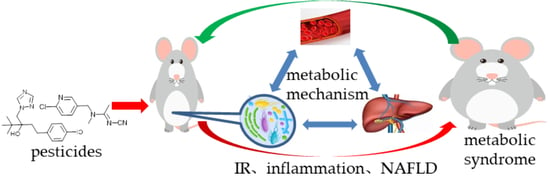
Gut Flora-Mediated Metabolic Health, the Risk Produced by Dietary Exposure to Acetamiprid and Tebuconazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. Sampling
2.3. Measurement of Mouse Glucose Tolerance
2.4. Measurement of Mouse Insulin Resistance
2.5. Serum Biochemical Assays
2.6. Inflammatory Factor Assays
2.7. Evaluation of In Vivo Intestinal Permeability
2.8. Histological Observation
2.9. Targeted and Untargeted Metabolic Profiling Analysis
2.10. Gut Flora Analysis
2.11. Intervention to Metabolic Disorders
2.12. Correlation and Statistical Analysis
3. Results
3.1. Effects of Exposure on Mouse Bodyweight
3.2. Effects of Exposure on Glucose Tolerance and Insulin Resistance
3.3. Serum Biochemistry Analysis
3.4. Evaluation of Mouse Inflammation
3.5. Detection of Lipopolysaccharide and Intestinal Permeability
3.6. Tissue Histology
3.7. Effects of Pesticide Exposure on the Diversity of Gut Flora Community Structure
3.8. Species Changes in Gut Flora
3.9. Metabolic Profiling of Gut Flora
3.10. Association of Gut Flora with Their Metabolism
3.11. Effects of Exposure on Host Circulation Metabolism
3.12. Association between Metabolites of Gut Flora and Host Circulation
3.13. Host Liver Metabolism and Gut–Liver Dialogue under Pesticide Exposure
3.14. Effects of Interventions on Physiology of Pesticide-Exposed Mice
4. Discussions
4.1. Effects of Pesticide Exposure on Physiological Phenotype
4.2. Relationships Among Gut Bacteria, Host and Metabolic Disease Risk
4.3. Effects of Interventions on Stressed Host
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Li, Z.; Hu, S.; Zhang, J.; Wu, J.; Shao, N.; Bo, X.; Ni, M.; Ying, X. Gut metagenomes of type 2 diabetic patients have characteristic single-nucleotide polymorphism distribution in Bacteroides coprocola. Microbiome 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Chassaing, B.; Zhang, L.; Yeoh, B.S.; Xiao, X.; Kumar, M.; Baker, M.T.; Cai, J.; Walker, R.; Borkowski, K.; et al. Microbiota-Dependent Hepatic Lipogenesis Mediated by Stearoyl CoA Desaturase 1 (SCD1) Promotes Metabolic Syndrome in TLR5-Deficient Mice. Cell Metab. 2015, 22, 983–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Kniss, A.R. Long-term trends in the intensity and relative toxicity of herbicide use. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.-H.; Lind, P.M.; Jacobs, D.R., Jr.; Salihovic, S.; van Bavel, B.; Lind, L. Polychlorinated Biphenyls and Organochlorine Pesticides in Plasma Predict Development of Type 2 Diabetes in the Elderly. Diabetes Care 2011, 34, 1778–1784. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhan, J.; Liu, D.; Luo, M.; Han, J.; Liu, X.; Liu, C.; Cheng, Z.; Zhou, Z.; Wang, P. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Trinder, M.; McDowell, T.W.; Daisley, B.A.; Ali, S.N.; Leong, H.S.; Sumarah, M.W.; Reid, G. Probiotic Lactobacillus rhamnosus Reduces Organophosphate Pesticide Absorption and Toxicity to Drosophila melanogaster. Appl. Environ. Microbiol. 2016, 82, 6204–6213. [Google Scholar] [CrossRef] [Green Version]
- Tsiaoussis, J.; Antoniou, M.N.; Koliarakis, I.; Mesnage, R.; Vardavas, C.I.; Izotov, B.N.; Psaroulaki, A.; Tsatsakis, A. Effects of single and combined toxic exposures on the gut microbiome: Current knowledge and future directions. Toxicol. Lett. 2019, 312, 72–97. [Google Scholar] [CrossRef]
- Nambu, T.; Matsuda, Y.; Matsuo, K.; Kanai, Y.; Yonemitsu, S.; Muro, S.; Oki, S. Liraglutide administration in type2 diabetic patients who either received no previous treatment or were treated with an oral hypoglycemic agent showed greater efficacy than that in patients switching from insulin. J. Diabetes Investig. 2013, 4, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Iovino, S.; Burkart, A.M.; Kriauciunas, K.; Warren, L.; Hughes, K.J.; Molla, M.; Lee, Y.-K.; Patti, M.-E.; Kahn, C.R. Genetic Insulin Resistance Is a Potent Regulator of Gene Expression and Proliferation in Human iPS Cells. Diabetes 2014, 63, 4130–4142. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.-H.; Chen, W.-B.; Shi, L.; Jiang, X.; Li, K.; Wang, Y.-X.; Liu, Y.-Q. The Underlying Mechanisms of Curcumin Inhibition of Hyperglycemia and Hyperlipidemia in Rats Fed a High-Fat Diet Combined With STZ Treatment. Molecules 2020, 25, 271. [Google Scholar] [CrossRef] [Green Version]
- Tie, Y.; Zheng, H.; He, Z.; Yang, J.; Shao, B.; Liu, L.; Luo, M.; Yuan, X.; Liu, Y.; Zhang, X.; et al. Targeting folate receptor beta positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct. Target. Ther. 2020, 5. [Google Scholar] [CrossRef]
- Belmadi, N.; Berchel, M.; Denis, C.; Berthe, W.; Sibiril, Y.; Le Gall, T.; Haelters, J.-P.; Jaffres, P.-A.; Montier, T. Evaluation of New Fluorescent Lipophosphoramidates for Gene Transfer and Biodistribution Studies after Systemic Administration. Int. J. Mol. Sci. 2015, 16, 26055–26076. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, P.; Shivappa, N.; Hebert, J.R.; Nasab, S.J.; Bahrami, A.; Hekmatdoost, A.; Rashidkhani, B.; Sadeghi, A.; Houshyari, M.; Hejazi, E. Dietary Inflammatory Index and Odds of Colorectal Cancer and Colorectal Adenomatous Polyps in a Case-Control Study from Iran. Nutrients 2019, 11, 1213. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.H.; Kosmina, R.; Ryden, M.; Baun, C.; Hvidsten, S.; Andersen, M.S.; Christensen, L.L.; Gastaldelli, A.; Marraccini, P.; Arner, P.; et al. The imprinted gene Delta like non-canonical notch ligand 1 (Dlk1) associates with obesity and triggers insulin resistance through inhibition of skeletal muscle glucose uptake. Ebiomedicine 2019, 46, 368–380. [Google Scholar] [CrossRef] [Green Version]
- Fabbiano, S.; Suarez-Zamorano, N.; Chevalier, C.; Lazarevic, V.; Kieser, S.; Rigo, D.; Leo, S.; Veyrat-Durebex, C.; Gaia, N.; Maresca, M.; et al. Functional Gut Microbiota Remodeling Contributes to the Caloric Restriction-Induced Metabolic Improvements. Cell Metab. 2018, 28, 907. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Littman, D.R. The Microbiome in Infectious Disease and Inflammation. Annu. Review Immunol. 2012, 30, 759–795. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.-J.; Zhang, Y.; Gerhard, M.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.-L.; Bajbouj, M.; Suchanek, S.; Liu, W.-D.; et al. Association Between Gut Microbiota and Helicobacter pylori-Related Gastric Lesions in a High-Risk Population of Gastric Cancer. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Zitvogel, L.; Kroemer, G. Immunostimulatory gut bacteria. Science 2019, 366, 1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.B.; Luo, C.; O’Connell, D.J.; Lefkovith, A.; Brown, E.M.; Yassour, M.; Varma, M.; Abelin, J.G.; Conway, K.L.; Jasso, G.J.; et al. Antigen discovery and specification of immunodominance hierarchies for MHCII-restricted epitopes. Nat. Med. 2018, 24, 1762. [Google Scholar] [CrossRef] [PubMed]
- McCracken, R.O.; Lipkowitz, K.B. Structure-activity relationships of benzothiazole and benzimidazole anthelmintics: A molecular modeling approach to in vivo drug efficacy. J. Parasitol. 1990, 76, 853–864. [Google Scholar] [CrossRef]
- Citi, S. Intestinal barriers protect against disease. Science 2018, 359, 1097–1098. [Google Scholar] [CrossRef]
- Manor, O.; Zubair, N.; Conomos, M.P.; Xu, X.; Rohwer, J.E.; Krafft, C.E.; Lovejoy, J.C.; Magis, A.T. A Multi-omic Association Study of Trimethylamine N-Oxide. Cell Rep. 2018, 24, 935–946. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; Molinaro, A.; Stahlman, M.; Khan, M.T.; Schmidt, C.; Manneras-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947. [Google Scholar] [CrossRef] [Green Version]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Lynes, M.I.; Leiria, L.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue (vol 23, pg 631, 2017). Nat. Med. 2017, 23, 1384. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859. [Google Scholar] [CrossRef]
- Koh, A.; Backhed, F. From Association to Causality: The Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef]
- Razquin, C.; Toledo, E.; Clish, C.B.; Ruiz-Canela, M.; Dennis, C.; Corella, D.; Papandreou, C.; Ros, E.; Estruch, R.; Guasch-Ferre, M.; et al. Plasma Lipidomic Profiling and Risk of Type 2 Diabetes in the PREDIMED Trial. Diabetes Care 2018, 41, 2617–2624. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428. [Google Scholar] [CrossRef]
- Meneses-León, J.; León-Maldonado, L.; Macías, N.; Torres-Ibarra, L.; Hernández-López, R.; Rivera-Paredez, B.; Flores, M.; Flores, Y.N.; Barrientos-Gutiérrez, T.; Quezada-Sánchez, A.D.; et al. Sugar-sweetened beverage consumption and risk of hyperuricemia: A longitudinal analysis of the Health Workers Cohort Study participants in Mexico. Am. J. Clin. Nutr. 2020, 112, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Morita, J.; Kano, K.; Kato, K.; Takita, H.; Sakagami, H.; Yamamoto, Y.; Mihara, E.; Ueda, H.; Sato, T.; Tokuyama, H.; et al. Structure and biological function of ENPP6, a choline-specific glycerophosphodiester-phosphodiesterase. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Oranje, A.P.; Riezebos, P.; van Toorenenbergen, A.W.; Mulder, P.G.H.; Heide, R.; Tank, B. Urinary N-methylhistamine as an indicator of bone marrow involvement in mastocytosis. Clin. Exp. Dermatol. 2002, 27, 502–506. [Google Scholar] [CrossRef]
- Vandekerckhove, E.; Janssens, F.; Tate, D.; De Looze, D. Treatment of Gut Fermentation Syndrome With Fecal Microbiota Transplantation. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Summers, S.A. Could Ceramides Become the New Cholesterol? Cell Metab. 2018, 27, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Taft, D.H.; Maldonado-Gomez, M.X.; Johnson, D.; Treiber, M.L.; LemayQ, D.G.; DePeters, E.J.; Mills, D.A. The fecal resistome of dairy cattle is associated with diet during nursing. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D. The Microbiome, Plasma Metabolites, Dietary Habits, and Cardiovascular Risk Unravelling Their Interplay. Circ. Res. 2019, 124, 1695–1696. [Google Scholar] [CrossRef] [PubMed]
- Kurilshikov, A.; van den Munckhof, I.C.L.; Chen, L.; Bonder, M.J.; Schraa, K.; Rutten, J.H.W.; Riksen, N.P.; de Graaf, J.; Oosting, M.; Sanna, S.; et al. Gut Microbial Associations to Plasma Metabolites Linked to Cardiovascular Phenotypes and Risk A Cross-Sectional Study. Circ. Res. 2019, 124, 1808–1820. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhao, F.; Xu, Y.; Qiu, J.; Qian, Y. Gut Flora-Mediated Metabolic Health, the Risk Produced by Dietary Exposure to Acetamiprid and Tebuconazole. Foods 2021, 10, 835. https://doi.org/10.3390/foods10040835
Liu J, Zhao F, Xu Y, Qiu J, Qian Y. Gut Flora-Mediated Metabolic Health, the Risk Produced by Dietary Exposure to Acetamiprid and Tebuconazole. Foods. 2021; 10(4):835. https://doi.org/10.3390/foods10040835
Chicago/Turabian StyleLiu, Jingkun, Fangfang Zhao, Yanyang Xu, Jing Qiu, and Yongzhong Qian. 2021. "Gut Flora-Mediated Metabolic Health, the Risk Produced by Dietary Exposure to Acetamiprid and Tebuconazole" Foods 10, no. 4: 835. https://doi.org/10.3390/foods10040835
APA StyleLiu, J., Zhao, F., Xu, Y., Qiu, J., & Qian, Y. (2021). Gut Flora-Mediated Metabolic Health, the Risk Produced by Dietary Exposure to Acetamiprid and Tebuconazole. Foods, 10(4), 835. https://doi.org/10.3390/foods10040835