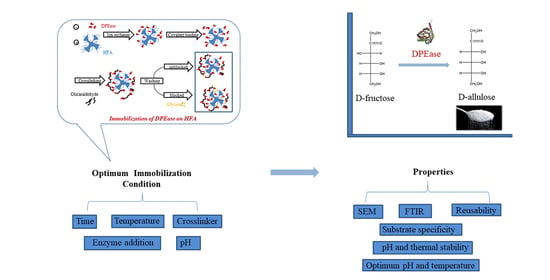
Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment
Abstract
Share and Cite
Bu, Y.; Zhang, T.; Jiang, B.; Chen, J. Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment. Foods 2021, 10, 831. https://doi.org/10.3390/foods10040831
Bu Y, Zhang T, Jiang B, Chen J. Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment. Foods. 2021; 10(4):831. https://doi.org/10.3390/foods10040831
Chicago/Turabian StyleBu, Yifan, Tao Zhang, Bo Jiang, and Jingjing Chen. 2021. "Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment" Foods 10, no. 4: 831. https://doi.org/10.3390/foods10040831
APA StyleBu, Y., Zhang, T., Jiang, B., & Chen, J. (2021). Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment. Foods, 10(4), 831. https://doi.org/10.3390/foods10040831