Assessment of Fennel Oil Microfluidized Nanoemulsions Stabilization by Advanced Performance Xanthan Gum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chromatographic Analysis
2.3. Emulsification Process
2.4. Gum Addition
2.5. Size Measurement
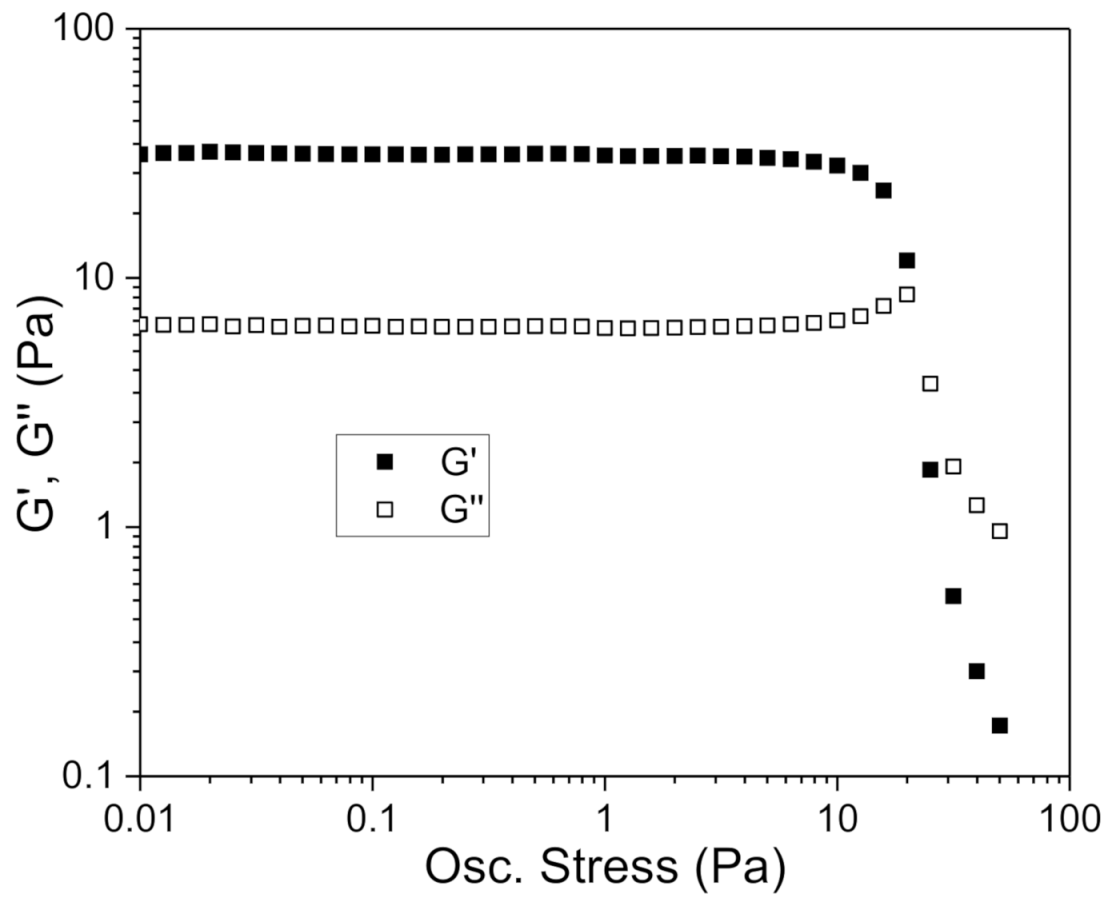
2.6. Oscillatory and Flow Curve Measurement
2.7. Microstructure of the Emulsions and Continuous Phases
2.8. Statistical Analysis
3. Results and Discussion
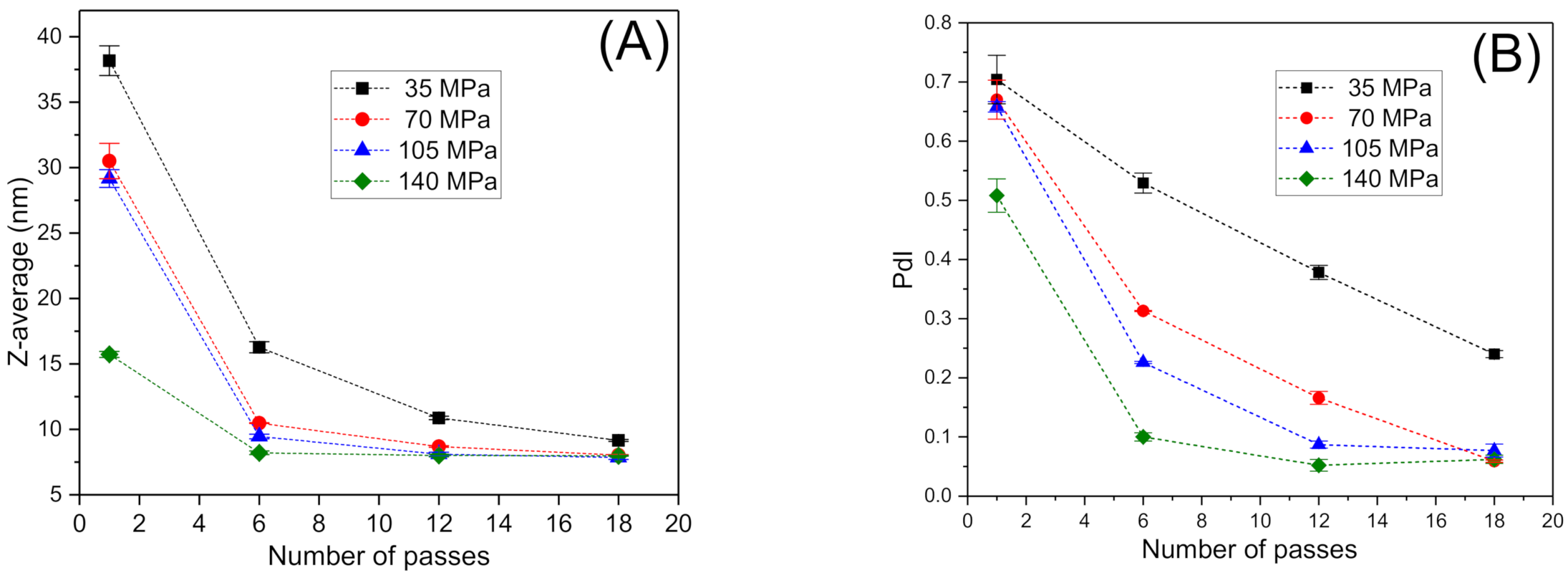
3.1. Influence of Energy Input on Droplet Size Distribution
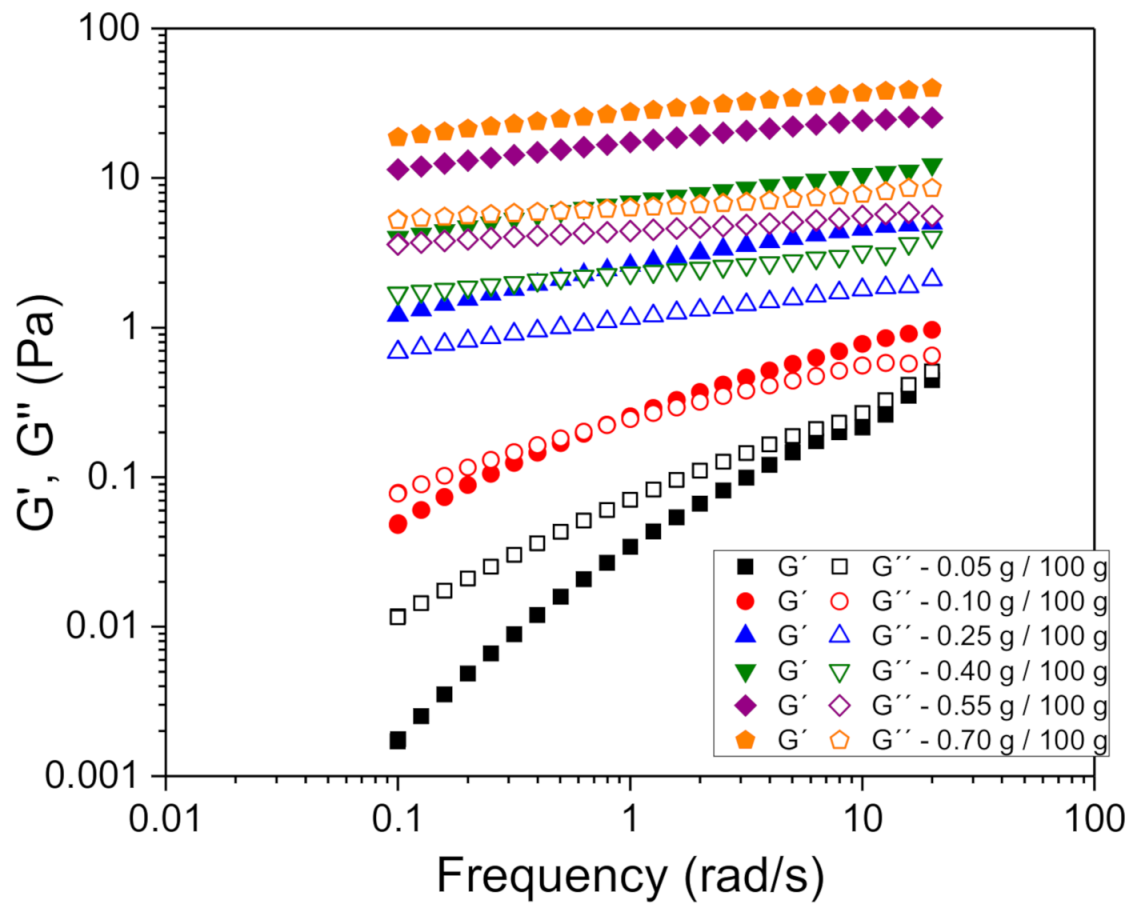
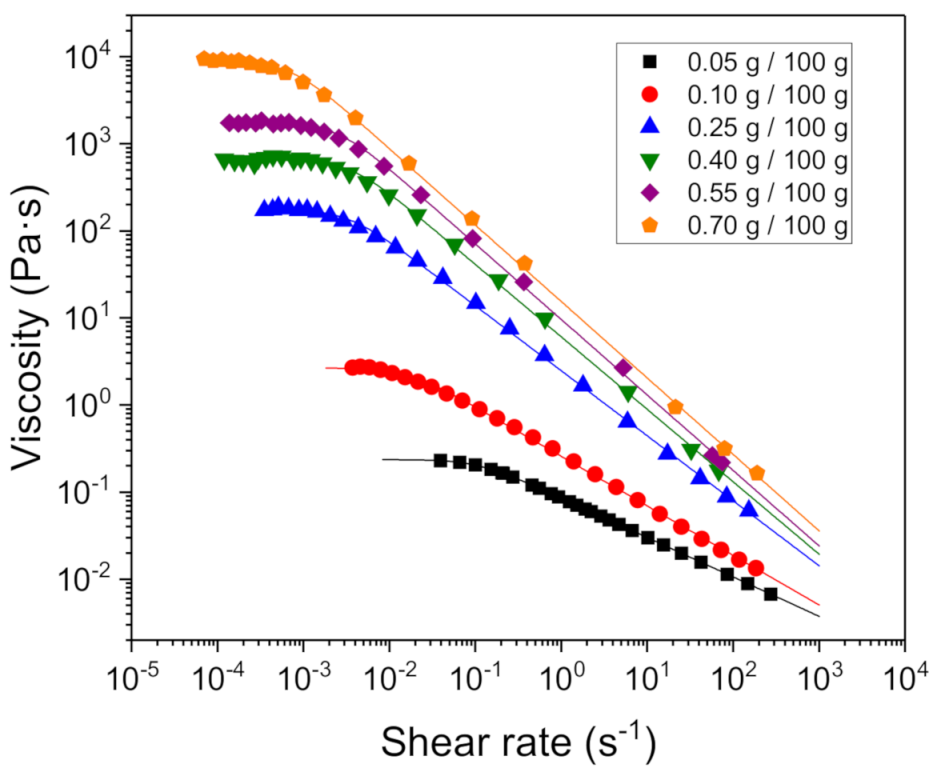
3.2. Rheological Behavior Influence of Xanthan Gum Concentration
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, Y.; McLandsborough, L.; McClements, D.J. Fabrication, stability and efficacy of dual-component antimicrobial nanoemulsions: Essential oil (thyme oil) and cationic surfactant (lauric arginate). Food Chem. 2015, 172, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.; Buchbauer, G. (Eds.) Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M.J. Assisted extraction of rosemary antioxidants with green solvents. J. Food Eng. 2012, 109, 98–103. [Google Scholar] [CrossRef]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Soliva-Fortuny, R.; Rojas-Graü, M.A.; McClements, D.J.; Martín-Belloso, O. Edible Nanoemulsions as Carriers of Active Ingredients: A Review. Annu. Rev. Food Sci. Technol. 2017, 8, 439–466. [Google Scholar] [CrossRef]
- Choi, M.-J.; Soottitantawat, A.; Nuchuchua, O.; Min, S.-G.; Ruktanonchai, U. Physical and light oxidative properties of eugenol encapsulated by molecular inclusion and emulsion–diffusion method. Food Res. Int. 2009, 42, 148–156. [Google Scholar] [CrossRef]
- Surassmo, S.; Min, S.-G.; Bejrapha, P.; Choi, M.-J. Effects of surfactants on the physical properties of capsicum oleoresin-loaded nanocapsules formulated through the emulsion–diffusion method. Food Res. Int. 2010, 43, 8–17. [Google Scholar] [CrossRef]
- Erramreddy, V.; Ghosh, S. Gelation in nanoemulsion: Structure formation and rheological behavior. In Emulsions; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 257–292. [Google Scholar]
- Barradas, T.N.; De Campos, V.E.B.; Senna, J.P.; Coutinho, C.D.S.C.; Tebaldi, B.S.; Silva, K.G.D.H.E.; Mansur, C.R.E. Development and characterization of promising o/w nanoemulsions containing sweet fennel essential oil and non-ionic sufactants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 214–221. [Google Scholar] [CrossRef]
- Mostafa, D.M.; El-Alim, S.H.A.; Asfour, M.H.; Al-Okbi, S.Y.; Mohamed, D.A.; Awad, G. Transdermal nanoemulsions of Foeniculum vulgare Mill. essential oil: Preparation, characterization and evaluation of antidiabetic potential. J. Drug Deliv. Sci. Technol. 2015, 29, 99–106. [Google Scholar] [CrossRef]
- Venkadesaperumal, G.; Rucha, S.; Sundar, K.; Shetty, P.H. Anti-quorum sensing activity of spice oil nanoemulsions against food borne pathogens. LWT 2016, 66, 225–231. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; Series in Contemporary Food Science; CRC Press: Boca Raton, FL, USA, 2005; p. 609. [Google Scholar]
- Dickinson, E. Stabilising emulsion-based colloidal structures with mixed food ingredients. J. Sci. Food Agric. 2012, 93, 710–721. [Google Scholar] [CrossRef]
- Rochefort, W.E.; Middleman, S. Rheology of Xanthan Gum: Salt, Temperature, and Strain Effects in Oscillatory and Steady Shear Experiments. J. Rheol. 1987, 31, 337–369. [Google Scholar] [CrossRef]
- Carnali, J.O. Original Contributions Gelation in physically associating biopolymer systems. Rheol. Acta 1992, 412, 399–412. [Google Scholar] [CrossRef]
- Lim, T.; Uhl, J.T.; Prudhomme, R.K. Rheology of Self-Associating Concentrated Xanthan Solutions. J. Rheol. 1984, 28, 367–379. [Google Scholar] [CrossRef]
- Carmona, J.; Ramirez, P.; Calero, N.; Munoz, J.M. Large amplitude oscillatory shear of xanthan gum solutions. Effect of sodium chloride (NaCl) concentration. J. Food Eng. 2014, 126, 165–172. [Google Scholar] [CrossRef]
- Carmona, J.A.; Lucas, A.; Ramírez, P.; Calero, N.; Muñoz, J. Nonlinear and linear viscoelastic properties of a novel type of xanthan gum with industrial applications. Rheol. Acta 2015, 54, 993–1001. [Google Scholar] [CrossRef]
- Carmona, J.A.; Calero, N.; Ramírez, P.; Muñoz, J. Rheology and structural recovery kinetics of an advanced performance xanthan gum with industrial application. Appl. Rheol. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- Miraldi, E. Comparison of the essential oils from ten Foeniculum vulgare Miller samples of fruits of different origin. Flavour Fragr. J. 1999, 14, 379–382. [Google Scholar] [CrossRef]
- Marotti, M.; Piccaglia, R.; Giovanelli, E.; Deans, S.G.; Eaglesham, E. Effects of Variety and Ontogenic Stage on the Essential Oil Composition and Biological Activity of Fennel (Foeniculum vulgare Mill.). J. Essent. Oil Res. 1994, 6, 57–62. [Google Scholar] [CrossRef]
- Ehsanipour, A.; Razmjoo, J.; Zeinali, H. Effect of nitrogen rates on yield and quality of fennel (Foeniculum vulgare Mill.) accessions. Ind. Crop. Prod. 2012, 35, 121–125. [Google Scholar] [CrossRef]
- García-Jiménez, N.; Péerez-Alonso, M.J.; Velasco-Negueruela, A. Chemical Composition of Fennel Oil, Foeniculum vulgare Miller, from Spain. J. Essent. Oil Res. 2000, 12, 159–162. [Google Scholar] [CrossRef]
- De, M.; De, A.K.; Sen, P.; Banerjee, A.B. Antimicrobial properties of star anise (Illicium verum Hook f). Phytother. Res. 2002, 16, 94–95. [Google Scholar] [CrossRef]
- Bilia, A.; Flamini, G.; Taglioli, V.; Morelli, I.; Vincieri, F. GC–MS analysis of essential oil of some commercial Fennel teas. Food Chem. 2002, 76, 307–310. [Google Scholar] [CrossRef]
- Trujillo-Cayado, L.A.; Santos, J.; Ramirez, P.; Alfaro, M.C.; Muñoz, J. Strategy for the development and characterization of environmental friendly emulsions by microfluidization technique. J. Clean. Prod. 2018, 178, 723–730. [Google Scholar] [CrossRef]
- Carmona, J.; Ramírez, P.; Trujillo-Cayado, L.; Caro, A.; Muñoz, J. Rheological and microstructural properties of sepiolite gels. Influence of the addition of ionic surfactants. J. Ind. Eng. Chem. 2018, 59, 1–7. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013, 30, 401–407. [Google Scholar] [CrossRef]
- Trujillo-Cayado, L.A.; Santos, J.; Alfaro, M.C.; Calero, N.; Muñoz, J. A further step in the development of oil-in-water emul-sions formulated with a mixture of green solvents. Ind. Eng. Chem. Res. 2016, 55, 7259–7266. [Google Scholar] [CrossRef]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, C.; Garcia, M.J.; Azemar, N.; Solans, C. Formation and Stability of Nano-Emulsions Prepared Using the Phase Inversion Temperature Method. Langmuir 2002, 18, 26–30. [Google Scholar] [CrossRef]
- Cuvelier, G.; Launay, B. Concentration regimes in xanthan gum solutions deduced from flow and viscoelastic properties. Carbohydr. Polym. 1986, 6, 321–333. [Google Scholar] [CrossRef]
- Wyatt, N.B.; Liberatore, M.W. Rheology and viscosity scaling of the polyelectrolyte xanthan gum. J. Appl. Polym. Sci. 2009, 114, 4076–4084. [Google Scholar] [CrossRef]
- Song, K.-W.; Kim, Y.-S.; Chang, G.-S. Rheology of concentrated xanthan gum solutions: Steady shear flow behavior. Fibers Polym. 2006, 7, 129–138. [Google Scholar] [CrossRef]




| Compound | Relative Concentration (%) |
|---|---|
| α-Pinene | 6.8 |
| β-Pinene | 0.3 |
| β-Phellandrene | 0.6 |
| α-Phellandrene | 1.2 |
| β-Myrcene | 0.8 |
| D-Limonene | 16.7 |
| p-Cymene | 0.7 |
| Fenchone | 7.4 |
| Estragole | 2.3 |
| Anethole | 60.6 |
| p-Anisaldehyde | 2.3 |
| p-Acetonylanisole | 0.3 |
| [XG] (g/100 g) | η0 (Pa·s) | (s−1) | n |
|---|---|---|---|
| 0.05 | 0.24 ± 0.01 | 0.11 ± 0.02 | 0.55 ± 0.01 |
| 0.10 | 2.7 ± 0.1 | 0.016 ± 0.002 | 0.43 ± 0.01 |
| 0.25 | 175 ± 9 | 3.3 × 10−3 ± 4 × 10−4 | 0.25 ± 0.01 |
| 0.40 | 660 ± 28 | 3.6 × 10−3 ± 5 × 10−4 | 0.17 ± 0.02 |
| 0.55 | 1730 ± 80 | 2.5 × 10−3 ± 3 × 10−4 | 0.13 ± 0.01 |
| 0.70 | 8900 ± 508 | 7 × 10−4 ± 1 × 10−4 | 0.12 ± 0.01 |
| [XG] (g/100 g) | η0 (Pa·s) * | η0 (Pa·s) ** |
|---|---|---|
| 0.25 | 175 ± 9 | 214 ± 5 |
| 0.40 | 660 ± 28 | 1638 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llinares, R.; Ramírez, P.; Carmona, J.A.; Trujillo-Cayado, L.A.; Muñoz, J. Assessment of Fennel Oil Microfluidized Nanoemulsions Stabilization by Advanced Performance Xanthan Gum. Foods 2021, 10, 693. https://doi.org/10.3390/foods10040693
Llinares R, Ramírez P, Carmona JA, Trujillo-Cayado LA, Muñoz J. Assessment of Fennel Oil Microfluidized Nanoemulsions Stabilization by Advanced Performance Xanthan Gum. Foods. 2021; 10(4):693. https://doi.org/10.3390/foods10040693
Chicago/Turabian StyleLlinares, Rubén, Pablo Ramírez, José Antonio Carmona, Luis Alfonso Trujillo-Cayado, and José Muñoz. 2021. "Assessment of Fennel Oil Microfluidized Nanoemulsions Stabilization by Advanced Performance Xanthan Gum" Foods 10, no. 4: 693. https://doi.org/10.3390/foods10040693
APA StyleLlinares, R., Ramírez, P., Carmona, J. A., Trujillo-Cayado, L. A., & Muñoz, J. (2021). Assessment of Fennel Oil Microfluidized Nanoemulsions Stabilization by Advanced Performance Xanthan Gum. Foods, 10(4), 693. https://doi.org/10.3390/foods10040693








