight [
54,
55]. This explains the fact that albumen weight was ranked first at the test of equality of group means.
Hen strain has been reported to significantly affect albumen ratio [
53,
56]. Albumen is critical for the survival of the chicken embryo and the variations in the content of albumen in hen eggs can generate differences in skeletal muscle or liver metabolism during embryonic development [
57]. In laying hens, albumen has great commercial importance, provided its unique functional properties and its use as an ingredient in a large number of culinary international preparations [
58]. In previous studies, the Leghorn has been demonstrated to have a higher albumen weight than the Utrerana avian breed, due to the Leghorn’s higher concentration of energy reserves [
14]. Contextually, Peña-Villalobos et al. [
57] suggested a significant reduction in metabolic rate occurs in the last fifth of embryonic life in albumen-removed eggs, which in turn derives into reduced catabolic activities in the skeletal muscle of chicks that eventually hatch.
Utrerana has been reported to present a lower eggshell weight and a higher yolk weight than Leghorns [
14]. These results agree with the present research since these parameters have a high discriminating power when clusters differentiate. Modern commercial breeds showed clear differences in terms of eggshell weight when compared to native poultry, due to the high selection of all egg traits of eggs for its transport and commercial purposes [
59,
60].
Differences in the proportion of egg yolk have been reported between breeds and within highly productive laying hens strains such as the White Leghorn, which may be indicative of the presence of sufficient additive genetic variation [
61]. Furthermore, selection based on additive genetic variation in yolk weight has been suggested as an option to promote seeking sustainability of local eggs [
62], as native breeds could satisfy the growing demand for more energetically efficient eggs in the market [
13,
14,
26].
Yolk diameter and Haugh units reported the best discriminating properties (fourth and fifth position in the rank) after weight-related traits (albumen, eggshell, and yolk weights). The relevance of these traits may be ascribed as suggested by Ukwu et al. [
63], who reported significant differences in yolk weight and albumen height among light (less than 49.99 g), medium (50–55 g), and heavy eggs (more than 55 g) of Isa Brown egg layer chickens in Nigeria. This has also been reported by Alkan et al. [
64], who addressed a parallel increase in yolk diameter as egg weight increases in partridge eggs. However, no differences between Utrerana and Leghorn breeds were detected in previous studies [
14].
Haugh units are used as an indicator of internal egg quality [
30]. Time of storage and storage conditions affect Haugh units values [
65]. However, the strains or breed of the hen have been reported to quantitatively affect them. For instance, several authors have reported higher values for Haugh units in local breeds than in commercial hybrid strains [
4,
13,
66]. In any case, albumen height is correlated with the percentage of albumen [
67]. Hence, commercial strains could present a certain advanced position, provided a larger percentage of albumen is found in hybrid strains in comparison to that in native breeds.
Values for pH-related traits showed the lowest values of F and highest for Wilks’ lambda. Egg pH allows the assessment of the egg’s freshness [
68,
69]. The loss of CO
2 and H
2O inside the egg produces an increase in albumen pH. The time of storage and high temperatures condition this loss of CO
2 and H
2O and promote a decrease in albumen viscosity and flavor with detrimental effects for egg quality [
19,
70]. Albumen and yolk pH can be slightly influenced by the hen strain [
13,
14,
71]. Nevertheless, in the present study, when all egg pH values were measured during the 24 h following oviposition, it was found that albumen pH and yolk pH have a low discriminating power between different groups of eggs, which may derive from the low variability in pH found. Such lack of variability may stem from the fact that the eggs considered in this study were fresh enough for those eggs presenting slightly lower values not to be detrimental on egg quality. Additionally, this finding may evidence a patent lack of importance provided to quality traits (such as the pH of the components of the egg) against quantitative traits among the current criteria that are considered for egg quality classification, as the quantity of the product may be better commercially valued than its quality. However, this commercial strategy may be erroneous given it may not match the current general trend of the customers preferring egg quality over quantity [
72].
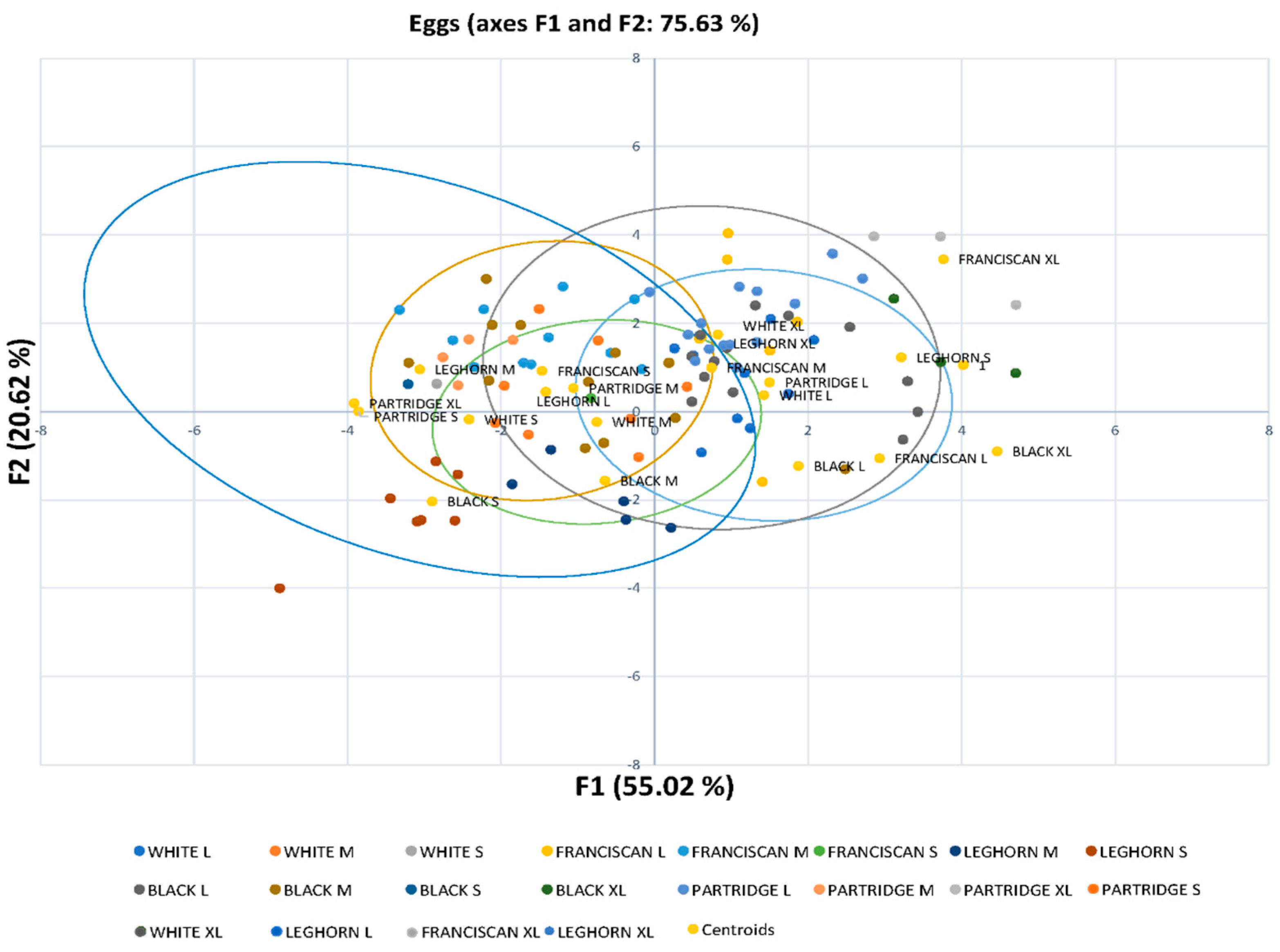
Figure 2 reports that egg quality classification clusters are mainly grouped depending on their commercial size. In addition, the Leghorn’s egg groups differed from the rest of those from the Utrerana varieties, except for those of White Utrerana XL and Leghorn XL eggs, which reported a certain closeness. This may be indicative of the hybridization of the White Utrerana with the Leghorn breed, both with white plumage, which may have been historically performed by breeders as an attempt to decrease the consanguinity of the white variety, which is the Utrerana variety accounting with the smallest number of animals and the one which faces the highest endangerment risk.
Additionally, the present study may confirm the fact that product differentiation could be a feasible opportunity for the eggs of Utrerana varieties, which could constitute a favorable point when compared to eggs from other breeds that have traditionally been sold in the market [
73].
The present discriminating tool allows to efficiently classify eggs based on quality-related traits as supported by the 73.2% of observations being correctly classified within their group. In this regard, weight traits play a pivotal role in the determination of the commercial quality of eggs.
All eggs belonging to the S category in White, Franciscan and Partridge, and L category of Franciscan and Partridge were correctly classified (
Supplementary Table S1). However, 45.5% of M category Partridge eggs were classified as Franciscan M. Previous research suggested Partridge and Franciscan varieties present a significantly heavier yolk and slightly lower weight than the rest of the varieties or the Leghorn breed [
14], hence similarities between egg quality-related traits of these two varieties could be expected. Moreover, 26.7% of XL category Leghorn eggs were classified as Leghorn L eggs, which may be explained as commercial genotypes have been selected to produce rather homogenous eggs, which may translate to a reduction in differences [
74,
75]. Furthermore, it may be worth mentioning that 23.8% of M category White Utrerana eggs were classified as M category Leghorn ones, with the likely hybridization between these two strains being the potential source for these similarities.