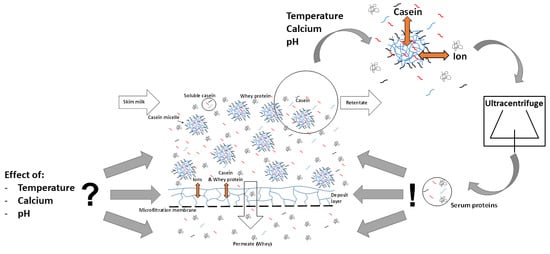
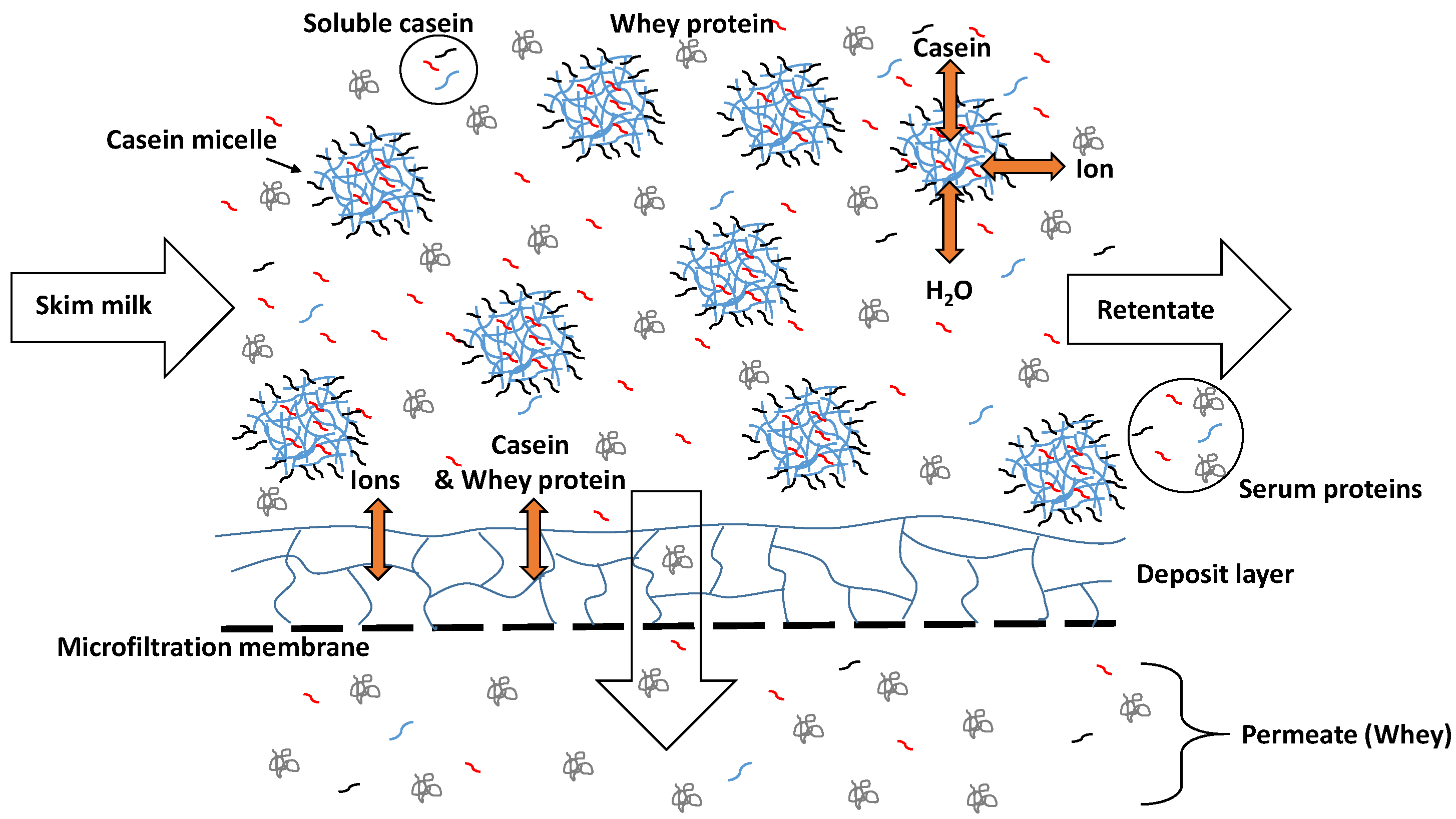
Effect of Temperature, Added Calcium and pH on the Equilibrium of Caseins between Micellar State and Milk Serum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Skim Milk and Preparation for Experiments
2.2. Separation of Micellar and Soluble Casein
2.3. Analyses
2.4. Data Evaluation and Statistics
3. Results and Discussion
3.1. Serum Concentration of β-Casein Lowers with Increasing Temperature
3.2. Effect of pH on the Serum Casein Concentration
3.3. Increasing Calcium and Phosphate Concentration in the Serum Phase with Decreasing pH
3.4. Decrease of the Serum Casein Concentration with Increasing Calcium Enrichemt
3.5. Decreasing Phosphate and Citrate Concentration after the Addition of Calcium
3.6. Influence of Calcium Concentration on the Serum Casein Concentration with pH Adjustment
3.7. Comparison of the Serum Casein Concentration after Ultracentrifugal Separation with the Concentration in Microfiltration Permeate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


| 6 °C | 8 °C | 10 °C | 12 °C | 14 °C | 16 °C | 18 °C | 20 °C | |
|---|---|---|---|---|---|---|---|---|
| pH [-] | 6.78 | 6.77 | 6.77 | 6.75 | 6.75 | 6.75 | 6.73 | 6.72 |
| Citrate [mg L−1] | Phosphate [mg L−1] | Chloride [mg L−1] |
|---|---|---|
| 1557 ± 81 | 1030 ± 39 | 838 ± 37 |
| Potassium [mg L−1] | Sodium [mg L−1] | Calcium [mg L−1] | Magnesium [mg L−1] |
|---|---|---|---|
| 1562 ± 70 | 391 ± 15 | 320 ± 31 | 79 ± 7 |


References
- Broyard, C.; Gaucheron, F. Modifications of structures and functions of caseins: A scientific and technological challenge. Dairy Sci. Technol. 2015, 95, 831–862. [Google Scholar] [CrossRef]
- Holt, C. Structure and Stability of Bovine Casein Micelles. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 1992; Volume 43, pp. 63–151. ISBN 9780120342433. [Google Scholar]
- De Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 2012, 171–172, 36–52. [Google Scholar] [CrossRef]
- Dickinson, E.; Hunt, J.A.; Horne, D.S. Calcium induced flocculation of emulsions containing adsorbed β-casein or phosvitin. Food Hydrocoll. 1992, 6, 359–370. [Google Scholar] [CrossRef]
- Mizuno, R.; Lucey, J.A. Effects of Emulsifying Salts on the Turbidity and Calcium-Phosphate–Protein Interactions in Casein Micelles. J. Dairy Sci. 2005, 88, 3070–3078. [Google Scholar] [CrossRef] [Green Version]
- Davies, D.T.; Law, A.J.R. Variation in the protein composition of bovine casein micelles and serum casein in relation to micellar size and milk temperature. J. Dairy Res. 1983, 50, 67–75. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Grinberg, V.Y.; de Kruif, C.G. Association behavior of β-casein. J. Colloid Interface Sci. 2003, 258, 33–39. [Google Scholar] [CrossRef]
- Ono, T.; Murayama, T.; Kaketa, S.; Odagiri, S. Changes in the Protein Composition and Size Distribution of Bovine Casein Micelles Induced by Cooling. Agric. Biol. Chem. 1990, 54, 1385–1392. [Google Scholar]
- Schiffer, S.; Matyssek, A.; Hartinger, M.; Bolduan, P.; Mund, P.; Kulozik, U. Effects of selective layer properties of ceramic multi-channel microfiltration membranes on the milk protein fractionation. Sep. Purif. Technol. 2020, 259, 118050. [Google Scholar] [CrossRef]
- France, T.C.; Kelly, A.L.; Crowley, S.V.; O’Mahony, J.A. The effects of temperature and transmembrane pressure on protein, calcium and plasmin partitioning during microfiltration of skim milk. Int. Dairy J. 2021, 114, 104930. [Google Scholar] [CrossRef]
- Zulewska, J.; Barbano, D.M. The effect of linear velocity and flux on performance of ceramic graded permeability membranes when processing skim milk at 50 °C. J. Dairy Sci. 2014, 97, 2619–2632. [Google Scholar] [CrossRef] [Green Version]
- Zulewska, J.; Newbold, M.; Barbano, D.M. Efficiency of serum protein removal from skim milk with ceramic and polymeric membranes at 50 °C. J. Dairy Sci. 2009, 92, 1361–1377. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, J.; Schubert, T.; Atamer, Z. Pilot-scale β-casein depletion from micellar casein via cold microfiltration in the diafiltration mode. Int. Dairy J. 2019, 97, 222–229. [Google Scholar] [CrossRef]
- Koutina, G.; Knudsen, J.C.; Andersen, U.; Skibsted, L.H. Temperature effect on calcium and phosphorus equilibria in relation to gel formation during acidification of skim milk. Int. Dairy J. 2014, 36, 65–73. [Google Scholar] [CrossRef]
- Chaplin, L.G.; Lyster, R.L.J. Effect of temperature on the pH of skim milk. J. Dairy Res. 1988, 55, 277–280. [Google Scholar] [CrossRef]
- Post, A.E.; Arnold, B.; Weiss, J.; Hinrichs, J. Effect of temperature and pH on the solubility of caseins: Environmental influences on the dissociation of α(S)- and β-casein. J. Dairy Sci. 2012, 95, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, S.; Vannieuwenhuyse, L.; Susianto, C.; Hartinger, M.; Kulozik, U. Influence of pH and calcium concentration on milk protein fractionation by 0.1 µm microfiltration at low temperatures. Int. Dairy J. 2021, 118, 105048. [Google Scholar] [CrossRef]
- Udabage, P.; McKinnon, I.R.; Augustin, M.A. Mineral and casein equilibria in milk: Effects of added salts and calcium-chelating agents. J. Dairy Res. 2000, 67, 361–370. [Google Scholar] [CrossRef]
- Deeth, H.C.; Lewis, M.J. Practical consequences of calcium addition to and removal from milk and milk products. Int. J. Dairy Technol. 2015, 68, 1–10. [Google Scholar] [CrossRef]
- Liu, D.Z.; Weeks, M.G.; Dunstan, D.E.; Martin, G.J.O. Temperature-dependent dynamics of bovine casein micelles in the range 10-40 °C. Food Chem. 2013, 141, 4081–4086. [Google Scholar] [CrossRef]
- Dumpler, J.; Wohlschläger, H.; Kulozik, U. Dissociation and coagulation of caseins and whey proteins in concentrated skim milk heated by direct steam injection. Dairy Sci. Technol. 2017, 96, 807–826. [Google Scholar] [CrossRef]
- Sedmerová, V.; Heleslcová, H.; Podrazký, V. On the composition of casein micelles isolated under different conditions of ultracentrifugation. Nahrung 1974, 18, 363–369. [Google Scholar] [CrossRef]
- Dumpler, J.; Kieferle, I.; Wohlschläger, H.; Kulozik, U. Milk ultrafiltrate analysis by ion chromatography and calcium activity for SMUF preparation for different scientific purposes and prediction of its supersaturation. Int. Dairy J. 2017, 68, 60–69. [Google Scholar] [CrossRef]
- Holt, C.; Davies, D.T.; Law, A.J.R. Effects of colloidal calcium phosphate content and free calcium ion concentration in the milk serum on the dissociation of bovine casein micelles. J. Dairy Res. 1986, 53, 557–572. [Google Scholar] [CrossRef]
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef] [Green Version]
- Swaisgood, H.E. Chemistry of the Caseins. In Advanced Dairy Chemistry—1 Proteins; Fox, P.F., McSweeney, P.L.H., Eds.; Springer US: Boston, MA, USA, 2003; pp. 139–201. ISBN 978-0-306-47271-8. [Google Scholar]
- Law, A.J.R.; Leaver, J. Effects of Acidification and Storage of Milk on Dissociation of Bovine Casein Micelles. J. Agric. Food Chem. 1998, 46, 5008–5016. [Google Scholar] [CrossRef]
- Gaucheron, F. The minerals of milk. Reprod. Nutr. Dev. 2005, 45, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.S. Casein Interactions: Casting Light on the Black Boxes, the Structure in Dairy Products. Int. Dairy J. 1998, 8, 171–177. [Google Scholar] [CrossRef]
- Philippe, M.; Gaucheron, F.; Le Graët, Y.; Michel, F.; Garem, A. Physicochemical characterization of calcium-supplemented skim milk. Lait 2003, 83, 45–59. [Google Scholar] [CrossRef]
- Philippe, M.; Le Graët, Y.; Gaucheron, F. The effects of different cations on the physicochemical characteristics of casein micelles. Food Chem. 2005, 90, 673–683. [Google Scholar] [CrossRef]
- Gaucheron, F. Milk Salts|Distribution and Analysis. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 908–916. ISBN 9780123744074. [Google Scholar]
- Hartinger, M.; Schiffer, S.; Heidebrecht, H.-J.; Dumpler, J.; Kulozik, U. Investigation on the spatial filtration performance in spiral-wound membranes–Influence and length-dependent adjustment of the transmembrane pressure. J. Membr. Sci. 2019, 591, 117311. [Google Scholar] [CrossRef]
- Heidebrecht, H.-J.; Toro-Sierra, J.; Kulozik, U. Concentration of Immunoglobulins in Microfiltration Permeates of Skim Milk: Impact of Transmembrane Pressure and Temperature on the IgG Transmission Using Different Ceramic Membrane Types and Pore Sizes. Foods 2018, 7, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Calcium [mg L−1] | Phosphate [mg L−1] | Citrate [mg L−1] | Magnesium [mg L−1] | |
|---|---|---|---|---|
| pH 6.3 | 460 ± 40 | 1316 ± 45 | 1768 ± 116 | 83 ± 5 |
| pH 6.5 | 400 ± 20 | 1189 ± 45 | 1479 ± 173 | 76 ± 4 |
| pH 7.0 | 315 ± 14 | 1090 ± 30 | 1500 ± 155 | 78 ± 4 |
| pH 7.3 | 314 ± 7 | 986 ± 27 | 1671 ± 59 | 75 ± 4 |
| Phosphate [mg L−1] | Citrate [mg L−1] | |
|---|---|---|
| 0.00 mM Ca2+ | 1029 ± 39 | 1557 ± 81 |
| 0.25 mM Ca2+ | 1014 ± 49 | 1541 ± 76 |
| 0.50 mM Ca2+ | 1068 ± 34 | 1597 ± 62 |
| 5.00 mM Ca2+ | 1013 ± 31 | 1427 ± 21 |
| 7.50 mM Ca2+ | 951 ± 26 | 1203 ± 65 |
| 0.5 mM Added Calcium | 5 mM Added Calcium | |
|---|---|---|
| Without pH adjustment | 0.27 ± 0.05 | 0.22 ± 0.04 |
| pH adjustment to 6.8 | 0.51 ± 0.02 | 0.47 ± 0.06 |
| pH adjustment to 7.3 | 0.51 ± 0.02 | 0.46 ± 0.05 |
| Native | 5 mM Added Calcium | ||||
|---|---|---|---|---|---|
| 10 [°C] | 14 [°C] | 10 [°C] | 14 [°C] | ||
| Serum casein conc. [g L−1] | MF permeate | 0.83 | 0.70 | 0.37 | 0.35 |
| Supernatant | 3.05 | 2.43 | 1.57 | 1.4 | |
| Relative serum casein permeation [%] | 27 | 29 | 24 | 25 | |
| Whey protein conc. in MF permeate [g L−1] | 1.9 | 2.1 | 1.3 | 1.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiffer, S.; Scheidler, E.; Kiefer, T.; Kulozik, U. Effect of Temperature, Added Calcium and pH on the Equilibrium of Caseins between Micellar State and Milk Serum. Foods 2021, 10, 822. https://doi.org/10.3390/foods10040822
Schiffer S, Scheidler E, Kiefer T, Kulozik U. Effect of Temperature, Added Calcium and pH on the Equilibrium of Caseins between Micellar State and Milk Serum. Foods. 2021; 10(4):822. https://doi.org/10.3390/foods10040822
Chicago/Turabian StyleSchiffer, Simon, Eva Scheidler, Tim Kiefer, and Ulrich Kulozik. 2021. "Effect of Temperature, Added Calcium and pH on the Equilibrium of Caseins between Micellar State and Milk Serum" Foods 10, no. 4: 822. https://doi.org/10.3390/foods10040822
APA StyleSchiffer, S., Scheidler, E., Kiefer, T., & Kulozik, U. (2021). Effect of Temperature, Added Calcium and pH on the Equilibrium of Caseins between Micellar State and Milk Serum. Foods, 10(4), 822. https://doi.org/10.3390/foods10040822