The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances
Abstract
:1. Introduction
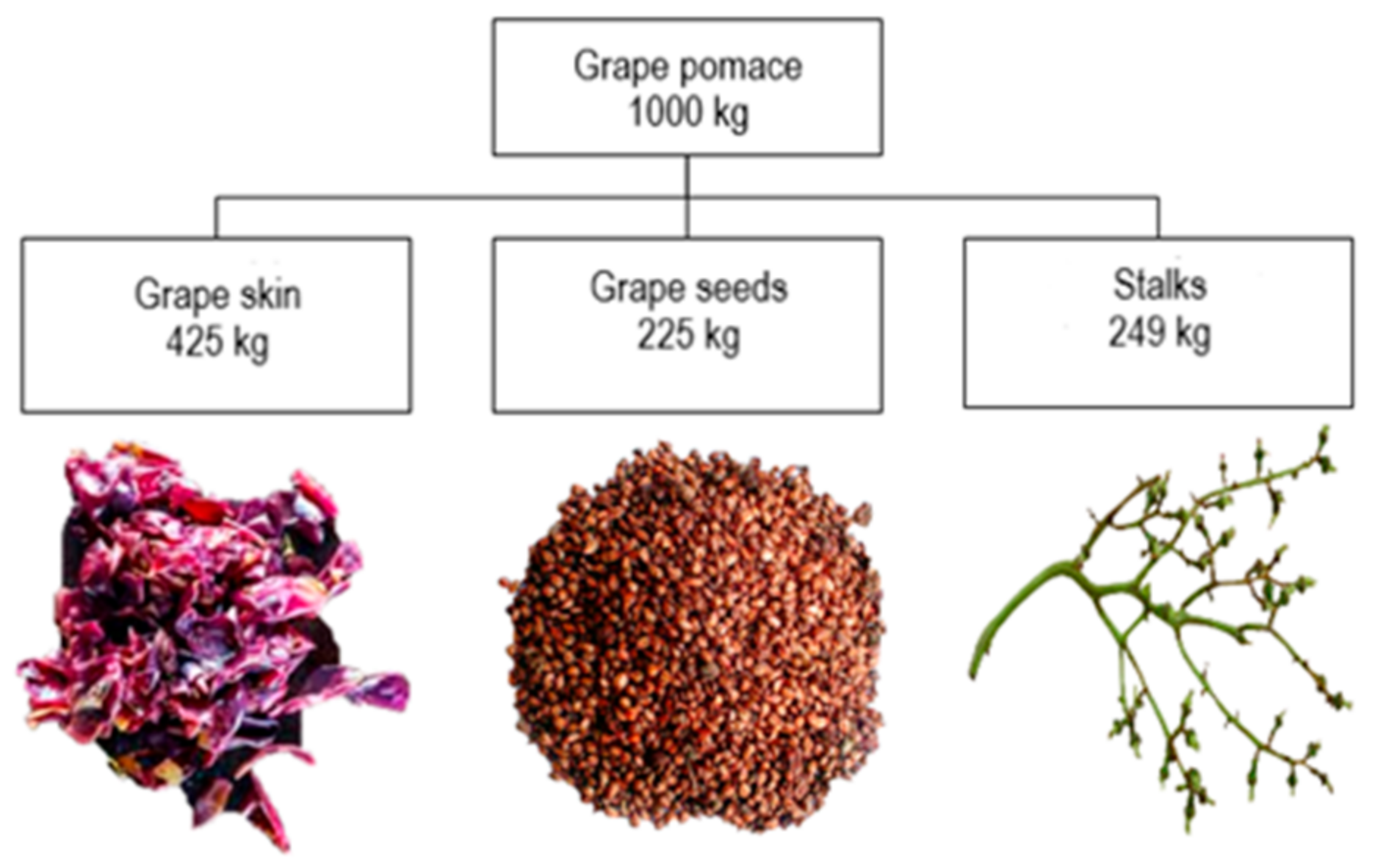
2. Structure and Composition of Grape Pomace
2.1. Structure and Composition of Grape Skin
2.1.1. Polysaccharide Structures in Grape Skin
The Cellulose–Xyloglucan Framework
Pectin Polysaccharides
Role of Ferulic Acid in Cross-Links among Sugars
Lignin
2.1.2. Phenolic Compounds
- phenolic compounds which are localized in the CW, are bounded to polysaccharides by hydrophobic interactions or hydrogen bonds
- phenolic compounds which are not localized in the CW, are retained in the vacuoles of plant cells or bounded to the cell nucleus
Cell-Wall Linked Phenolic Compounds
Non-Cell-Wall Phenolic Compounds
2.2. Structure and Composition of Grape Seeds
2.2.1. Grape Seed Oil
Hydrophilic Compounds
Lipophilic Compounds
2.2.2. Phenolic Compounds
2.3. Structure and Composition of Grape Inflorescence Architectures
2.3.1. Ligno-Cellulosic Compounds
2.3.2. Phenolic Compounds
3. Grape Pomace Pectic Substances
3.1. Extraction of Pectin from Grape Pomace
3.2. Pectin Characterization of Grape Pomace
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nerantzis, E.; Tataridis, P. Integrated enology utilization of winery by-products into high added value products. E-J. Sci. Technol. 2006, 1, 79–89. [Google Scholar]
- Dávila, I.; Robles, E.; Egüés, I.; Labidi, J.; Gullón, P. The biorefinery concept for the industrial valorization of grape processing by-products. In Handbook of Grape Processing By-Products—Suitable Solutions; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 29–53. ISBN 9780128098707. [Google Scholar]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Amendola, D.; De Faveri, D.M.; Egües, I.; Serrano, L.; Labidi, J.; Spigno, G. Autohydrolysis and organosolv process for recovery of hemicelluloses, phenolic compounds and lignin from grape stalks. Bioresour. Technol. 2012, 107, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.C.; Meza-Velázquez, J.A.; Simal, S.; Rosselló, C. Ultrasound-assisted extraction of pectins from grape pomace using citric acid: A response surface methodology approach. Carbohydr. Polym. 2014, 106, 179–189. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [Green Version]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [Green Version]
- Boussetta, N.; Lanoisellé, J.L.; Bedel-Cloutour, C.; Vorobiev, E. Extraction of soluble matter from grape pomace by high voltage electrical discharges for polyphenol recovery: Effect of sulphur dioxide and thermal treatments. J. Food Eng. 2009, 95, 192–198. [Google Scholar] [CrossRef]
- Panouillé, M.; Ralet, M.-C.; Bonnin, E.; Thibault, J.-F. Recovery and reuse of trimmings and pulps from fruit and vegetable processing. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Waldron, K.W., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2007; pp. 417–447. ISBN 9781845690250. [Google Scholar]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Ye, Z.; Harrison, R.; Cheng, V.J.; Bekhit, A.E.A. Wine making by-products. In Valorization of Wine Making By-Products; CRC Press: Boca Raton, FL, USA, 2016; pp. 73–116. ISBN 9781482255331. [Google Scholar]
- Sanhueza, L.; Tello, M.; Vivanco, M.; Mendoza, L.; Wilkens, M. Relation between antibacterial activity against food transmitted pathogens and total phenolic compounds in grape pomace extracts from Cabernet Sauvignon and Syrah varieties. Adv. Microbiol. 2014, 4, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Lafka, T.-I.; Sinanoglou, V.; Lazos, E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Mendes, J.A.S.; Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Towards comprehensive utilization of winemaking residues: Characterization of grape skins from red grape pomaces of variety Touriga Nacional. Ind. Crops Prod. 2013, 43, 25–32. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape seed oil compounds: Biological and chemical actions for health. Nutr. Metab. Insights 2016, 9, NMI.S32910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beres, C.; Simas-Tosin, F.F.; Cabezudo, I.; Freitas, S.P.; Iacomini, M.; Mellinger-Silva, C.; Cabral, L.M.C. Antioxidant dietary fibre recovery from Brazilian Pinot noir grape pomace. Food Chem. 2016, 201, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Brazinha, C.; Cadima, M.; Crespo, J.G. Optimization of extraction of bioactive compounds from different types of grape pomace produced at wineries and distilleries. J. Food Sci. 2014, 79, E1142–E1149. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Guchu, E.; Ebeler, S.E.; Lee, J.; Mitchell, A.E. Monitoring selected monomeric polyphenol composition in pre- and post-fermentation products of Vitis vinifera L. cv. Airén and cv. Grenache noir. LWT Food Sci. Technol. 2015, 60, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; Tommasi, N. De Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L.) byproducts. J. Food Sci. Technol. 2015, 52, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Amico, V.; Chillemi, R.; Mangiafico, S.; Spatafora, C.; Tringali, C. Polyphenol-enriched fractions from Sicilian grape pomace: HPLC-DAD analysis and antioxidant activity. Bioresour. Technol. 2008, 99, 5960–5966. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef] [PubMed]
- De Campos, L.M.A.S.; Leimann, F.V.; Pedrosa, R.C.; Ferreira, S.R.S. Free radical scavenging of grape pomace extracts from Cabernet sauvingnon (Vitis vinifera). Bioresour. Technol. 2008, 99, 8413–8420. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.d.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Bravo, L.; Saura-Calixto, F. Characterization of dietary fiber and the in vitro indigestible fraction of grape pomace. Am. J. Enol. Vitic. 1998, 49, 135–141. [Google Scholar]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Effect of grape antioxidant dietary fibre on the prevention of lipid oxidation in minced fish: Evaluation by different methodologies. Food Chem. 2007, 101, 372–378. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Ziarati, P.; Moshiri, I.M.; Sadeghi, P.; Mohammadi, S. Grape pomace flour (Vitis spp.) from Shiraz in South of Iran by high trace mineral elements as food supplements. SciFed Drug Deliv. Res. J. 2017, 1, 1–9. [Google Scholar]
- Boonchu, T.; Utama-ang, N. Optimization of extraction and microencapsulation of bioactive compounds from red grape (Vitis vinifera L.) pomace. J. Food Sci. Technol. 2015, 52, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; de Morais, S.M.; de Lima, A.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Hernández-Hierro, J.M.; José Heredia, F.; Byrne, H.J. Linking ATR-FTIR and Raman features to phenolic extractability and other attributes in grape skin. Talanta 2017, 167, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulao, L.F.; Fernandes, J.C.; Lopes, P.; Amâncio, S. Tackling the cell wall of the grape berry. In The Biochemistry of the Grape Berry; Bentham Science Publishers: Bussum, The Netherlands, 2012; pp. 172–193. ISBN 978-1-60805-540-1. [Google Scholar]
- Spigno, G.; Pizzorno, T.; De Faveri, D.M. Cellulose and hemicelluloses recovery from grape stalks. Bioresour. Technol. 2008, 99, 4329–4337. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Evaluation of grape stalks as a bioresource. Ind. Crops Prod. 2011, 33, 200–204. [Google Scholar] [CrossRef]
- Prozil, S.O.; Costa, E.V.; Evtuguin, D.V.; Cruz Lopes, L.P.; Domingues, M.R.M. Structural characterization of polysaccharides isolated from grape stalks of Vitis vinifera L. Carbohydr. Res. 2012, 356, 252–259. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Gómez-Plaza, E.; Terrier, N.; Doco, T.; Ros-García, J.M. The composition of cell walls from grape skin in Vitis vinifera intraspecific hybrids. J. Sci. Food Agric. 2017, 97, 4029–4035. [Google Scholar] [CrossRef]
- Gao, Y.; Zietsman, A.J.J.; Vivier, M.A.; Moore, J.P. Deconstructing wine grape cell walls with enzymes during winemaking: New insights from glycan microarray technology. Molecules 2019, 24, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef] [Green Version]
- Kannam, S.K.; Oehme, D.P.; Doblin, M.S.; Gidley, M.J.; Bacic, A.; Downton, M.T. Hydrogen bonds and twist in cellulose microfibrils. Carbohydr. Polym. 2017, 175, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Posé, S.; Paniagua, C.; Matas, A.J.; Gunning, A.P.; Morris, V.J.; Quesada, M.A.; Mercado, J.A. A nanostructural view of the cell wall disassembly process during fruit ripening and postharvest storage by atomic force microscopy. Trends Food Sci. Technol. 2019, 87, 47–58. [Google Scholar] [CrossRef]
- Brahem, M.; Renard, C.M.G.C.; Gouble, B.; Bureau, S.; Le Bourvellec, C. Characterization of tissue specific differences in cell wall polysaccharides of ripe and overripe pear fruit. Carbohydr. Polym. 2017, 156, 152–164. [Google Scholar] [CrossRef]
- Arnous, A.; Meyer, A.S. Quantitative prediction of cell wall polysaccharide composition in grape (Vitis vinifera L.) and apple (Malus domestica) skins from acid hydrolysis monosaccharide profiles. J. Agric. Food Chem. 2009, 57, 3611–3619. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 2015, 56, 180–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Zhao, J.; Wang, X.; Guo, Y.; Han, Y.; Zhou, J. Lignin structure and solvent effects on the selective removal of condensed units and enrichment of S-type lignin. Polymers 2018, 10, 967. [Google Scholar] [CrossRef] [Green Version]
- Gil Cortiella, M.; Peña-Neira, Á. Extraction of soluble polysaccharides from grape skins. Cienc. Investig. Agrar. 2017, 44, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hanlin, R.L.; Hrmova, M.; Harbertson, J.F.; Downey, M.O. Review: Condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Aust. J. Grape Wine Res. 2010, 16, 173–188. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Peled, M.; Urbanowicz, B.R.; O’Neill, M.A.; Debolt, S.; Scheller, H.; Berkeley, L. The synthesis and origin of the pectic polysaccharide rhamnogalacturonan II—Insights from nucleotide sugar formation and diversity. Front. Plant Sci. 2012, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fasoli, M.; Dell’Anna, R.; Dal Santo, S.; Balestrini, R.; Sanson, A.; Pezzotti, M.; Monti, F.; Zenoni, S. Pectins, Hemicelluloses and Pectins, hemicelluloses and celluloses show specific dynamics in the internal and external surfaces of grape berry skin during ripening. Plant Cell Physiol. 2016, 57, 1332–1349. [Google Scholar] [CrossRef] [Green Version]
- Zietsman, A.J.J.; Moore, J.P.; Fangel, J.U.; Willats, W.G.T.; Trygg, J.; Vivier, M.A. Following the compositional changes of fresh grape skin cell walls during the fermentation process in the presence and absence of maceration enzymes. J. Agric. Food Chem. 2015, 63, 2798–2810. [Google Scholar] [CrossRef]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, S.E.; Schols, H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018, 192, 263–272. [Google Scholar] [CrossRef]
- Zdunek, A.; Kozioł, A.; Pieczywek, P.M.; Cybulska, J. Evaluation of the nanostructure of pectin, hemicellulose and cellulose in the cell walls of pears of different texture and firmness. Food Bioprocess Technol. 2014, 7, 3525–3535. [Google Scholar] [CrossRef] [Green Version]
- Bunzel, M.; Ralph, J.; Steinhart, H. Phenolic compounds as cross-links of plant derived polysaccharides. Czech J. Food Sci. 2004, 22, S64–S67. [Google Scholar] [CrossRef]
- Mnich, E.; Bjarnholt, N.; Eudes, A.; Harholt, J.; Holland, C.; Jørgensen, B.; Larsen, F.H.; Liu, M.; Manat, R.; Meyer, A.S.; et al. Phenolic cross-links: Building and de-constructing the plant cell wall. Nat. Prod. Rep. 2020, 37, 919–961. [Google Scholar] [CrossRef]
- De O. Buanafina, M.M.; Fescemyer, H.W. Modification of esterified cell wall phenolics increases vulnerability of tall fescue to herbivory by the fall armyworm. Planta 2012, 236, 513–523. [Google Scholar] [CrossRef]
- Grabber, J.H.; Ralph, J.; Hatfield, R.D. Model studies of ferulate-coniferyl alcohol cross-product formation in primary maize walls: Implications for lignifaction in grasses. J. Agric. Food Chem. 2002, 50, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Waldron, K.W.; Parr, A.J.; Ng, A.; Ralph, J. Cell wall esterified phenolic dimers: Identification and quantification by reverse phase high performance liquid chromatography and diode array detection. Phytochem. Anal. 1996, 7, 305–312. [Google Scholar] [CrossRef]
- Hatfield, R.; Ralph, J.; Grabber, J.H. A potential role for sinapyl p-coumarate as a radical transfer mechanism in grass lignin formation. Planta 2008, 228, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-X.; Sun, R.; Sun, X.-F.; Su, Y. Fractional and physico-chemical characterization of hemicelluloses from ultrasonic irradiated sugarcane bagasse. Carbohydr. Res. 2004, 339, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chantreau, M.; Sibout, R.; Hawkins, S. Plant cell wall lignification and monolignol metabolism. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murciano Martínez, P.; Punt, A.M.; Kabel, M.A.; Gruppen, H. Deconstruction of lignin linked p -coumarates, ferulates and xylan by NaOH enhances the enzymatic conversion of glucan. Bioresour. Technol. 2016, 216, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Sun, R.-C.; Sun, J.-X.; Liu, C.-F.; He, B.-H.; Fan, J.-S. Determination of cell wall ferulic and p-coumaric acids in sugarcane bagasse. Anal. Chim. Acta 2005, 552, 207–217. [Google Scholar] [CrossRef]
- Grabber, J.H. How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Sci. 2005, 45, 820–831. [Google Scholar] [CrossRef] [Green Version]
- Timokhin, V.I.; Regner, M.; Motagamwala, A.H.; Sener, C.; Karlen, S.D.; Dumesic, J.A.; Ralph, J. Production of p-coumaric ccid from corn GVL-lignin. ACS Sustain. Chem. Eng. 2020, 8, 17427–17438. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Interactions between cell wall polysaccharides and polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1808–1831. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber-polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of polyphenols to plant cell wall analogues—Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; Van Breusegem, F.; Munné-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Saslowsky, D.E.; Warek, U.; Winkel, B.S.J. Nuclear localization of flavonoid enzymes in Arabidopsis. J. Biol. Chem. 2005, 280, 23735–23740. [Google Scholar] [CrossRef] [Green Version]
- Bouriche, H.; Kada, S.; Senator, A.; Demirtas, I.; Ozen, T.; Toptanci, B.Ç.; Kizil, G.; Kizil, M. Phenolic content and biomolecule oxidation protective activity of Globularia alypum extracts. Arch. Biol. Technol. 2017, 60, 17160409. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J. Antioxidant and antiproliferative properties of a tocotrienol-rich fraction from grape seeds. Food Chem. 2009, 114, 1386–1390. [Google Scholar] [CrossRef]
- Mironeasa, S.; Leahu, A.; Codină, G.-G.; Stroe, G.-S.; Mironeasa, C. Grape seed: Physico-chemical, structural characteristics and oil content. J. Agroaliment. Process. Technol. 2010, 16, 1–6. [Google Scholar]
- Shinagawa, F.B.; de Santana, F.C.; Torres, L.R.O.; Mancini-Filho, J. Grape seed oil: A potential functional food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Beveridge, T.H.J.; Girard, B.; Kopp, T.; Drover, J.C.G. Yield and composition of grape seed oil extracted by supercritical carbon dioxide and petroleum ether: Varietal effects. J. Agric. Food Chem. 2005, 53, 1799–1804. [Google Scholar] [CrossRef]
- Bjelica, M.; Vujasinović, V.; Rabrenović, B.; Dimić, S. Some chemical characteristics and oxidative stability of cold pressed grape seed oils obtained from different winery waste. Eur. J. Lipid Sci. Technol. 2019, 121, 1800416. [Google Scholar] [CrossRef]
- Bordiga, M. Valorization of Wine Making By-Products; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429158902. [Google Scholar]
- Licev, V.; Bojinov, A.; Dimov, C. Izlndovane na lignina v grazdovite semki. Lozar. Vinar. 1974, 5, 29–32. [Google Scholar]
- Cotea, V.D. Tratat de Oenologie; Ceres: Bucharest, Romania, 1985; pp. 147–152. [Google Scholar]
- Shinagawa, F.B.; de Santana, F.C.; Araujo, E.; Purgatto, E.; Mancini-Filho, J. Chemical composition of cold pressed Brazilian grape seed oil. Food Sci. Technol. 2017, 38, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Cadot, Y.; Miñana-Castelló, M.T.; Chevalier, M. Anatomical, histological, and histochemical changes in grape seeds from Vitis vinifera L. cv Cabernet franc during fruit development. J. Agric. Food Chem. 2006, 54, 9206–9215. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Castello, J.; Van Hecke, E.; Lanoisellé, J.-L. Optimization of oil yield and oil total phenolic content during grape seed cold screw pressing. Ind. Crops Prod. 2015, 63, 26–33. [Google Scholar] [CrossRef]
- Duba, K.S.; Fiori, L. Supercritical CO2 extraction of grape seed oil: Effect of process parameters on the extraction kinetics. J. Supercrit. Fluids 2015, 98, 33–43. [Google Scholar] [CrossRef]
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Karasu, S.; Tornuk, F.; Toker, O.S.; Geçgel, Ü.; Sagdic, O.; Ozcan, N.; Gül, O. Recovery potential of cold press byproducts obtained from the edible oil industry: Physicochemical, bioactive, and antimicrobial properties. J. Agric. Food Chem. 2015, 63, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelmann, W. Quantification of the main constituents of some authentic grape-seed oils of different origin. J. Agric. Food Chem. 2006, 54, 6261–6265. [Google Scholar] [CrossRef]
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Kamiloglu, O.; Demirkeser, O. Fatty acid composition, total phenolic content and antioxidamt activity of grape seed oils obtained by cold-pressed and solvent extraction. Indian J. Pharm. Educ. Res. 2019, 53, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, L.; Casal, S.; Cruz, R.; Pereira, J.A.; Ramalhosa, E. Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Res. Int. 2013, 50, 161–166. [Google Scholar] [CrossRef]
- Assumpção, C.F.; Nunes, I.L.; Mendonça, T.A.; Bortolin, R.C.; Jablonski, A.; Flôres, S.H.; de Oliveira Rios, A. Bioactive compounds and stability of organic and conventional Vitis labrusca grape seed oils. J. Am. Oil Chem. Soc. 2016, 93, 115–124. [Google Scholar] [CrossRef]
- Navas, P.B. Chemical composition of the virgin oil obtained by mechanical pressing form several grape seed varieties (Vitis vinifera L.) with emphasis on minor constituents. Arch. Latinoam. Nutr. 2009, 59, 214–219. [Google Scholar]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of Muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Nawaz, H.; Shi, J.; Mittal, G.S.; Kakuda, Y. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep. Purif. Technol. 2006, 48, 176–181. [Google Scholar] [CrossRef]
- Guendez, R.; Kallithraka, S.; Makris, D.P.; Kefalas, P. Determination of low molecular weight polyphenolic constituents in grape (Vitis vinifera sp.) seed extracts: Correlation with antiradical activity. Food Chem. 2005, 89, 1–9. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Weber, H.A.; Hodges, A.E.; Guthrie, J.R.; O’Brien, B.M.; Robaugh, D.; Clark, A.P.; Harris, R.K.; Algaier, J.W.; Smith, C.S. Comparison of proanthocyanidins in commercial antioxidants: Grape seed and pine bark extracts. J. Agric. Food Chem. 2007, 55, 148–156. [Google Scholar] [CrossRef]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Rodríguez Montealegre, R.; Romero Peces, R.; Chacón Vozmediano, J.L.; Martínez Gascueña, J.; García Romero, E. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- De Freitas, V.A.P.; Glories, Y. Concentration and compositional changes of procyanidins in grape seeds and skin of white Vitis vinifera varieties. J. Sci. Food Agric. 1999, 79, 1601–1606. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Changes in grape seed polyphenols during fruit ripening. Phytochemistry 2000, 55, 77–85. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- De Bossoreille de Ribou, S.; Douam, F.; Hamant, O.; Frohlich, M.W.; Negrutiu, I. Plant science and agricultural productivity: Why are we hitting the yield ceiling? Plant Sci. 2013, 210, 159–176. [Google Scholar] [CrossRef]
- Kirchoff, B.K.; Claßen-Bockhoff, R. Inflorescences: Concepts, function, development and evolution. Ann. Bot. 2013, 112, 1471–1476. [Google Scholar] [CrossRef] [Green Version]
- Périlleux, C.; Lobet, G.; Tocquin, P. Inflorescence development in tomato: Gene functions within a zigzag model. Front. Plant Sci. 2014, 5, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanderbali, A.S.; Berger, B.A.; Howarth, D.G.; Soltis, P.S.; Soltis, D.E. Evolving ideas on the origin and evolution of flowers: New perspectives in the genomic era. Genetics 2016, 202, 1255–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Klein, L.L.; Duncan, K.E.; Jiang, N.; Chitwood, D.H.; Londo, J.P.; Miller, A.J.; Topp, C.N. Characterizing 3D inflorescence architecture in grapevine using X-ray imaging and advanced morphometrics: Implications for understanding cluster density. J. Exp. Bot. 2019, 70, 6261–6276. [Google Scholar] [CrossRef]
- Cárcel, J.A.; García-Pérez, J.V.; Mulet, A.; Rodríguez, L.; Riera, E. Ultrasonically assisted antioxidant extraction from grape stalks and olive leaves. Phys. Procedia 2010, 3, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Perez, J.V.; García-Alvarado, M.A.; Carcel, J.A.; Mulet, A. Extraction kinetics modeling of antioxidants from grape stalk (Vitis vinifera var. Bobal): Influence of drying conditions. J. Food Eng. 2010, 101, 49–58. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour. Technol. 2007, 98, 2963–2967. [Google Scholar] [CrossRef] [PubMed]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Fiol, N.; Olivella, M.À.; Gominho, J.; Villaescusa, I.; Pereira, H. Chemical characterization of different granulometric fractions of grape stalks waste. Ind. Crops Prod. 2013, 50, 494–500. [Google Scholar] [CrossRef]
- Spigno, G.; Maggi, L.; Amendola, D.; Dragoni, M.; De Faveri, D.M. Influence of cultivar on the lignocellulosic fractionation of grape stalks. Ind. Crops Prod. 2013, 46, 283–289. [Google Scholar] [CrossRef]
- Fernándes-Cano, L.H.; Togores, J.H. Tratado de Viticultura. Tomo I; Ediciones: Madrid, Spain, 2011; ISBN 8484764257. [Google Scholar]
- Hon, D.N.-S. Cellulose: A random walk along its historical path. Cellulose 1994, 1, 1–25. [Google Scholar] [CrossRef]
- Ioelovitch, M.Y.; Tupureine, A.D.; Veveris, G.P. Study on the cellulose cocrystallinization during it isolation from plant raw materials. Khimiya Drev. 1991, 4, 27–33. [Google Scholar]
- Rodríguez Couto, S.; Rodríguez, R.; Gallego, P.P.; Sanromán, A. Biodegradation of grape cluster stems and ligninolytic enzyme production by Phanerochaete chrysosporium during semi-solid-state cultivation. Acta Biotechnol. 2003, 23, 65–74. [Google Scholar] [CrossRef]
- Prozil, S.O.; Evtuguin, D.V.; Silva, A.M.S.; Lopes, L.P.C. Structural characterization of lignin from grape stalks (Vitis vinifera L.). J. Agric. Food Chem. 2014, 62, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Souquet, J.-M.; Labarbe, B.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. Phenolic composition of grape stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef]
- Cruz, J.M.; Domínguez, H.; Parajó, J.C. Assessment of the production of antioxidants from winemaking waste solids. J. Agric. Food Chem. 2004, 52, 5612–5620. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, S.; Wang, X.; Zheng, X. Modification and application of dietary fiber in food. J. Chem. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tejada-Ortigoza, V.; García-Amezquita, L.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. The dietary fiber profile of fruit peels and functionality modifications induced by high hydrostatic pressure treatments. Food Sci. Technol. Int. 2017, 23, 396–402. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—A review. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Christiaens, S.; Uwibambe, D.; Uyttebroek, M.; Van Droogenbroeck, B.; Van Loey, A.M.; Hendrickx, M.E. Pectin characterisation in vegetable waste streams: A starting point for waste valorisation in the food industry. LWT Food Sci. Technol. 2015, 61, 275–282. [Google Scholar] [CrossRef]
- Gul, H.; Acun, S.; Sen, H.; Nayir, N.; Turk, S. Antioxidant activity, total phenolics and some chemical properties of Öküzgözü and Narince grape pomace and grape seed flour. J. Food Agric. Environ. 2013, 11, 28–34. [Google Scholar]
- Yu, X.C.; Sun, D.-L. Microwave and enzymatic extraction of orange peel pectin. Asian J. Chem. 2013, 25, 5333–5336. [Google Scholar] [CrossRef]
- Bayar, N.; Friji, M.; Kammoun, R. Optimization of enzymatic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal. Food Chem. 2018, 241, 127–134. [Google Scholar] [CrossRef]
- Sundari, N. Extrication of pectin from waste peels: A review. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1841–1848. [Google Scholar]
- Jamsazzadeh Kermani, Z.; Shpigelman, A.; Pham, H.T.T.; Van Loey, A.M.; Hendrickx, M.E. Functional properties of citric acid extracted mango peel pectin as related to its chemical structure. Food Hydrocoll. 2015, 44, 424–434. [Google Scholar] [CrossRef]
- Min, B.; Lim, J.; Ko, S.; Lee, K.-G.; Lee, S.H.; Lee, S. Environmentally friendly preparation of pectins from agricultural byproducts and their structural/rheological characterization. Bioresour. Technol. 2011, 102, 3855–3860. [Google Scholar] [CrossRef] [PubMed]
- Da Gama, B.M.V.; Silva, C.E.d.F.; da Silva, L.M.O.; Abud, A.K.d.S. Extraction and characterization of pectin from citric waste. Chem. Eng. Trans. 2015, 44, 259–264. [Google Scholar] [CrossRef]
- Methacanon, P.; Krongsin, J.; Gamonpilas, C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters andits properties. Food Hydrocoll. 2014, 35, 383–391. [Google Scholar] [CrossRef]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A. Extraction and characterization of pectin from passion fruit peels. Agric. Agric. Sci. Procedia 2014, 2, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Shakila Banu, M.; Bharathi Kannamma, G.; Gayatrri, P.; Nadezhda, H.; Nandhini, J. Comparative studies of pectin yield from fruits using different acids. Food Sci. 2012, 42, 6349–6351. [Google Scholar]
- Bhat, S.A.; Singh, E.R. Extraction and characterization of pectin from guava fruit peel. Int. J. Res. Eng. Adv. Technol. 2014, 2, 1–7. [Google Scholar]
- Devanooru Krishnamurthy, B.; Shrilakshmi, S.; Suraksha, R. Value added products from agriculture: Extraction of pectin from agro waste product Musa Acuminata and Citrus fruit. Res. J. Agric. For. Sci. 2015, 3, 13–18. [Google Scholar]
- Castillo-Israel, K.A.T.; Baguio, S.F.; Diasanta, M.D.B.; Lizardo, R.C.M.; Dizon, E.I.; Mejico, M.I.F. Extraction and characterization of pectin from Saba banana [Musa ‘saba’(Musa acuminata × Musa balbisiana)] peel wastes: A preliminary study. Int. Food Res. J. 2015, 22, 202–207. [Google Scholar]
- Panouillé, M.; Thibault, J.-F.; Bonnin, E. Cellulase and protease preparations can extract pectins from various plant byproducts. J. Agric. Food Chem. 2006, 54, 8926–8935. [Google Scholar] [CrossRef]
- Rahmati, S.; Abdullah, A.; Momeny, E.; Kang, O.L. Optimization studies on microwave assisted extraction of dragon fruit (Hylocereus polyrhizus) peel pectin using response surface methodology. Int. Food Res. J. 2015, 22, 233–239. [Google Scholar]
- Maran, J.P.; Swathi, K.; Jeevitha, P.; Jayalakshmi, J.; Ashvini, G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohydr. Polym. 2015, 123, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Jerman Klen, T.; Mozetič Vodopivec, B. Optimisation of olive oil phenol extraction conditions using a high-power probe ultrasonication. Food Chem. 2012, 134, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef]
- Du, B.; Li, F.Y.; Fan, C.J.; Zhu, F.M. Response surface methodology for optimization of extraction process for soluble dietary fibre from grape pomace with hydrochloric acid. Food Sci. 2011, 32, 128–134. [Google Scholar]
- Ferreira, C.S.; Pinho, M.; Cabral, L.C. Solid-Liquid Extraction and Concentration with Processes of Membrane Technology of Soluble Fibers from Wine Grape Pomace; Técnico Lisboa: Lisbon, Portugal, 2013. [Google Scholar]
- Sun, Y.; Fang, Y.L.; Zhang, A.; Dai, L.M.; Liu, S.W.; Lai, J.W. Extraction technology of soluble dietary fiber from grape pomace. J. Northwest A F Univ. Sci. Ed. 2010, 38, 145–158. [Google Scholar]
- Colodel, C.; Vriesmann, L.C.; Teófilo, R.F.; de Oliveira Petkowicz, C.L. Optimization of acid-extraction of pectic fraction from grape (Vitis vinifera cv. Chardonnay) pomace, a Winery Waste. Int. J. Biol. Macromol. 2020, 161, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Llobera, A.; Cañellas, J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Antioxidant activity and dietary fibre of Prensal Blanc white grape (Vitis vinifera) by-products. Int. J. Food Sci. Technol. 2008, 43, 1953–1959. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Effect of different drying methods and storage time on the retention of bioactive compounds and antibacterial activity of wine grape pomace (Pinot Noir and Merlot). J. Food Sci. 2012, 77, H192–H201. [Google Scholar] [CrossRef] [PubMed]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; da Silva, M.V.; Pontes, P.V.d.A.; Haminiuk, C.W.I.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Bencivenni, M.; Caligiani, A.; Tedeschi, T.; Bruggeman, G.; Bosch, M.; Petrusan, J.; Van Droogenbroeck, B.; Elst, K.; Sforza, S. Pectin content and composition from different food waste streams. Food Chem. 2016, 201, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J.L. Valorisation potential of Cabernet grape pomace for the recovery of polyphenols: Process intensification, optimisation and study of kinetics. Food Bioprod. Process. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Limareva, N.; Donchenko, L.; Vlaschik, L. Comparative evaluation of properties of pectin substances in pomace of grape varieties for development of functional foods. In Advances in Engineering Research. Proceedings of the IV International Scientific and Practical Conference “Anthropogenic Transformation of Geospace: Nature, Economy, Society” (ATG 2019), Volgograd, Russia, 1–4 October 2019; Atlantis Press: Paris, France, 2020; pp. 160–164. [Google Scholar]



| Compound | Dry Matter Content | References |
|---|---|---|
| Physico-chemical parameters | [37,38,39] | |
| Ash | 4.65 ± 0.05 g/100 g | |
| Moisture content | 3.33 ± 0.04 g/100 g | |
| Fiber | 46.17 ± 0.80 g/100 g | |
| Lipids | 8.16 ± 0.01 g/100 g | |
| Proteins | 8.49 ± 0.02 g/100 g | |
| Carbohydrates | 29.20 g/100 g | |
| Fructose | 8.91 ± 0.08 g/100 g | |
| Glucose | 7.95 ± 0.07 g/100 g | |
| Energy value | 224.00 Kcal/100 g | |
| Mineral substances | ||
| Ca | 9.90 g/kg | |
| P | 2.70 g/kg | |
| Mg | 0.80 g/kg | |
| K | 13.90 g/kg | |
| Na | 0.22 g/kg | |
| S | 1.50 g/kg | |
| Mn | 13.00 mg/kg | |
| Zn | 25.00 mg/kg | |
| Cu | 49.00 mg/kg | |
| Fe | 361.00 mg/kg | |
| Se | 0.20 mg/kg | |
| Co | 0.40 mg/kg | |
| Bioactive compounds | ||
| Vitamin E | 5.00 mg/kg | |
| Vitamin C | 26.25 ± 0.01 mg AAE a/g | |
| Soluble fiber | 9.76 ± 0.03 g/100 g | |
| Insoluble fiber | 36.40 ± 0.84 g/100 g | |
| Total anthocyanin content | 131.00 ± 0.40 mg/100 g | |
| Total phenolic content | 60.10 ± 0.10 mg GAE b/g | |
| Catehic tannins | 13.10 ± 0.80 mg CE c/g | |
| Hydrolysable tannins | 3.70 ± 0.10 mg TAE d/g | |
| Quercitin | 128.70 ± 5.90 μg/g | |
| Gallic acid | 607.00 ± 9.00 μg/g | |
| Catechin | 1973.40 ± 17.60 μg/g | |
| Procyanidin B2 | 1071.00 ± 17.70 μg/g | |
| Phenolic Compounds | Content, mg/g | References | |
|---|---|---|---|
| Free phenolic compounds | Gallic acid | 13.7 ± 0.6 | [3,75] |
| Caftaric acid | 40.4 ± 3.6 | ||
| Protocatechuic acid | 11.0 ± 1.0 | ||
| Vanillic acid | 9.2 ± 2.4 | ||
| Caffeic acid | n.d. | ||
| Syringic acid | 4.3 ± 0.1 | ||
| p-Coumaric acid | n.d. | ||
| (+)-Catechin | 16.5 ± 0.6 | ||
| (−)-Epicatechin | 23.7 ± 1.4 | ||
| Rutin | 143.1 ± 7.6 | ||
| Isoquercitrin | 212.1 ± 12.8 | ||
| Kaempferol | 362.7 ± 45.0 | ||
| Resveratrol | 149.2 ± 11.0 | ||
| Bound phenolic compounds | Gallic acid | 2.6 ± 0.1 | |
| Caftaric acid | 7.4 ± 0.1 | ||
| Protocatechuic acid | 4.4 ± 0.2 | ||
| Vanillic acid | 4.9 ± 0.6 | ||
| Caffeic acid | 7.2 ± 0.3 | ||
| Syringic acid | 2.2 ± 0.1 | ||
| p-Coumaric acid | 6.4 ± 1.9 | ||
| (+)-Catechin | 13.6 ± 1.0 | ||
| (−)-Epicatechin | 10.6 ± 1.9 | ||
| Rutin | 4.5 ± 1.2 | ||
| Isoquercitrin | 3.4 ± 1.1 | ||
| Kaempferol | 46.4 ± 0.9 | ||
| Resveratrol | 0.3 ± 0.1 | ||
| Phytosterols | Content, mg/kg Oil | References |
|---|---|---|
| Cholesterol | n.d.-10.00 | [87,106] |
| Cholestanol | n.d. | |
| Brassicasterol | 0.60–0.90 | |
| 2,4-methylene-cholesterol | n.d.-0.18 | |
| Campesterol | 0.10–9.30 | |
| Campestanol | n.d. | |
| Stigmasterol | 10.20–10.80 | |
| Δ-7 campesterol | 0.16–0.27 | |
| Δ-5 2,3-stigmastadienol | n.d. | |
| Clerosterol | 0.90–0.94 | |
| β-sitosterol | 66.60–67.40 | |
| Sitostanol | 3.92–4.70 | |
| Δ-5 avenasterol | 1.98–2.09 | |
| Δ-5 2,4-stigmastadienol | 0.41–0.47 | |
| Δ-7 estigmastenol | 1.99–2.30 | |
| Δ-7 avenasterol | 0.98–1.10 |
| Grape Pomace Variety | Yield of Soluble Fiber/Pectin Recovery | Extraction Technique and Operating Conditions | References | |
|---|---|---|---|---|
| Vitis vinifera L. | 2.3–4.4 g/100 g pectin | n.d. | [33] | |
| Manto Negro | 6.20 ± 0.30% soluble pectins | Solvent extraction Solvent: 0.5 M hydrochloric acid; temperature: 80 °C | [164] | |
| Prensal Blanc | 45.0 ± 1.6 g/kg soluble pectins | Solvent extraction Solvent: 0.5 M hydrochloric acid; temperature: 80 °C | [165] | |
| Gamay Noir | Soluble dietary fiber | 455.2 mg/g | Solvent extraction Solvent: hydrochloric acid; solid/liquid ratio: 1:20; temperature: 75 °C; time: 75 min | [162] |
| Chardonnay | 438.6 mg/g | |||
| Syrah | 277.2 mg/g | |||
| Gamay Noir | 421.0 mg/g | Enzyme-assisted extraction Enzyme: cellulase; solid/liquid ratio: 1:20; temperature: 55 °C; time: 210 min | ||
| Chardonnay | 401.8 mg/g | |||
| Syrah | 242.8 mg/g | |||
| Vitis vinifera L. | 37–54 mol% pectic substances of CWP a | Solvent extraction Solvent: sulphuric acid; temperature: 20 °C; time: 3 h | [4] | |
| Vitis vinifera L. | 47% soluble fiber | Solvent extraction Solvent: hydrochloric acid; solid/liquid ratio: 1:12; temperature: 75 °C; time: 90 min | [160] | |
| RWGP b (Cabernet Sauvignon, Merlot, Pinot Noir) | 32.3–41.2 mg GUAE d/g total extractable pectins | Solvent extraction Solvent: deionized water; solid/liquid ratio: 1:20; time: 10 min | [35] | |
| WWGP c (Muller Thurgau, Morio Muscat) | 50.6–56.4 mg GUAE/g total extractable pectins | |||
| Pinot Noir Merlot | 3.68 ± 0.05% pectin 5.82 ± 0.81% pectin | Solvent extraction Solvent: water; time: 10 min | [166] | |
| Chardonnay | 0.904 ± 0.045 g/100 g mass of sugar | Solvent extraction Solvent: water; solid/liquid ratio: 1:4; temperature: 90 °C; time: 3 h | [161] | |
| 2.156 ± 0.012 g/100 g mass of sugar | Solvent extraction Solvent: 2% alkali solution; solid/liquid ratio: 1:4; temperature: 90 °C; time: 5 h | |||
| 0.757 ± 0.010 g/100 g mass of sugar | Enzyme-assisted extraction Enzyme: cellulase; solid/liquid ratio: 1:4; temperature: 50 °C; time: 3 h | |||
| Cabernet Sauvignon | 32.4 ± 1.4% pectin | Ultrasound-assisted extraction Solvent: citric acid; pH 2.0; solid/liquid ratio: 1:10; temperature: 75 °C; time: 60 min; frequency: 37 kHz | [7] | |
| Benitaka | 3.92 ± 0.02 g calcium pectate/100 g | Neutralization the overall charge of free uronic acid residues by calcium ions | [38] | |
| Merlot | 6.99 ± 0.19% pectin | Solvent extraction Solvent: phosphate-citrate buffer; pH 3.0; solid/liquid ratio: 1:50; temperature: 80 °C; time: 2 h | [167] | |
| Tanat | 4.59 ± 0.18% pectin | |||
| Cabernet | 3.46 ± 0.21% pectin | |||
| Pinot Noir | 10.93% total sugar | Solvent extraction Solvent: water; solid/liquid ratio: 1:12; temperature: 100 °C; time: 1 h; particle size: <249 μm | [21] | |
| Vitis vinifera L. | 11 g CASS e/g pectic polysaccharides 8 mg DASS f/g pectic polysaccharides | Solvent extraction Solvent (CASS): 50 mM trans-1,2-diaminocyclohexane-N,N,N,N-tetraacetic acid; time: 6 and 12 h Solvent (DASS): 50 mM sodium carbonate/20 mM sodium borohydride; temperature: 4 °C; time: 6 and 12 h | [168] | |
| Cabernet | 11.25 g/100 g soluble dietary fiber | n.d. | [169] | |
| Chardonnay | 11.1% pectin | Solvent extraction Solvent: nitric acid; pH: 2.08; solid/liquid ratio: 35.11 mL/g; time: 135.23 min | [163] | |
| Cabernet Sauvignon | 1.9% protopectin 0.9% hydropectin | Calcium-pectatism method | [170] | |
| Saperavi Severnyi | 2.85% protopectin 2.01% hydropectin | |||
| Moldova | 3.95% protopectin 1.8% hydropectin | |||
| Aligote | 2.4 protopectin 1.23% hydropectin | |||
| Chardonnay | 2.3 protopectin 1.3% hydropectin | |||
| Rkatsiteli | 2.2% protopectin 1.2% hydropectin | |||
| Pervenets Magarachea | 2.6 protepctin 1.1% hydropectin | |||
| Grape Pomace Variety | Galacturonic Acid Content | Degree of Esterification | Molecular Weight | Neutral Monosaccharide Content | References |
|---|---|---|---|---|---|
| Manto Negro | 6.21 ± 0.18% | n.d. | n.d. | 4.60 ± 0.12% | [164] |
| Prensal Blanc | 41.8 ± 1.6 g/kg (uronic acids) | n.d. | n.d. | 61.2 ± 1.7 g/kg | [165] |
| Cabernet Sauvignon | n.d. | 21–39% | n.d. | 0.2 Rha a, 0.4 Fuc b, 5.1 Ara c, 11.6 Xyl d, 7.4 Man e, 3.6 Gal f, 40.7 Glc g (mol%) | [4] |
| Callet | 0.2 Rha, 1.0 Fuc, 4.9 Ara, 17.2 Xyl, 6.2 Man, 3.3 Gal, 38.3 Glc (mol%) | ||||
| Manto Negro | 0.3 Rha, 0.3 Fuc, 6.9 Ara, 11.5 Xyl, 5.6 Man, 4.9 Gal, 38.5 Glc (mol%) | ||||
| Merlot | 0.1 Rha, 0.3 Fuc, 5.5 Ara, 19.2 Xyl, 7.4 Man, 3.3 Gal, 34.6 Glc (mol%) | ||||
| Tempranillo | 0.2 Rha, 0.3 Fuc, 4.8 Ara, 12.8 Xyl, 4.6 Man, 3.5 Gal, 38.4 Glc (mol%) | ||||
| Syrah | 0.2 Rha, 0.1 Fuc, 4.8 Ara, 18.4 Xyl, 6.0 Man, 3.5 Gal, 37.7 Glc (mol%) | ||||
| Chardonnay | 0.1 Rha, 0.1 Fuc, 6.4 Ara, 14.1 Xyl, 4.8 Man, 3.9 Gal, 29.8 Glc (mol%) | ||||
| Macabeu | 0.1 Rha, 0.1 Fuc, 6.0 Ara, 8.4 Xyl, 5.6 Man, 4.0 Gal, 35.9 Glc (mol%) | ||||
| Parellada | 0.2 Rha, 0.2 Fuc, 6.9 Ara, 12.0 Xyl, 5.7 Man, 4.0 Gal, 38.7 Glc (mol%) | ||||
| Manto Negro | n.d. | 21–39% | n.d. | 0.1 Rha, 0.1 Fuc, 6.2 Ara, 11.5 Xyl, 4.7 Man, 3.9 Gal, 35.6 Glc (mol%) | [4] |
| Muller Thurgau | 0.43 ± 0.06 mg GUAE h/g | n.d. | n.d. | 0.07% Ara, 0.02% Xyl, 0.05% Man, 0.12% Gal, 0.20% Glc | [35] |
| Morio Muscat | 0.26 ± 0.03 mg GUAE/g | 0.11% Ara, 0.02% Xyl, 0.01% Man, 0.13% Gal, 0.15% Glc | |||
| Cabernet Sauvignon | 0.27 ± 0.04 mg GUAE/g | 0.07% Ara, 0.03% Xyl, 0.05% Man, 0.07% Gal, 0.33% Glc | |||
| Merlot | 0.73 ± 0.07 mg GUAE/g | 0.13% Ara, 0.04% Xyl, 0.09% Man, 0.15% Gal, 0.38% Glc | |||
| Pinot Noir | 0.72 ± 0.06 mg GUAE/g | 0.16% Ara, 0.02% Xyl, 0.22% Man, 0.18% Gal, 0.43% Glc | |||
| Merlot | 0.50 ± 0.07% (uronic acid) | n.d. | n.d. | 0.73 ± 0.01% (total content of neutral sugar) | [166] |
| Pinot Noir | 0.35 ± 0.04% (uronic acid) | 1.09 ± 0.01% (total content of neutral sugar) | |||
| Chardonnay | n.d. | n.d. | n.d. | 4.6–8.7 Rha, 3.1 Fuc, 22.1–30.9 Ara, 3.5–7.6 Xyl, 7.6–9.8 Man, 8.6–19.7 Gal, 20.2–51.4 Glc (mol%) | [161] |
| Cabernet Sauvignon | n.d. | 55.2% | 163.9 kDa | n.d. | [7] |
| Pinot Noir | 31 mol% (GalA i) | n.d. | n.d. | 2.0–40.5 Rha, 20.4–38.4 Ara, 1.2–3.3 Xyl, 10.3–14.9 Man, 6.6–28.3 Gal, 6.0–37.0 Glc (mol%) | [21] |
| Chardonnay | 56.8 ± 0.3% (GalA) | 43.3% | 1.597 × 105 g/mol | 2.7% Rha, 0.2% Fuc, 2.9% Ara, 2.5% Xyl, 2.2% Man, 7.2% Gal, 25.5% Glc | [163] |
| Saperavi Severnyi | 42.8–69.09% | 52–65% | n.d. | n.d. | [170] |
| Moldova | |||||
| Cabernet Sauvignon | |||||
| Aligote | |||||
| Chardonnay | |||||
| Rkatsiteli | |||||
| Pervenets Magarachea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. https://doi.org/10.3390/foods10040867
Spinei M, Oroian M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods. 2021; 10(4):867. https://doi.org/10.3390/foods10040867
Chicago/Turabian StyleSpinei, Mariana, and Mircea Oroian. 2021. "The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances" Foods 10, no. 4: 867. https://doi.org/10.3390/foods10040867
APA StyleSpinei, M., & Oroian, M. (2021). The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods, 10(4), 867. https://doi.org/10.3390/foods10040867






