Gastric Digestion of Milk Proteins in Adult and Elderly: Effect of High-Pressure Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. High-Pressure Treatments
2.3. Protein Composition Analysis by Gel Electrophoresis
2.4. In Vitro Gastric Digestion for Adults and the Elderly
2.5. Proteomic Analysis Using LC–MS
2.6. Data Analysis
3. Results and Discussion
3.1. Characterization of the Milks before Digestion
3.1.1. Milk Composition
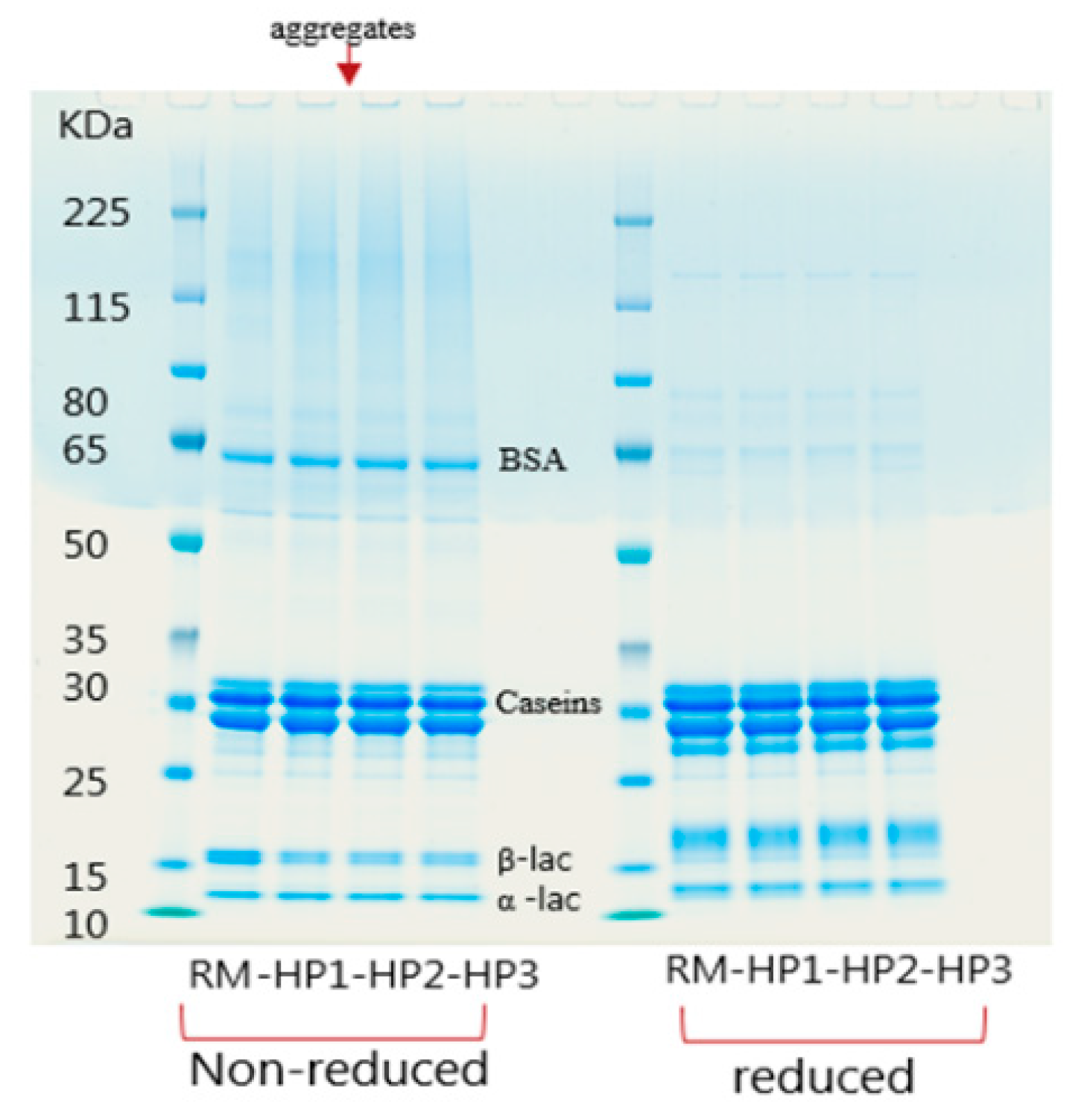
3.1.2. Denaturation of Milk Proteins after Application of HPP
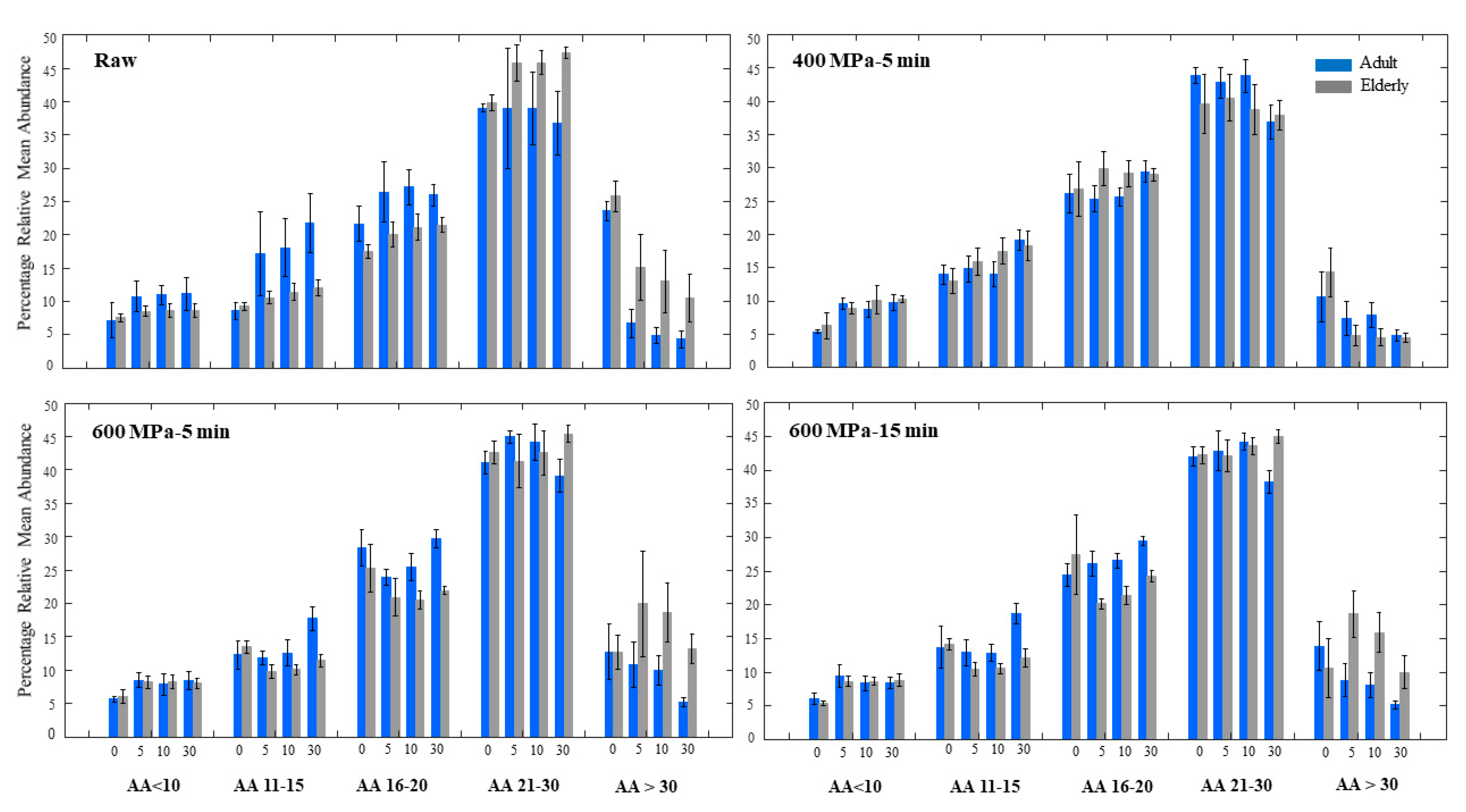
3.2. In Vitro Digestion of Proteins in Adult and Elderly Model
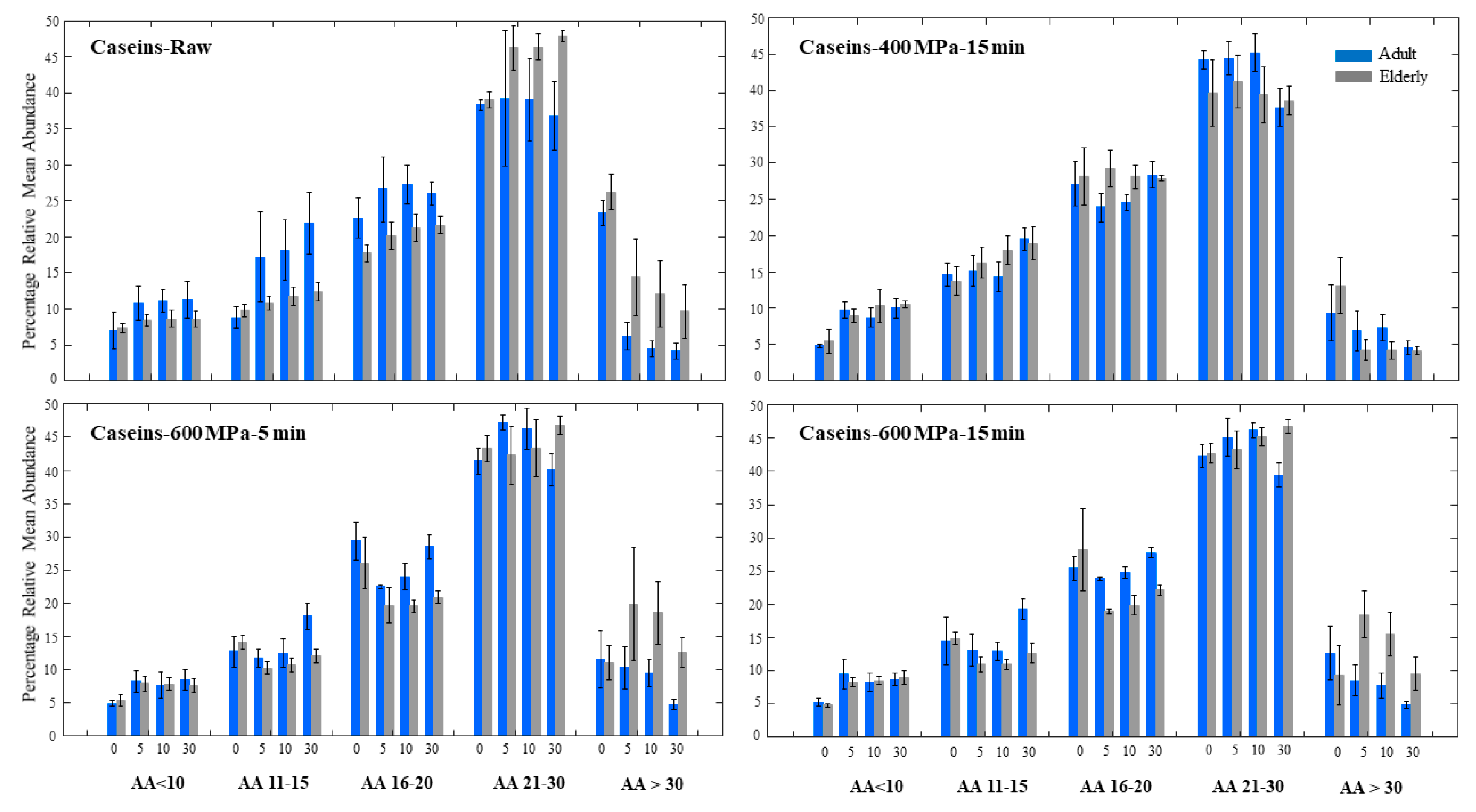
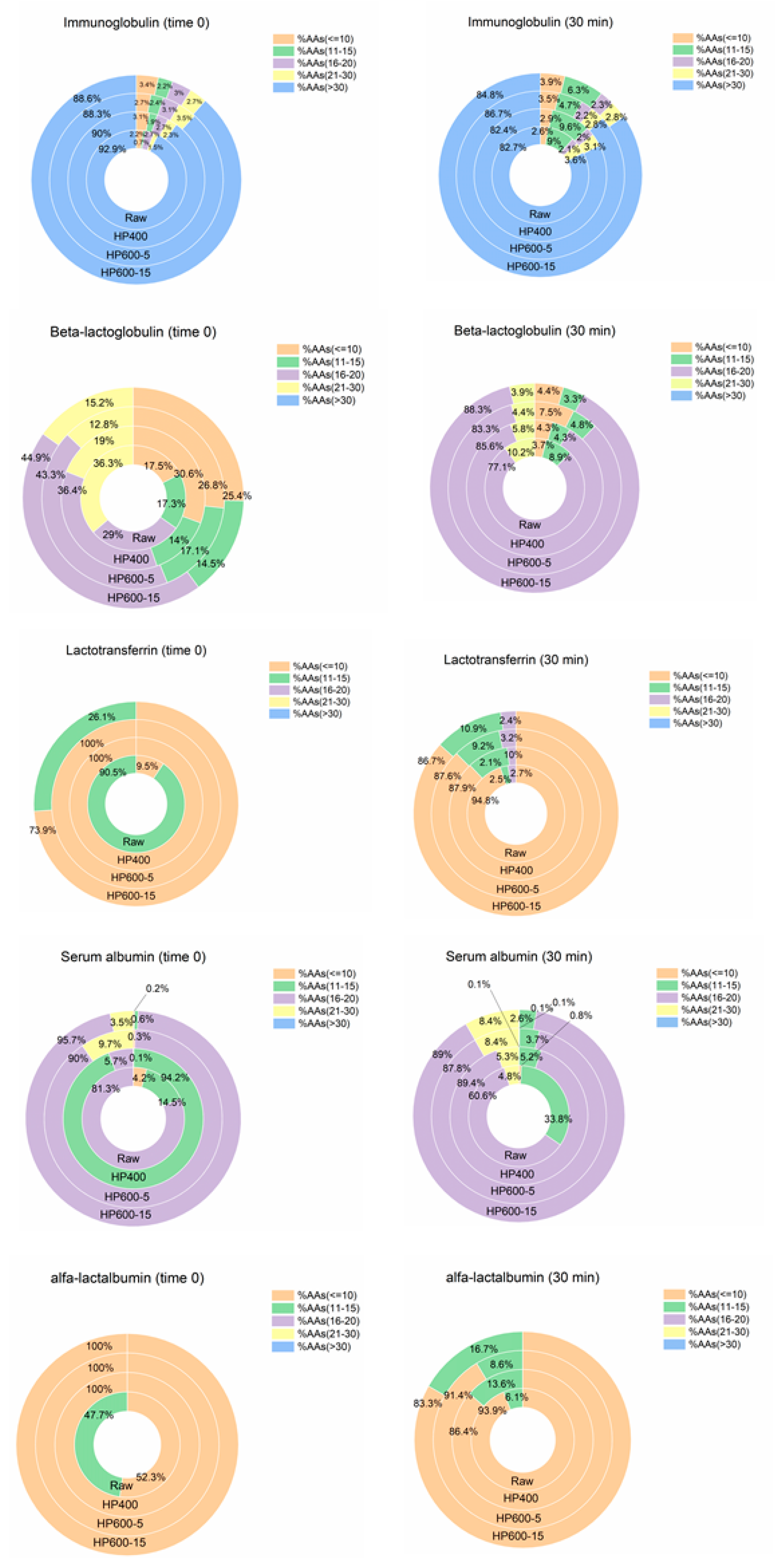
3.3. Caseins Digestion
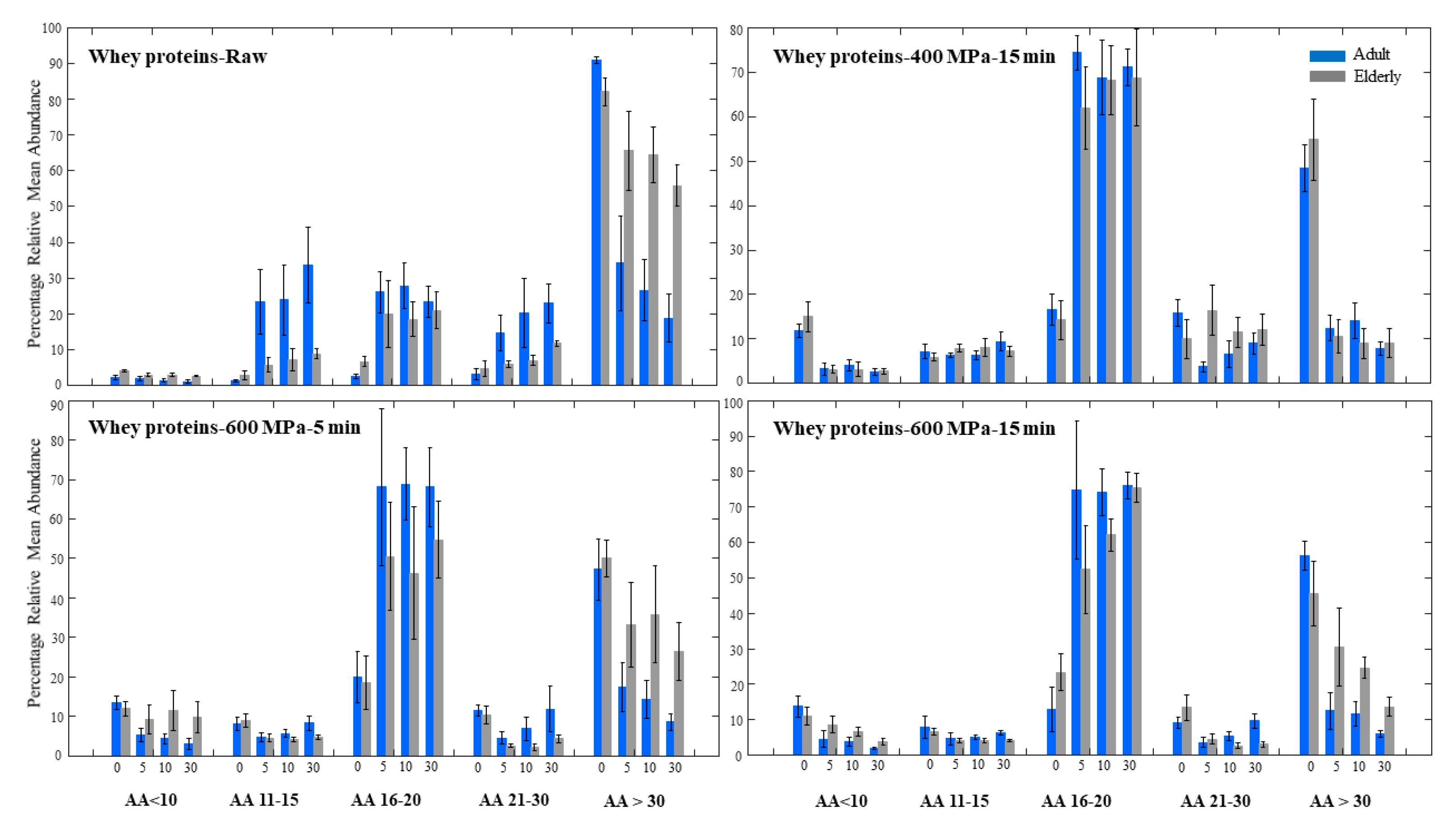
3.4. Whey Proteins Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Voigt, D.D.; Kelly, A.L.; Huppertz, T. High-pressure processing of milk and dairy products. Emerg. Dairy Process. Technol. 2015, 1, 71–92. [Google Scholar] [CrossRef]
- Cornell, A. Inventor HPP Process for Dairy Foods. Australian Patent AU, Application number PCT/AU2016/050921, 6 April 2017. [Google Scholar]
- Huppertz, T.; Kelly, A.L.; Fox, P.F. Effects of high pressure on constituents and properties of milk. Int. Dairy J. 2002, 12, 561–572. [Google Scholar] [CrossRef]
- Maynard, F.; Weingand, A.; Hau, J.; Jost, R. Effect of high-pressure treatment on the tryptic hydrolysis of bovine β;-lactoglobulin ab. Int. Dairy J. 1998, 8, 125–133. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Inguglia, E.S.; Linton, M.; Tollerton, J.; Murphy, L.; Corcionivoschi, N.; Koidis, A.; Tiwari, B.K. Effect of high pressure processing on the safety, shelf life and quality of raw milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 325–333. [Google Scholar] [CrossRef]
- Cadesky, L.; Walkling-Ribeiro, M.; Kriner, K.T.; Karwe, M.V.; Moraru, C.I. Structural changes induced by high-pressure processing in micellar casein and milk protein concentrates. J. Dairy Sci. 2017, 100, 7055–7070. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G.; Lowe, E.K.; Stockmann, R. Particle size changes and casein solubilisation in high-pressure-treated skim milk. Food Hydrocoll. 2005, 19, 257–267. [Google Scholar] [CrossRef]
- Anema, S.G.; Stockmann, R.; Lowe, E.K. Denaturation of β-lactoglobulin in pressure-treated skim milk. J. Agric. Food Chem. 2005, 53, 7783–7791. [Google Scholar] [CrossRef]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. Properties of casein micelles in high pressure-treated bovine milk. Food Chem. 2004, 87, 103–110. [Google Scholar] [CrossRef]
- Mazri, C.; Sánchez, L.; Ramos, S.; Calvo, M.; Pérez, M. Effect of high-pressure treatment on denaturation of bovine lactoferrin and lactoperoxidase. J. Dairy Sci. 2012, 95, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Orlien, V. 1.06-Structural changes induced in foods by HPP. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 112–129. [Google Scholar]
- Olsen, K.; Ipsen, R.; Otte, J.; Skibsted, L. Effect of high pressure on aggregation and thermal gelation of beta-lactoglobolin. Milchwissenschaft 1999, 54, 543–546. [Google Scholar]
- Lopez-Fandiño, R.; Carrascosa, A.; Olano, A. The effects of high pressure on whey protein denaturation and cheese-making properties of raw milk. J. Dairy Sci. 1996, 79, 929–936. [Google Scholar] [CrossRef]
- Kopf-Bolanz, K.A.; Schwander, F.; Gijs, M.; Vergères, G.; Portmann, R.; Egger, L. Impact of milk processing on the generation of pep-tides during digestion. Int. Dairy J. 2014, 35, 130–138. [Google Scholar] [CrossRef]
- Lorieau, L.; Halabi, A.; Ligneul, A.; Hazart, E.; Dupont, D.; Floury, J. Impact of the dairy product structure and protein nature on the proteolysis and amino acid bioaccessiblity during in vitro digestion. Food Hydrocoll. 2018, 82, 399–411. [Google Scholar] [CrossRef]
- Mat, D.J.L.; Le Feunteun, S.; Michon, C.; Souchon, I. In vitro digestion of foods using pH-stat and the INFOGEST protocol: Impact of matrix structure on digestion kinetics of macronutrients, proteins and lipids. Food Res. Int. 2016, 88, 226–233. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Mackie, A.R.; Wilde, P.J.; Fenelon, M.A.; Brodkorb, A. Structural mechanism and kinetics of in vitro gastric di-gestion are affected by process-induced changes in bovine milk. Food Hydrocolloid. 2018, 86, 172–183. [Google Scholar] [CrossRef]
- Almaas, H.; Cases, A.-L.; Devold, T.G.; Holm, H.; Langsrud, T.; Aabakken, L.; Aadnoey, T.; Vegarud, G.E. In vitro digestion of bovine and caprine milk by human gastric and duodenal enzymes. Int. Dairy J. 2006, 16, 961–968. [Google Scholar] [CrossRef]
- Felfoul, I.; Jardin, J.; Gaucheron, F.; Attia, H.; Ayadi, M. Proteomic profiling of camel and cow milk proteins under heat treatment. Food Chem. 2017, 216, 161–169. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Montilla, A.; Mulet-Cabero, A.-I.; Rigby, N.; Olano, A.; Mackie, A.; Villamiel, M. Study on the digestion of milk with prebiotic carbohydrates in a simulated gastrointestinal model. J. Funct. Foods 2017, 33, 149–154. [Google Scholar] [CrossRef]
- He, Z.; Yuan, B.; Zeng, M.; Tao, G.; Chen, J. Effect of simulated processing on the antioxidant capacity and in vitro protein digestion of fruit juice-milk beverage model systems. Food Chem. 2015, 175, 457–464. [Google Scholar] [CrossRef]
- Nehir El, S.; Karakaya, S.; Simsek, S.; Dupont, D.; Menfaatli, E.; Eker, A.T. In vitro digestibility of goat milk and kefir with a new standardised static digestion method (INFOGEST cost action) and bioactivities of the resultant peptides. Food Func. 2015, 6, 2322–2330. [Google Scholar] [CrossRef]
- Rinaldi, L.; Gauthier, S.F.; Britten, M.; Turgeon, S.L. In vitro gastrointestinal digestion of liquid and semi-liquid dairy matrixes. LWT Food Sci. Technol. 2014, 57, 99–105. [Google Scholar] [CrossRef]
- Sánchez-Rivera, L.; Ménard, O.; Recio, I.; Dupont, D. Peptide mapping during dynamic gastric digestion of heated and unheated skimmed milk powder. Food Res. Int. 2015, 77, 132–139. [Google Scholar] [CrossRef]
- Chicón, R.; Belloque, J.; Alonso, E.; López-Fandiño, R. Immunoreactivity and digestibility of high-pressure-treated whey proteins. Int. Dairy J. 2008, 18, 367–376. [Google Scholar] [CrossRef]
- Hu, G.; Zheng, Y.; Liu, Z.; Xiao, Y.; Deng, Y.; Zhao, Y. Effects of high hydrostatic pressure, ultraviolet light-C, and far-infrared treatments on the digestibility, antioxidant and antihypertensive activity of α-casein. Food Chem. 2017, 221, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Carøe, C.; Qin, Z.; Munk, D.M.; Crafack, M.; Petersen, M.A.; Ahrné, L. Comparative study on quality of whole milk processed by high hydrostatic pressure or thermal pasteurization treatment. LWT 2020, 127, 109370. [Google Scholar] [CrossRef]
- Russell, R.M. Changes in gastrointestinal function attributed to aging. Am. J. Clin. Nutr. 1992, 55, 1203S–1207S. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Cryer, B.; McArthur, K.E.; Huet, B.A.; Lee, E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: A prospective study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Löhr, J.-M.; Panic, N.; Vujasinovic, M.; Verbeke, C.S. The ageing pancreas: A systematic review of the evidence and analysis of the consequences. J. Intern. Med. 2018, 283, 446–460. [Google Scholar] [CrossRef]
- Salles, N. Basic mechanisms of the aging gastrointestinal tract. Dig. Dis. 2007, 25, 112–117. [Google Scholar] [CrossRef]
- Vellas, B.; Balas, D.; Moreau, J.; Bouisson, M.; Senegas-Balas, F.; Guidet, M.; Ribet, A. Exocrine pancreatic secretion in the elderly. Int. J. Pancreatol. 1988, 3, 497–502. [Google Scholar]
- Levi, C.S.; Lesmes, U. Bi-compartmental elderly or adult dynamic digestion models applied to interrogate protein digestibility. Food Funct. 2014, 5, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- Aalaei, K.; Khakimov, B.; De Gobba, C.; Ahrné, L. Digestion patterns of proteins in pasteurized and ultra-high temperature milk using in vitro gastric models of adult and elderly. J. Food Eng. 2021, 292, 110305. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Yang, S.; Liu, G.; Munk, D.M.; Qin, Z.; Petersen, M.A.; Cardoso, D.R.; Otte, J.; Ahrné, L. Cycled high hydrostatic pressure processing of whole and skimmed milk: Effects on physicochemical properties. Innov. Food Sci. Emerg. Technol. 2020, 63, 102378. [Google Scholar] [CrossRef]
- Bogahawaththa, D.; Buckow, R.; Chandrapala, J.; Vasiljevic, T. Comparison between thermal pasteurization and high pressure processing of bovine skim milk in relation to denaturation and immunogenicity of native milk proteins. Innov. Food Sci. Emerg. Technol. 2018, 47, 301–308. [Google Scholar] [CrossRef]
- Olsen, K.; Orlien, V. 11-High-pressure processing for modification of food biopolymers. In Innovative Food Processing Technologies; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 291–313. [Google Scholar]
- Scollard, P.G.; Beresford, T.P.; Needs, E.C.; Murphy, P.M.; Kelly, A.L. Plasmin activity, β-lactoglobulin denaturation and proteolysis in high pressure treated milk. Int. Dairy J. 2000, 10, 835–841. [Google Scholar] [CrossRef]

| Composition % | Fat | Protein | Lactose | Total Solid | Solid Non-Fat |
|---|---|---|---|---|---|
| Raw milk | 5.26 ± 0.001 | 3.27 ± 0.003 | 4.41 ± 0.000 | 14.28 ± 0.010 | 9.07 ± 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aalaei, K.; Khakimov, B.; De Gobba, C.; Ahrné, L. Gastric Digestion of Milk Proteins in Adult and Elderly: Effect of High-Pressure Processing. Foods 2021, 10, 786. https://doi.org/10.3390/foods10040786
Aalaei K, Khakimov B, De Gobba C, Ahrné L. Gastric Digestion of Milk Proteins in Adult and Elderly: Effect of High-Pressure Processing. Foods. 2021; 10(4):786. https://doi.org/10.3390/foods10040786
Chicago/Turabian StyleAalaei, Kataneh, Bekzod Khakimov, Cristian De Gobba, and Lilia Ahrné. 2021. "Gastric Digestion of Milk Proteins in Adult and Elderly: Effect of High-Pressure Processing" Foods 10, no. 4: 786. https://doi.org/10.3390/foods10040786
APA StyleAalaei, K., Khakimov, B., De Gobba, C., & Ahrné, L. (2021). Gastric Digestion of Milk Proteins in Adult and Elderly: Effect of High-Pressure Processing. Foods, 10(4), 786. https://doi.org/10.3390/foods10040786








