Abstract
The addition of phosphates to meat products improves the emulsifying and gelling properties of meat proteins, in turn enhancing overall product quality. The current market trend towards additive-free products and the health issues related to phosphate challenge the industry to develop phosphate-free meat products. The aim of this study was to evaluate the potential of seven protein-based ingredients (pea, blood plasma, gelatin, soy, whey, egg, and potato) to remediate quality losses of emulsified meat products (cooked sausages) upon phosphate elimination. First, the intrinsic gelling and emulsifying characteristics of the proteins were assessed. Next, the proteins were added to phosphate-free sausages, of which quality characteristics during production (viscoelastic behavior and emulsion stability) and of the final products (texture, cooking loss, and pH) were screened. Blood plasma and soy were superior in phosphate-free cooked sausages, as no significant differences in hardness, cooking yield, or stability were found compared to phosphate-containing sausages. Egg and pea also improved the previously mentioned quality characteristics of phosphate-free sausages, although to a lesser extent. These insights could not entirely be explained based on the intrinsic gelling and emulsifying capacity of the respective proteins. This indicated the importance of a well-defined standardized meat matrix to determine the potential of alternative proteins in meat products.
1. Introduction
Food phosphates exist in different types (mono-, di-, tri-, and polyphosphates) and are often used in the meat industry due to their impact on pH, chelation, ionic strength, and antibacterial activity. They fulfill several functional properties in meat products such as a good buffering capacity (monophosphates) and the ability to dissociate the actomyosin complex of meat (diphosphates) and activate the meat proteins by chelating the protein-bound Mg2+ and Ca2+, leading to increased solubilization of the meat proteins and depolymerization of the thick and thin filaments (tri- and polyphosphates) [1,2]. Due to these effects, meat proteins can maximally exert their emulsifying and gelling properties, which are very important with regard to water holding capacity (WHC) and fat emulsification. In addition, most di- or polyphosphates contribute to an increase in pH or ionic strength, respectively. Both effects result in increased electrostatic repulsion and consequently more space to bind water and fat between the meat proteins, which again contributes to increased water and fat stabilization. The different phosphate types (or blends) in meat products can be added to a maximum amount of 0.5% (expressed as P2O5) according to European legislation [3]. However, in a former study of Ritz, et al. [4], an association was found between a high intake of phosphate additives and cardiovascular morbidity and mortality. This health issue was already recognized for chronic kidney disease patients, but questions arose with regard to the general population. The EFSA Panel on Food Additives and Flavorings further investigated the matter and provided a scientific opinion re-evaluating the safety of phosphates as food additives in 2019. They considered phosphates to be of low acute oral toxicity, and there was no concern with respect to genotoxicity and carcinogenicity. Furthermore, the Panel considered an acceptable daily intake (ADI) of 40 mg/kg body weight per day. However, this ADI does not apply to humans with a reduction in renal function. Ten percent of the general population might have chronic kidney disease with reduced renal function and they may not tolerate the proposed ADI [5].
In a recent study, it was shown that the current amount of P2O5 added to emulsified meat products (cooked sausages) can be strongly reduced with minimal loss in product quality [6]. Nevertheless, the market trend towards additive-free products [2,7] and the negative effect of phosphates on human health for certain population groups justify attempts to develop phosphate-free emulsified meat products. Unfortunately, phosphate elimination results in decreased meat protein functionality, which causes quality defects such as compromised water and fat stabilization [6]. Therefore, alternative ingredients or even innovative technologies are needed to compensate for this functionality loss. These include pH improving ingredients, starches, hydrocolloids, or the use of high-pressure technology [2,8,9,10,11]. Additionally, proteins, from both animal and vegetable sources, can act as enhancers to compensate for the loss of functionalized meat proteins due to phosphate elimination in meat products. This is mainly related to their gelling and emulsification properties. They have already been proven useful to boost the quality characteristics of meat products related to water and fat binding properties, gel network formation, texture, and/or sensorial properties. In this respect, they have been successfully deployed as fat-replacers, processing aids of low-cost meat products, and substitutes for meat proteins [12,13,14,15,16,17,18,19,20].
However, only a limited number of studies aimed to investigate the opportunities of reduction/replacement of phosphate in meat products [11], especially with regard to the use of alternative proteins. Hurtado, et al. [21] concluded that porcine blood plasma was a useful functional ingredient to replace phosphate and caseinate in frankfurters. Pereira, et al. [22] stated that the addition of collagen fibers improved cooking yield and hardness in phosphate-free sausages. Enhancement of water holding capacity, sensorial attributes, color, and microbial stability could also be achieved by replacing phosphates with a purified beef collagen powder in injected beef strip loins [23]. Furthermore, Muguruma, et al. [24] stated that the addition of biopolymers containing soybean and milk proteins may permit a reduction in phosphate content without a loss of the texture of chicken sausages.
In summary, alternative proteins have been proven to function as functional ingredients in different meat systems on account of their gelling and emulsifying capacities. In contrast, the more specific ability to act as a phosphate replacer, in order to manage the loss of functionalized meat proteins, has only been studied for a limited number of proteins. Furthermore, standardized comparison between proteins remains difficult, because these surveys were conducted on different meat matrices (difference in meat product class, composition of recipes, processing conditions) and different analyzing techniques were applied, making it impossible to identify the most promising protein. Xiong [25] stated that a valid comparison between proteins is only possible if the screening is made under identical processing and storage conditions. Therefore, the aim of this study was to evaluate the potential of seven protein-based ingredients, from both animal and vegetable sources, to counter the loss of quality due to phosphate elimination in emulsified meat products (cooked sausages). In the first stage, the intrinsic protein characteristics (gelation and emulsification potential), related to improving the quality of meat products, are studied. In the second stage, the ability of the proteins to enhance the properties of phosphate-free cooked sausages (viscoelastic and textural properties, emulsion stability, cooking loss, and pH) is evaluated. This research is of important industrial relevance, since phosphate elimination in emulsified meat products will decrease potential health concerns and is a promising step towards clean-label products.
2. Materials and Methods
2.1. Determination of the Intrinsic Characteristics of Selected Proteins
The proteins discussed in this study are egg white (Pulviver), pea (Nutralys), potato (KMC), soy concentrate (Pulviver), blood plasma (Veos), gelatin (Rousselot), and whey (Caldic) protein. Proteins were selected based on their industrial relevance. In addition, a balanced distribution between animal and vegetable proteins was envisioned. In order to learn more about their intrinsic properties related to enhancing meat product quality, their gelation and emulsification potential were studied in a watery environment. It is well described that the salt level and acidity of a medium have an important impact on protein characteristics [12,26,27]. In order to create an aqueous medium that reflects the composition of emulsified meat products, proteins were suspended in a 0.05 M Na phosphate buffer (pH = 6) containing 3.5% NaCl and stirred (800 rpm) for 105 min at room temperature before analysis. The applied protein concentration was dependent on the screening technique used, as described below. The concentration of salt corresponds to its quantity in the cooked sausage model (see Section 2.2) expressed in the water phase, and the pH value is in the range of common meat products.
2.1.1. Gelation Potential
The gelation properties of the proteins were determined through rheological measurements using an AR2000ex stress-controlled rheometer (TA instruments, New Castle, DE, USA) equipped with a 40-mm parallel plate system. A crosshatched upper plate and a lower plate were used to prevent slippage of the sample. The gap was set at 500 μm. The AR2000ex was supplemented with an efficient Peltier temperature control system and an upper heated plate (TA Instruments) to control the sample temperatures accurately. Temperature sweeps were conducted to investigate structural changes of the protein suspensions (4.5% protein) during a heating and cooling process, representative of the manufacturing process of emulsified meat products. The following profile was applied: (1) a heating step from 20 to 76 °C at a constant heating rate of 2 °C/min; (2) an isothermal heating step at 76 °C for 3 min; (3) a cooling step from 76 to 20 °C at a constant cooling rate of 2 °C/min. Oscillation measurements during the entire process were performed at a fixed frequency of 1 Hz and a strain of 0.025, a value found to be within the linear viscoelastic region based on preliminary experiments. The storage modulus (G’) and phase angle (δ, with δ of 90° representing a fully viscous material and δ of 0° representing a fully elastic material) at the end of the isothermal heating step and cooling step (G’76 °C,suspension, δ76 °C,suspension and G’end,suspension, δend,suspension, respectively) were derived from the temperature sweep profiles using the software (Rheology Advantage Data Analysis, v. 5.7.0, TA Instruments). All G’-values are expressed logarithmically. Protein suspensions were made in duplicate for each protein, and determination of the gelling potential was performed in duplicate per suspension.
2.1.2. Emulsification
The emulsifying properties of the proteins were screened according to the procedure described by Steen, et al. [28], which was based upon the turbidimetric method of Pearce and Kinsella [29]. Emulsions were prepared by mixing 2.0 mL sunflower oil and 8.0 mL protein suspensions (0.15% protein) for 1 min at a speed of 12,000 rpm and room temperature using an Ultra-Turrax homogenizer (model T25, IKA-Werke GmbH, Staufen, Germany). Immediately and 10 min after emulsion formation, 50 μL of the emulsion was taken and diluted with 5 mL of 0.1% sodium dodecyl sulfate (SDS) solution. Absorbance values were measured at 500 nm (A500) and used to calculate the emulsifying activity index (EAI, m2/g) according to the equations below.
where A500 represents the absorbance at 500 nm, L the light path length (L = 0.01 m), φ the volume fraction (v/v) of the dispersed phase (φ = 0.20), C the protein concentration (C = 1500 g/m3) before emulsification, T the turbidity, and F the dilution factor (F = 100). The emulsion activity index immediately after emulsion formation is represented by the abbreviation EAI0. Emulsion stability (ES) was the percentage of emulsion turbidity remaining after 10 min. Emulsions were made in duplicate for each protein, and determination of the emulsifying properties was performed in duplicate for each emulsion.
2.2. Manufacturing of Cooked Sausage
Cooked sausages were prepared in the pilot plant of the research group “Technology and Quality of Animal Products” (KU Leuven Technology Campus Gent, Belgium). Raw materials (pork shoulder and pork backfat) were obtained from a local industrial meat supplier (De Lausnay Rene bvba, Destelbergen, Belgium), chopped, homogenized to generate one batch, vacuum-packed, and stored at –18 °C until preparation. Cooked sausages contained pork shoulder (35/100 g), pork backfat (35/100 g), and ice (30/100 g), together with nitrite curing salt (1.5/100 g), sugar (0.5/100 g), white pepper (0.2/100 g), foil (0.05/100 g), ascorbic acid (0.05/100 g), glutamate (0.05/100 g), coriander (0.025/100 g), and cardamom (0.025 g/100 g). All non-meat ingredients were purchased from Solina Group (Eke-Nazareth, Belgium). Ingredients were calculated relative to the total mass of meat raw materials (pork shoulder and pork backfat) and ice. First, a standardized reference treatment was prepared, containing 0.32/100 g tetrasodium pyrophosphate (TSPP) (Solina Group, Eke-Nazareth, Belgium), which is equal to 0.171% P2O5, a standard amount used in the meat industry for emulsified meat products. The reference containing phosphate will be referred to as M+TSPP. Secondly, TSPP was eliminated and standardized phosphate-free cooked sausages were prepared. These cooked sausages will be referred to as M-TSPP. Finally, the seven above-described protein-based ingredients were added to the phosphate-free treatment. All proteins were added in a mass fraction of 2/100 g, a commonly used dosage [17]. Proteins were calculated relative to the total mass of meat raw materials (pork shoulder and pork backfat) and ice. These phosphate-free treatments containing protein-based ingredients will be referred to as M-TSPP+“corresponding protein source”. During manufacturing of the M+TSPP preparations, the raw lean meat was first pre-chopped together with ice, salt, and TSPP in a bowl cutter for 7 min and 30 s (Stephan cutter UM12, Hameln, Germany), corresponding to a final temperature of 5 °C. Next, the pork backfat was added to the meat batter together with the remaining food ingredients. The total mass was ground under vacuum for 4 min and 30 s to obtain a homogenous batter. The temperature did not exceed 14 °C during processing to avoid protein denaturation and fat coalescence. Phosphate-free sausages were prepared the same way, but without the addition of TSPP. When phosphate-free sausages containing protein-based ingredients were prepared, these proteins were added during the first grinding step of the manufacturing process instead of TSPP. Part of the batter, prepared in duplicate per treatment, was immediately analyzed for dynamic viscoelastic properties (Section 2.3) and emulsion stability (Section 2.4). In order to standardize the cooking process, the remainder of the batter was filled into cans of standardized dimensions (diameter 7 cm, height 5 cm, mass ± 250 g, Crown Verpakking België NV, Hoboken, Belgium), cooked at 76 °C (core temperature 72 °C) for 90 min in a cooking chamber (Rational Climaplus Combi CPC 61, Claes Machines, Paal, Belgium) and finally cooled to 4 °C. The resulting meat products, cooked in cans, served as a model product for cooked sausage and will be referred to as “cooked sausages”. Each treatment, including the reference products with or without TSPP, was manufactured in duplicate. One week after the manufacturing process, three sausages per replicate were analyzed for cooking loss (Section 2.5), pH (Section 2.6), and textural properties (Section 2.7). The number of measuring points is described in the respective analyses below.
2.3. Dynamic Viscoelastic Properties
The dynamic viscoelastic properties of the batters were analyzed using the same equipment as described in Section 2.1.1. The gap was set at 1000 μm for both rheological procedures described below (stress sweep and temperature sweeps).
Stress sweeps were conducted at a temperature of 13 °C, between 0.1 and 1000 Pa, and at a fixed frequency of 1 Hz to determine the linear viscoelastic region (LVR). Hereby, parameters G’, G” (storage and loss modulus respectively), and δ were directly obtained from the software. The complex modulus (G*), representing the materials’ overall rigidity or resistance to deformation, was calculated by the following formula,
The LVR represents the stress range within which G* (and thus G’,G”) is independent of the imposed stress amplitude and is determined according to Glorieux, Goemaere, Steen and Fraeye [6]. LVR is determined in duplo per replicate of each treatment and referred to as LVRbatter. Furthermore, the corresponding G*batter, expressed logarithmically, and δbatter within the LVR are reported.
Temperature sweeps were conducted to investigate the impact of phosphate elimination and use of alternative proteins on the structure formation of meat batters during a heating and cooling procedure, representative of the manufacturing process of cooked sausages. Similar profiles and conditions were applied as described in Section 2.1.1., except for the initial (before heating) and final (end of cooling) temperatures, which were both set at 13 °C in accordance with the final temperature of the raw batter at the end of the cutter process. The parameters G’ and δ at the end of the isothermal heating step and cooling step (G’76°C,batter, δ76°C, batter and G’end,batter δend,batter, respectively) were derived from the temperature sweep profiles using the software. G’-values are expressed logarithmically. All rheological parameters (G’76°C,batter, δ76°C,batter, G’end,batter, δend,batter) were determined in duplo per replicate of each treatment.
2.4. Emulsion Stability
Emulsion stability of the meat batter was determined immediately after the grinding process, according to Glorieux, Goemaere, Steen and Fraeye [6] with slight modifications. Summarized, emulsion stability is expressed as drip loss upon heating (30 min, 70 °C) and centrifugation at 4230× g (6000 rpm in a rotor Cat. No. 1620 A, Hettich, Germany) at 25 °C for 3 min, of a pre-weighed amount of raw batter. The percentage of total expressible fluid (TEF) was expressed as follows:
Furthermore, the relative amount of water, next to the fat in the drip, was determined. Therefore drip loss after centrifugation was weighed before and after drying in an oven (Typ U 40, Memmert, Germany) for 24 h. The relative amount of water in the drip loss was expressed as follows:
TEF and Relative amount of H2O in drip were determined six times per replicate of each treatment.
2.5. Cooking Loss
Cooking loss (CL) of the cooked sausages of each treatment was measured according to Glorieux, Goemaere, Steen and Fraeye [6]. CL was calculated as follows:
Measurements were determined in triplicate per replicate of each treatment.
2.6. pH Measurement
The pH of the cooked sausages was measured three times on three different sausages (nine measurements) per replicate of each treatment, according to the methods described in Glorieux, Goemaere, Steen and Fraeye [6].
2.7. Texture
The hardness of the cooked sausages was analyzed using a Lloyd Texture Analyzer (Model LF plus, Lloyd Instruments, Bognor Regis, UK) and expressed as the maximum force (N) to penetrate the sample, as described in Glorieux, Goemaere, Steen and Fraeye [6]. Per replicate of each treatment, hardness was measured three times on three different sausages (nine measurements).
2.8. Statistical Analysis
Results are expressed as mean ± standard deviation. All results were evaluated by one-way ANOVA. A Tukey’s post hoc test was performed with a significance level of p < 0.05 to identify significant differences. Statistical analysis was performed using the software IBM SPSS Statistics 25 (IBM, Armonk, NY, USA).
3. Results and Discussion
3.1. Intrinsic Characteristics of Selected Proteins
Screening of the intrinsic characteristics of functional ingredients is often executed in watery media. It is a rather quick and easy method to evaluate ingredient functionality that requests no specific and often expensive process equipment to imitate industrial food products. Moreover, it can provide a broad view of the application potential of the ingredients in several food products. Food proteins are mainly applied in meat products in relation to their gelling and emulsifying properties, enabling them to improve overall meat product quality. Results regarding these intrinsic characteristics are described below in Section 3.1.1 and Section 3.1.2.
3.1.1. Gelation Potential
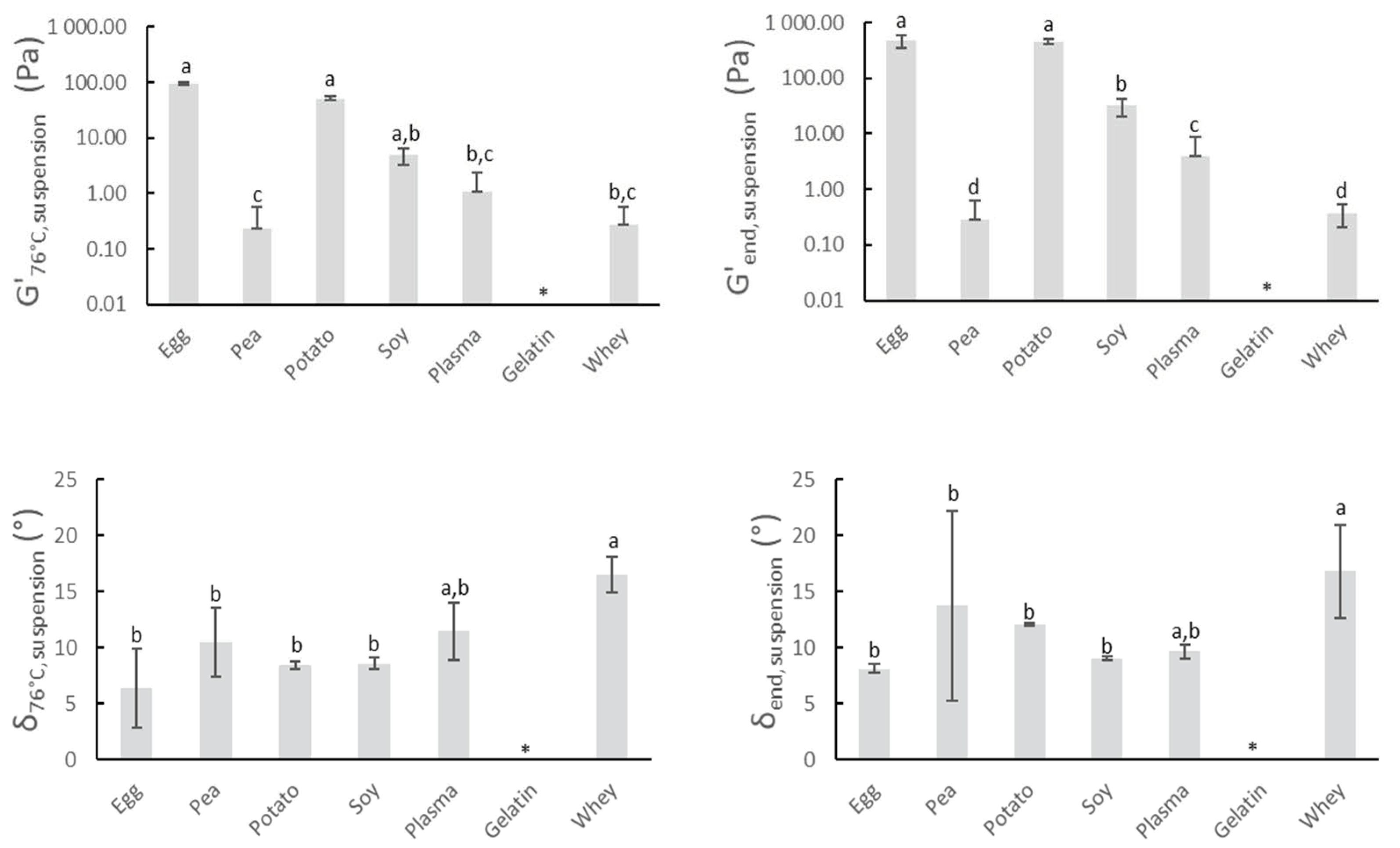
The gelling characteristics of proteins are one of the key reasons they are applied for meat product improvement. Figure 1 shows the gelling properties of the proteins upon heating, with the exception of gelatin. Gelatin is a cold-gelling protein that solubilizes during heating [30] and can therefore only participate in gel network formation at sufficiently low temperatures. The critical temperature below which gelling can occur is dependent on gelatin concentration, cooling rate, and maturing temperature [31]. The applied thermal processing and used gelatin (concentration, source) did not allow the expression of the cold gelling character of gelatin. For all other protein suspensions, the heating and subsequent cooling process caused in general an overall increase in G’ and a decrease of δ. This suggests the formation of a gel-like structure and increased elastic behavior. G’76°C,suspension and G’end,suspension are highest for potato and egg white protein, indicating the strongest gelling potential of all screened proteins. The irreversible heat coagulation of egg white proteins involves the formation of spherical aggregates via hydrophobic interactions, which are further stiffened through sulfhydryl–disulfide reactions to finally give rise to a gel, which explains the rather high values of G’76°C,suspension. Furthermore, the G’-values of egg white protein still increase (G’76°C,suspension vs. G’end,suspension) during cooling, which can be attributed to the numerous hydrogen bonds that are formed at lower temperatures [32]. The suspension of potato protein also exerted very good gelling properties upon heating. The low denaturation temperature of patatin, one of the main potato protein fractions, may be partially responsible for this. The denaturation temperature is roughly 20 °C lower compared to common food proteins as ovalbumin (egg) or soy glycinin [33,34]. Figure 1 reveals no significant difference between G’76°C,suspension of the egg white protein and G’76°C,suspension of soy concentrate, indicating good gelation characteristics of the latter. Pea proteins are mainly composed of globulins. Pea globulins are recognized for their lower gelling ability compared to their soy counterparts. This can also be observed in Figure 1, where G’76°C,suspension and G’end,suspension of pea proteins are significantly lower than the values of soy concentrate. The gelation of pea proteins appeared to be governed mainly by nonspecific interactions, whereas the involvement of disulfide bonds was reported for soy proteins [35]. Furthermore, high temperatures are required to induce the gelation of the pea proteins because of their high denaturation temperature (>85 °C) [36]. The applied thermal processing in this research was therefore not sufficient to obtain proper gelling of pea proteins. Whey protein suspensions start to form gels at concentrations higher than 80 mg whey protein/g H2O when heated above 75 °C [37]. The rather short heating time above 75 °C and applied concentration could therefore explain the somewhat low values of G’76°C,suspension, and G’end,suspension of whey protein. Blood plasma also showed relatively low values of both G’76°C,suspension, and G’end,suspension and is probably attributed to the same reasons as described for whey protein. Research stated that heating to 75 °C was a necessity to create strong gels from 4% w/v plasma protein solutions [38]. Other sources claimed that suspensions containing 4–5% blood plasma already tend to form firm and irreversible gels when temperatures over 70 °C are applied [39].
Figure 1.
G’76°C,suspension and δ76°C,suspension represent the elasticity modulus and phase angle of protein suspensions at the end of the isothermal heating at 76 °C for 3 min. G’end,suspension and δend,suspension represent the elasticity modulus and phase angle of protein suspensions after further cooling from 76 to 20 °C. (*) = no value of gelatin could be obtained. Mean values and standard deviations are presented (n = 4). Letters a–d: different letters indicate significant differences (p < 0.05) between different proteins.
Based upon the gathered data and literature study, potato and egg white protein show the most potential for use in meat products. Their gelling properties may lead to a better structure formation of the meat gel and as a consequence to improved water binding or texture of the sausages.
3.1.2. Emulsification
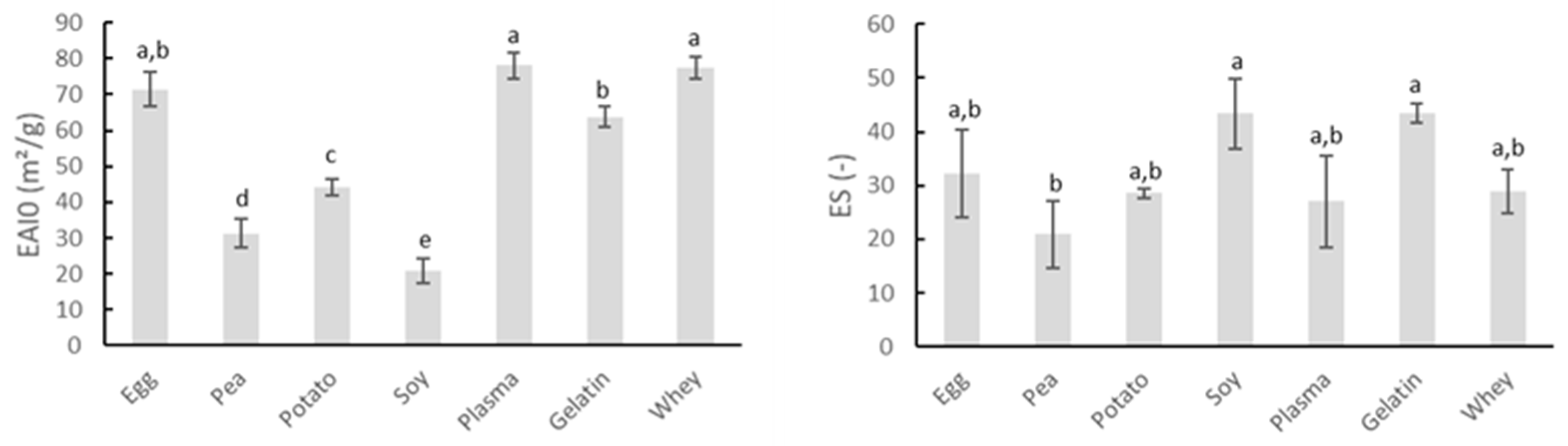
In addition to their gelation potential, proteins are also of interest to the meat industry because of their ability to stabilize emulsions. The intrinsic emulsifying and emulsion-stabilizing properties of the proteins can be derived from Figure 2. The EAI0 indicates the area of interface stabilized per unit weight of protein (m2/g) and is associated with the ability of the protein to coat the water–oil interface immediately after emulsion formation. ES represents the percentage of emulsion turbidity remaining after 10 min and therefore refers to the ability of an emulsion to resist changes in its properties over time, e.g., droplet coalescence, creaming, and/or flocculation [28].
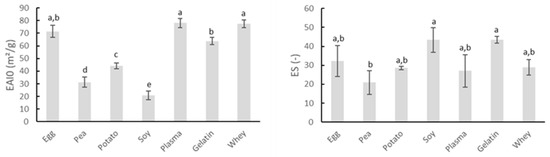
Figure 2.
Comparison of emulsifying activity index immediately after emulsion formation (EAI0, m2/g) and emulsion stability (ES) of several protein sources. Mean values and standard deviations are presented (n = 4). Letters a–e: different letters indicate significant differences (p < 0.05) between different proteins.
Gelatin possesses good emulsifying properties, as can be noticed by the high values of both EAI0 and ES in Figure 2. Gelatin is capable of reducing the surface tension of aqueous environments and forming the necessary identically charged film around the fat droplets of the dispersed phase. Therefore, the isoelectric point (IEP) is of great importance in the surface activity effects of the used gelatin [40]. The protein carried a net negative charge under the conditions in which this analysis was performed. Whey proteins are well-known for their ability to stabilize interfaces, explaining their great emulsifying properties, as seen in the present research [41]. Figure 2 also shows that blood plasma and egg white protein exerted excellent emulsifying properties. Research by Rodriguez Furlán, et al. [42] confirmed the good emulsifying properties of blood plasma. Yet, literature stated that ovalbumin, the major protein in egg white, may perform good emulsifying ability and stability under extreme acidic conditions, which is in contrast to the watery suspensions applied in this research, while under neutral and alkaline pH the stability of egg white emulsions was limited [43]. The emulsifying capacity of soy concentrate was rather limited, as indicated by the low value of EAI0 in Figure 2. The study of Amine, et al. [44] also indicated soy protein as a poor emulsifier for oil in water emulsions, based upon the measurement of oil droplet particle sizes. The same research presented potato protein as the better emulsifier compared to soy and pea proteins, as was the case in this study. Pea protein also exhibited poor emulsifying properties, as seen in Figure 2. Several studies concluded that pea proteins are usually inferior to traditional emulsifiers such as milk and egg proteins [45].
Results indicated the use of egg white protein, blood plasma, gelatin, or whey protein may be more beneficial in stabilizing meat emulsion regarding water and fat binding compared to the other screened proteins because of their high initial emulsion activity in combination with their good emulsion stability.
3.2. Impact of Seven Different Protein-Based Ingredients on the Quality Characteristics of Cooked Sausage
The results presented in the following sections deal with the impact of the selected proteins on several quality characteristics of phosphate-free sausage.
3.2.1. Dynamic Viscoelastic Properties of Meat Batters Influenced by Protein Source
Stress sweeps were performed to study the structure of the raw meat batter immediately after the grinding process, prior to thermal processing. Data (Table 1, Stress sweeps) indicated that the LVR, the stress range in which the structure of the sample remains intact, significantly (p < 0.05) increased when TSPP was eliminated (M-TSPP) compared to the model preparation containing phosphate (M+TSPP). Since TSPP has the ability to dissociate the actomyosin complex [1], the M+TSPP batter was presumably more sensitive to external deformation. This is reflected in a significantly (p < 0.05) lower δbatter value compared to the M-TSPP sample, the latter having more “solid-like” behavior. In parallel, the G*batter of M-TSPP was significantly higher compared to M+TSPP, indicating that M-TSPP showed high resistance to deformation. A higher LVRbatter, lower δbatter, and higher G*batter as a result of phosphate elimination were also seen in our previous study [6].

Table 1.
Structural parameters of sausage batters (stress sweeps) and hardness, pH, water, and fat binding characteristics (CL, TEF, and relative amount of H2O) of cooked sausages with phosphate (M+TSPP), without phosphate (M-TSPP), and several protein-based ingredients instead of phosphate (M-TSPP+protein). Mean values and standard deviations are presented (n = 2). Different letters indicate significant differences at p < 0.05. Abbreviations used: M (Model), TSPP (tetrasodium pyrophosphate), LVRbatter (linear viscoelastic region of the sausage batter), G*batter (complex modulus of the sausage batter), δbatter (phase angle of the sausage batter), TEF (total expressible fluid), and CL (cooking loss). Superscripts a–e: different letters indicate significant differences (p < 0.05) between different treatments.
The addition of protein-based ingredients to phosphate-free raw sausage batters did not affect the LVRbatter or G*batter compared to the M-TSPP preparations, with the exception of the preparation with gelatin (M-TSPP+gelatin) and egg white protein (M-TSPP+egg). The addition of gelatin to phosphate-free raw sausage batter (M-TSPP+gelatin) significantly increased the G*batter, which can possibly be attributed to the cold gelling capacity of the protein [46]. Raw phosphate-free sausage batter containing 2% egg white protein (M-TSPP+egg) gave rise to a significantly lower LVRbatter, and, at the same time, a remarkably high G*batter compared to M-TSPP. An explanation of this striking observation is given in Appendix A.
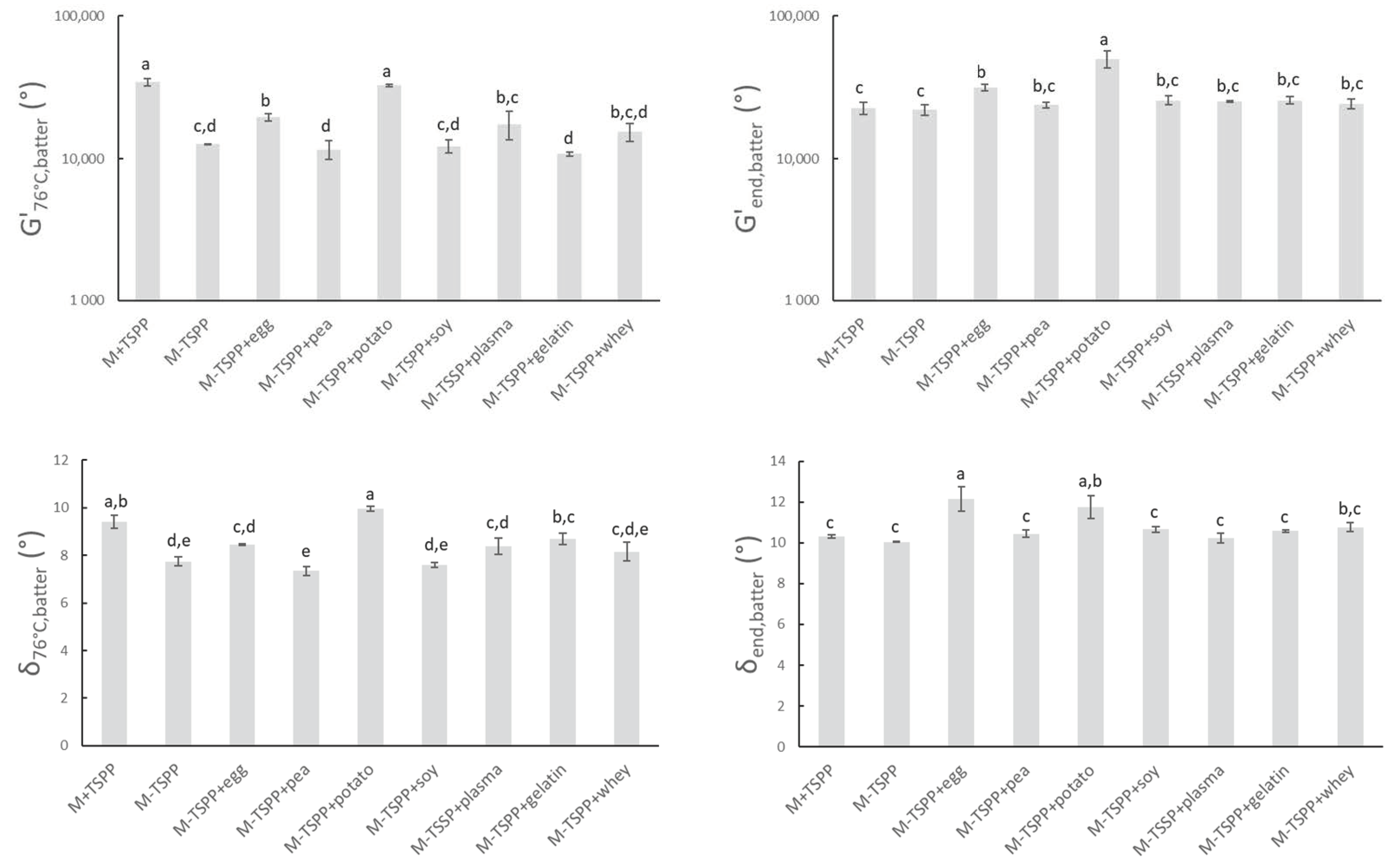
To study the rheological properties of the sausage batters during thermal processing, all samples were subjected to a temperature sweep as described in Section 2.3. The heat causes the myofibrillar proteins to unfold and/or dissociate, followed by association and aggregation, resulting in a gelled system in which water and fat are entrapped [47,48]. The high G’batter-values in Figure 3 confirmed the formation of gel structures. δ76°C,batter is lower than 10° for all batters, indicating a strong elastic behavior of the formed network. Significant differences in δ76°C,batter between batters have little relevance.
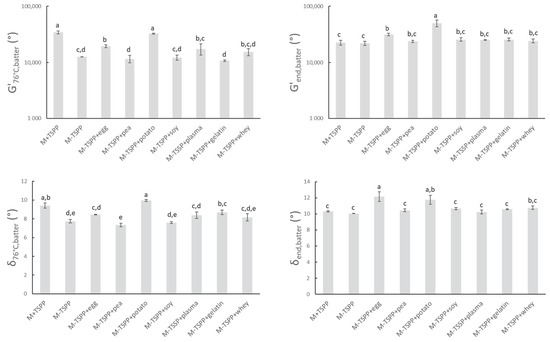
Figure 3.
G’76°C,batter and δ76°C,batter represent the elasticity modulus and phase angle, respectively, of sausage batters during rheological measurements at the end of isothermal heating at 76 °C. G’end,batter and δend,batter represent the elasticity modulus and phase angle of sausage batters after further cooling from 76 to 20 °C. Mean values and standard deviations are presented (n = 2). Abbreviations used: M (model) and TSPP (tetrasodium pyrophosphate). Letters a–e: different letters indicate significant differences (p < 0.05) between treatments.
Comparison between M+TSPP and M-TSPP indicated that the elimination of phosphate had an effect on the viscoelastic behavior of the meat batter during heating (G’76°C,batter δ76°C,batter). Values of G’76,batters revealed that phosphate elimination significantly reduced gel strength at the end of heating prior to cooling. On the other hand, upon subsequent cooling, no significant differences could be observed anymore between M+TSPP and M-TSPP (G’end,batter and δend,batter). The stronger increase of G’ during heating upon the addition of phosphates was possibly caused by conformation transitions, exposure of hydrophobic groups, and the formation of more disulfide bonds of the meat proteins [49]. In other words, TSPP promoted gelation, as it aids in the extraction of myofibrillar proteins that will subsequently aggregate and gel upon thermal processing [48,50,51,52]. However, Sun and Holley [48] also reported that it was possible that polyphosphates do not influence myofibrillar gel strength, as this is dependent on the applied protein source and preparation and gelation conditions that are used.
The strong gelling properties of potato protein nullified the drop in G’76°C,batter due to phosphate elimination (M-TSPP+potato). A similar observation can be made when egg white protein (M-TSPP+egg) is used, although the total impact of phosphate elimination on G’76,batters could not be compensated, since a significant difference in G’76°C,batters between M+TSPP and M-TSPP+egg remained. Furthermore, G’end,batter and δend,batter significantly increased (p < 0.05) with the addition of egg white (M-TSPP+egg) and potato proteins (M-TSPP+potato) compared to M-TSPP. Potato and egg white proteins probably formed additional protein networks or improved interactions for gel formation compared to the other proteins, leading to increased structure formation. Studies on the impact of egg albumin on the thermal gelation of myofibrillar proteins are contradictory. Some authors concluded egg proteins caused disruption of the meat gel by interfering with the gelling process of the myofibrillar proteins or by the formation of mixed egg–myofibrillar protein gels, while others reported egg proteins participated in meat gel network formation [53]. Hunt, et al. [54] also observed a positive effect on gelation characteristics of Alaska pollock fish protein upon the addition of dried egg white protein. No significant difference in G’76°C,batters, G’end,batter, or δend,batter between M-TSPP and preparations with pea, gelatin, whey, blood plasma, or soy concentrate (M-TSPP+pea, M-TSPP+gelatin, M-TSPP+whey, M-TSPP+plasma, and M-TSPP+soy, respectively) could be observed. On the other hand, the studies of Wang, et al. [55] and Li, et al. [56] claimed an improvement of the gelling characteristics and structural strength of myofibrillar protein gels upon the addition of soy protein. Additionally, the addition of blood plasma has been shown to affect the thermal gelation of myofibrils and therefore influence the final gel strength [57,58]. Sun and Holley [48] stated that due to a lack of interaction between nonmeat and muscle proteins, it is possible that texture is negatively affected by interference with the gelation of the myofibrillar proteins. This could not be deducted from Figure 3, as the final gel strength (G’end,batter) of all phosphate-free batches with different proteins is similar or higher on average compared to M-TSPP.
The rather strong gelation potential of egg white and potato protein in the meat matrix during heating was also seen in the watery medium (Figure 1), as described in Section 3.1.1. Despite this similarity, batter parameters G’76°C,batter and G’end,batter were significantly higher for potato protein compared to egg white protein, which was not the case for G’76°C, suspension and G’end,suspension of the same proteins. Furthermore, blood plasma and whey protein resulted in similar values of G’76°C,batter and G’end,batter compared to egg white protein, which was not observed in G’76°C, suspension and G’end,suspension of the same proteins. In contrast, soy concentrate resulted in a significantly lower value of G’76°C,batter compared to egg white protein, while this was not the case for G’76°C,suspension. On the other hand, G’76°C,batter was lowest for preparations with pea protein and gelatin, which was identically reflected in G’76°C,suspension.
Evaluating these insights, it seems that the gelation potential of the different proteins, as determined in an aqueous medium during thermal processing (Section 3.1.1), was not always clearly noticeable in a meat system. This suggests the importance of a well-defined meat matrix, imitating industrial meat products, to determine and understand the impact of ingredient functionality. A food environment is a more complex system, where ingredients and other components (i.e., salts, lipids, and proteins) may interact, thus modifying the added value to the product quality of the functional ingredient.
3.2.2. Emulsion Stability of Meat Batters and Cooking Loss of Cooked Sausages Influenced by Protein Source
Significant (p < 0.05) differences in emulsion stability and cooking loss (CL) were found between the different preparations (Table 1). Elimination of TSPP (M-TSPP) resulted in a significant increase of total expressible fluid (TEF) and thus lower emulsion stability, and increased CL compared to M+TSPP. These findings are in line with our former study [6]. It is known from the literature that TSPP is able to dissociate the actomyosin complex, releasing myosin, which can act as a natural emulsifier. Additionally, more myofibrillar proteins are extracted by TSPP, helping to stabilize the protein matrix in which water and fat are entrapped [1].
Preparations containing egg white protein (M-TSPP+egg), pea (M-TSPP+pea), soy concentrate (M-TSPP+soy), and blood plasma proteins (M-TSPP+plasma) significantly (p < 0.05) reduced TEF compared to M-TSPP and even resulted in similar percentages of TEF as the preparation containing phosphate (M+TSPP), indicating an equal stabilization of moisture and fat in the meat matrix. These proteins were thus able to compensate for the decreased emulsion stability due to phosphate elimination. On the other hand, there was no significant difference in TEF between M-TSPP and preparations with the addition of potato (M-TSPP+potato), whey proteins (M-TSPP+whey), and gelatin (M-TSPP+gelatin). Furthermore, the use of some proteins also caused a shift in composition (water vs. fat) of the drip loss. The relative amount of fat in the drip loss was significantly higher when adding blood plasma (M-TSPP+plasma), gelatin (M-TSPP+gelatin), soy concentrate (M-TSPP+soy), or whey protein (M-TSPP+whey) to phosphate-free sausages (M-TSPP). This could mean that fat stabilization in the meat matrix could be altered by using additional proteins, which could affect the final product characteristics such as texture or mouthfeel [59].
In almost all cases, CL significantly (p < 0.05) decreased with the addition of protein-based ingredients compared to M-TSPP. The addition of blood plasma (M-TSPP+plasma), whey proteins (M-TSPP+whey), egg white proteins (M-TSPP+egg), and soy concentrate (M-TSPP+soy) even resulted in similar CL as the cooked sausages containing phosphate (M+TSPP). Blood plasma proteins are good emulsifiers [9] and were found to be a useful substitute for polyphosphate in frankfurters, as they did not affect the water holding capacity and cooking losses compared to frankfurters containing 0.5% sodium tripolyphosphate [21]. Research by Prabhu [60] also indicated blood plasma was suitable to improve the emulsion stability, texture, flavor, and juiciness of comminuted meat products. Additionally, the use of pea protein (M-TSPP+pea) could significantly decrease CL compared to M-TSPP, although to a lesser extent than the previously mentioned proteins. On the other hand, the addition of gelatin (M-TSPP+gelatin) or potato protein (M-TSPP+potato) did not change CL compared to M-TSPP. This was in contrast with the study by Nieto, Castillo, Xiong, Álvarez, Payne and Garrido [20] in which cooking losses were reduced when 2.5% hydrolyzed potato proteins were added to phosphate-free meat emulsions.
Comparison between the intrinsic properties of the proteins discussed in Section 3.1 and their impact on fat and water binding characteristics of cooked sausages indicated limited analogy. Stronger gelation potential of the protein samples as measured in the watery medium would suggest better water and especially fat binding in meat products. Furthermore, proteins with good emulsifying capacities are expected to be able to stabilize emulsified meat products to a greater extent and contribute to reducing cooking loss (especially fat release). While potato and egg white protein both showed very good gelation properties, only the latter could positively improve the cooking yield. In contrast, pea proteins showed both low emulsifying capacity and gelling behavior in the watery medium, while in the cooked sausages, they could reduce cooking loss and TEF. Results even surpassed those of potato protein. Blood plasma proteins, showing an average gelation potential and good attribution to emulsion stability in the watery environment, outperformed the other proteins, with the exception of soy concentrate, regarding water and fat binding in cooked sausages. Again, these results underline the importance of a well-defined meat matrix, close to industrial meat products, to determine and fully understand the impact of ingredient functionality.
3.2.3. pH of Cooked Sausages Influenced by Alternative Protein Source
Data (Table 1) showed that the elimination of TSPP (M-TSPP) resulted in significantly lower pH values compared to the model system containing TSPP (M+TSPP), which was in line with our former study [6]. The pH of TSPP (1% solution) is equal to 10.2 [1], which explains the pH difference between preparations M-TSPP and M+TSPP. Due to phosphate elimination, the pH of the meat product was decreased and was closer to the iso-electric pH of the myofibrillar proteins. This led to a reduction in their net charge and repulsion between proteins, causing a negative impact on water and fat binding, as seen in Section 3.2.2 [61]. The decrease in pH by phosphate elimination could not be compensated by the addition of protein-based ingredients, as seen in Table 1. Velemir, et al. [62] determined no significant difference in pH upon the addition of 1.5% whey or soy protein to sausages. Blood plasma, despite its higher pH, could also not remediate the lower pH of phosphate-free sausages, which was also seen in the research of Hurtado, Saguer, Toldrà, Parés and Carretero [21]. The proteins could therefore not contribute to water binding by generating a higher concentration of negative meat protein charges.
3.2.4. Textural Properties of Cooked Sausages Influenced by Protein Type
The differences in hardness of the different preparations are limited (Table 1). Phosphate elimination (M-TSPP) did not significantly affect the hardness of the cooked sausages, which was in line with our former study [6]. A lower hardness might be expected when TSPP is eliminated, since TSPP dissociates the actomyosin complex, resulting in more proteins being available for emulsification and the formation of a more stable gel matrix during heating. Yet, gel strength at the end of thermal processing (see Section 3.2.1) also revealed no difference in G’end,batter between M+TSPP and M-TSPP. On the other hand, the increase in CL when phosphate is eliminated could lead to a firmer meat product. The addition of gelatin (M-TSPP+gelatin) increased hardness compared to M-TSPP, despite no significant difference in CL being measured. Therefore, it could be concluded that gelatin itself had an impact on the final hardness of the phosphate-free cooked sausage, which could probably be attributed to its cold gelling properties [46]. The addition of the other protein-based ingredients did not significantly affect hardness compared to M-TSPP, but a significant increase in hardness compared to the reference sausage containing phosphate (M+TSPP) was determined upon the addition of pea, potato, egg white protein, and again gelatin. Nieto, Castillo, Xiong, Álvarez, Payne and Garrido [20] found that the addition of 2.5% hydrolyzed potato proteins had no effect on the hardness of phosphate-free frankfurters, which was also in line with our results. Furthermore, Youssef and Barbut [14] concluded that soy protein could increase or decrease the product texture depending on the type of soy used. The impact of whey proteins on hardness is linked to their degree of denaturation, which is dependent on their production process. In general, undenatured whey protein preparations deteriorate textural properties, while partially denatured whey concentrates enhance the binding and texture of sausages and other comminuted meat products [25]. This could possibly explain the mild impact on the observed hardness of whey proteins. Fernandez, et al. [63] also found no difference in hardness when 2% of dried egg white was added to chicken meat batters. Cofrades, Guerra, Carballo, Fernández-Martín and Colmenero [19] noted an increase in the product hardness of Bologna sausages when blood plasma was applied. This observation could not be established in this research.
4. Conclusions
The elimination of phosphate had a negative impact on several quality characteristics of cooked sausages. Next to an increase in cooking loss and reduced emulsion stability, a change in gel network formation during thermal processing could be observed, although the final gel strength was not influenced. The cause of these quality losses is mainly related to the reduced functionality of the myofibrillar proteins due to phosphate elimination. This research indicated that the addition of specific proteins could remediate the negative impact of phosphate elimination. However, it is important to keep in mind that different protein sources exhibit varying potential in this respect. Hereby, it is crucial to evaluate the potential of the proteins in a well-defined standardized meat matrix. The intrinsic protein properties, gelation and emulsification, related to improving meat quality are often evaluated in aqueous media. This study showed that protein characteristics determined in this manner did not entirely reflect their capacity to enhance the characteristics of phosphate-free emulsified meat products.
In phosphate-free cooked sausages, blood plasma and soy protein overall showed the most promising results, as no significant differences in terms of product hardness, cooking yield, or emulsion stability could be found compared to standard phosphate-containing sausages. These proteins may therefore provide an added value for the meat industry to further reduce E-numbers and contribute to the healthy image of meat products. Other screened proteins, such as egg white, pea, and whey protein, also proved to be beneficial, yet the quality level of the phosphate-containing sausages could not be equaled. Potato protein and gelatin showed the least improvement to the phosphate-free cooked sausages.
Future research can be conducted on the use of combinations of different protein sources or mixtures of proteins with certain hydrocolloids to further remediate the loss of quality due to phosphate elimination in emulsified meat products.
Author Contributions
Conceptualization: O.G. and I.F.; formal analysis: S.G.; funding acquisition: I.F.; investigation: O.G., M.G., and L.S.; methodology: O.G. and I.F.; project administration: I.F.; resources: I.F.; supervision: I.F.; validation: M.G. and L.S.; visualization: O.G.; writing—original draft: O.G. and S.G.; writing—review and editing: M.G., L.S., and I.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was 80% funded by Flanders Food and the Agency for Innovation by Science and Technology (IWT), grant number IWT 130500, and 20% co-funded by the Flemish meat and ingredient producing industry.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Participating companies in the research (co-funding partners) had the opportunity to propose ingredients to be included in the study. However, the final decision of ingredient choice was solely and independently made by the researchers. Protein-based ingredients were chosen to obtain a good mix of different animal and vegetable sources and with relevance to the broad industry. Furthermore, they had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Raw phosphate-free sausage batter containing 2% egg white protein (M-TSPP+egg) gave rise to a significantly lower LVRbatter, and, at the same time, a remarkably high G*batter compared to M-TSPP, as seen in Table 1 (main text). In order to gain insight into this striking observation, the stress sweeps as seen in Figure A1 were evaluated.
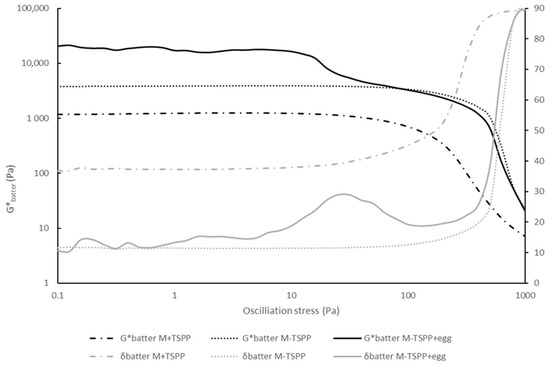
Figure A1.
Stress sweep curves of meat batters containing phosphate (M+TSPP, dash-dot line), without phosphate (M-TSPP, dotted line), and without phosphate-containing egg white proteins (M-TSPP+egg, full line). The complex modulus (G*, black color) and phase angle (δ, grey color) are displayed on the primary and secondary y-axis, respectively. Confidence intervals are not included to enhance the readability of the graph (n = 2).
Figure A1.
Stress sweep curves of meat batters containing phosphate (M+TSPP, dash-dot line), without phosphate (M-TSPP, dotted line), and without phosphate-containing egg white proteins (M-TSPP+egg, full line). The complex modulus (G*, black color) and phase angle (δ, grey color) are displayed on the primary and secondary y-axis, respectively. Confidence intervals are not included to enhance the readability of the graph (n = 2).
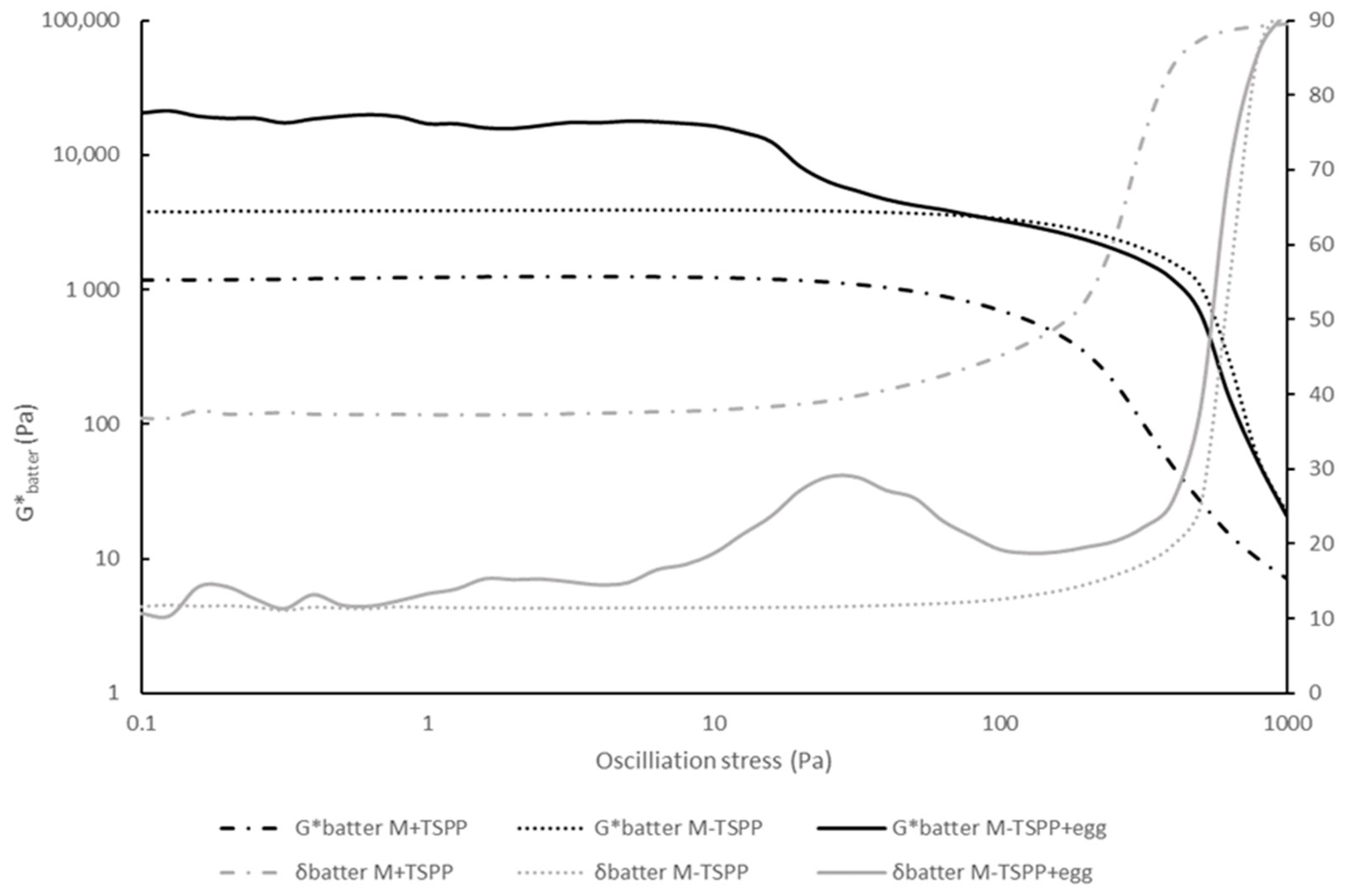
According to Glorieux, Goemaere, Steen and Fraeye [6], the LVR is calculated as the stress level at which G* deviates more than 5% from a constant G* (plateau) value and indicates irreversible structure breakdown. However, the raw sausage batter containing egg white proteins (M-TSPP+egg white) contained two plateau regions in which G’ and G” (and thus G*) were independent of the applied stress amplitude. The first plateau was characterized by a high Gbatter* value and ranged up to ±10 Pa, the stress value at which structure breakdown occurred. However, from around a stress value of 30 Pa, the structure stabilized again, resulting in another plateau that reached stress values of ±200 Pa until irreversible structure breakdown occurred (Figure A1). The two LVR regions could possibly be explained by the presence of two distinct protein structures. The first plateau is characterized by G*batter higher than G*batter of M-TSPP, which may be attributed to the presence of the egg white proteins. The second plateau is characterized by G*batter values in the same order of magnitude as M-TSPP, and the LVR ends at a comparable stress value, presumably indicating that this part of the LVR was stabilized independently of the added protein-based ingredient.
References
- Long, N.H.B.S.; Gál, R.; Buňka, F. Use of phosphates in meat products. Afr. J. Biotechnol 2011, 10, 19874–19882. [Google Scholar] [CrossRef]
- Balestra, F.; Petracci, M. Technofunctional Ingredients for Meat Products: Current Challenges. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–68. [Google Scholar] [CrossRef]
- European Food Safety Authority. Assessment of one published review on health risks associated with phosphate additives in food. EFSA J. 2013, 11, n. [Google Scholar] [CrossRef]
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate Additives in Food-a Health Risk. Dtsch. Arztebl. Int. 2012, 109, 49–55. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; Mennes, W.; et al. Re-evaluation of phosphoric acid–phosphates – di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Glorieux, S.; Goemaere, O.; Steen, L.; Fraeye, I. Phosphate Reduction in Emulsified Meat Products: Impact of Phosphate Type and Dosage on Quality Characteristics. Food Technol. Biotechnol. 2017, 55, 390–397. [Google Scholar] [CrossRef] [PubMed]
- van Gunst, A.; Roodenburg, A.J.C. Consumer Distrust about E-numbers: A Qualitative Study among Food Experts. Foods 2019, 8, 178. [Google Scholar] [CrossRef]
- Sen, A.R.; Naveena, B.M.; Muthukumar, M.; Babji, Y.; Murthy, T.R.K. Effect of chilling, polyphosphate and bicarbonate on quality characteristics of broiler breast meat. Br. Poult. Sci. 2005, 46, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Bianchi, M.; Mudalal, S.; Cavani, C. Functional ingredients for poultry meat products. Trends Food Sci. Technol. 2013, 33, 27–39. [Google Scholar] [CrossRef]
- Solomon, M.B.; Liu, M.N.; Patel, J.; Paroczay, E.; Eastridge, J.; Coleman, S.W. Tenderness improvement in fresh or frozen/thawed beef steaks treated with hydrodynamic pressure processing. J. Muscle Foods 2008, 19, 98–109. [Google Scholar] [CrossRef]
- Thangavelu, K.P.; Kerry, J.P.; Tiwari, B.K.; McDonnell, C.K. Novel processing technologies and ingredient strategies for the reduction of phosphate additives in processed meat. Trends Food Sci. Technol. 2019, 94, 43–53. [Google Scholar] [CrossRef]
- Phillips, G.O.; Williams, P.A. Handbook of Food Proteins; Elsevier Science & Technology: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Effects of caseinate, whey and milk proteins on emulsified beef meat batters prepared with different protein levels. J. Muscle Foods 2010, 21, 785–800. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Effects of two types of soy protein isolates, native and preheated whey protein isolates on emulsified meat batters prepared at different protein levels. Meat Sci. 2011, 87, 54–60. [Google Scholar] [CrossRef]
- Egbert, W.R.; Payne, C.T. Plant Proteins. In Ingredients in Meat Products: Properties, Functionality and Applications; Tarté, R., Ed.; Springer: New York, NY, USA, 2009; pp. 111–129. [Google Scholar] [CrossRef]
- Tarté, R. Meat-Derived Protein Ingredients. In Ingredients in Meat Products: Properties, Functionality and Applications; Tarté, R., Ed.; Springer: New York, NY, USA, 2009; pp. 145–171. [Google Scholar] [CrossRef]
- Feiner, G. Additives: Proteins, carbohydrates, fillers and other additives. In Meat Products Handbook; Feiner, G., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 89–141. [Google Scholar] [CrossRef]
- Choe, J.-H.; Kim, H.-Y.; Lee, J.-M.; Kim, Y.-J.; Kim, C.-J. Quality of frankfurter-type sausages with added pig skin and wheat fiber mixture as fat replacers. Meat Sci. 2013, 93, 849–854. [Google Scholar] [CrossRef]
- Cofrades, S.; Guerra, M.A.; Carballo, J.; Fernández-Martín, F.; Colmenero, F.J. Plasma Protein and Soy Fiber Content Effect on Bologna Sausage Properties as Influenced by Fat Level. J. Food Sci. 2000, 65, 281–287. [Google Scholar] [CrossRef]
- Nieto, G.; Castillo, M.; Xiong, Y.L.; Álvarez, D.; Payne, F.A.; Garrido, M.D. Antioxidant and emulsifying properties of alcalase-hydrolyzed potato proteins in meat emulsions with different fat concentrations. Meat Sci. 2009, 83, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, S.; Saguer, E.; Toldrà, M.; Parés, D.; Carretero, C. Porcine plasma as polyphosphate and caseinate replacer in frankfurters. Meat Sci. 2012, 90, 624–628. [Google Scholar] [CrossRef]
- Pereira, A.G.T.; Ramos, E.M.; Teixeira, J.T.; Cardoso, G.P.; Ramos, A.d.L.S.; Fontes, P.R. Effects of the addition of mechanically deboned poultry meat and collagen fibers on quality characteristics of frankfurter-type sausages. Meat Sci. 2011, 89, 519–525. [Google Scholar] [CrossRef]
- Lowder, A.C.; Goad, C.L.; Lou, X.; Morgan, J.B.; Mireles DeWitt, C.A. Evaluation of a dehydrated beef protein to replace sodium-based phosphates in injected beef strip loins. Meat Sci. 2011, 89, 491–499. [Google Scholar] [CrossRef]
- Muguruma, M.; Tsuruoka, K.; Katayama, K.; Erwanto, Y.; Kawahara, S.; Yamauchi, K.; Sathe, S.K.; Soeda, T. Soybean and milk proteins modified by transglutaminase improves chicken sausage texture even at reduced levels of phosphate. Meat Sci. 2003, 63, 191–197. [Google Scholar] [CrossRef]
- Xiong, Y.L. Dairy Proteins. In Ingredients in Meat Products: Properties, Functionality and Applications; Tarté, R., Ed.; Springer: New York, NY, USA, 2009; pp. 131–144. [Google Scholar] [CrossRef]
- Dàvila, E.; Parés, D.; Cuvelier, G.; Relkin, P. Heat-induced gelation of porcine blood plasma proteins as affected by pH. Meat Sci. 2007, 76, 216–225. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Dynamic oscillatory rheological measurement and thermal properties of pea protein extracted by salt method: Effect of pH and NaCl. J. Food Eng. 2011, 105, 577–582. [Google Scholar] [CrossRef]
- Steen, L.; Glorieux, S.; Goemaere, O.; Brijs, K.; Paelinck, H.; Foubert, I.; Fraeye, I. Functional Properties of Pork Liver Protein Fractions. Food Bioprocess. Tech. 2016, 9, 970–980. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Sultana, S.; Ali, M.E.; Ahamad, M.N.U. Gelatine, collagen, and single cell proteins as a natural and newly emerging food ingredients. In Preparation and Processing of Religious and Cultural Foods; Ali, M.E., Nizar, N.N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 215–239. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I. 5-Gelatin. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 92–115. [Google Scholar] [CrossRef]
- Strixner, T.; Kulozik, U. 7-Egg proteins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 150–209. [Google Scholar] [CrossRef]
- Alting, A.C.; Pouvreau, L.; Giuseppin, M.L.F.; van Nieuwenhuijzen, N.H. 12-Potato proteins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 316–334. [Google Scholar] [CrossRef]
- Katzav, H.; Chirug, L.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. Comparison of Thermal and High-Pressure Gelation of Potato Protein Isolates. Foods 2020, 9, 1041. [Google Scholar] [CrossRef]
- Mession, J.-L.; Sok, N.; Assifaoui, A.; Saurel, R. Thermal denaturation of pea globulins (Pisum sativum L.)-molecular interactions leading to heat-induced protein aggregation. J. Agric. Food Chem. 2013, 61, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, W.; Feizollahi, E.; Roopesh, M.S.; Chen, L. Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innovative Food Sci. Emerg. Technol. 2021, 67. [Google Scholar] [CrossRef]
- Boye, J.I.; Alli, I.; Ismail, A.A.; Gibbs, B.F.; Konishi, Y. Factors affecting molecular characteristics of whey protein gelation. Int. Dairy J. 1995, 5, 337–353. [Google Scholar] [CrossRef]
- O’Riordan, D.; Kinsella, J.E.; Mulvihill, D.M.; Morrissey, P.A. Gelation of plasma proteins. Food Chem. 1989, 33, 203–214. [Google Scholar] [CrossRef]
- Tarté, R. Meat protein ingredients. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 56–91. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. From Collagen to Gelatine. In Gelatine Handbook; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007; pp. 45–117. [Google Scholar] [CrossRef]
- Boland, M. Whey proteins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 30–55. [Google Scholar] [CrossRef]
- Rodriguez Furlán, L.; Rinaldoni, A.N.; Padilla, A.; Campderros, M. Assessment of Functional Properties of Bovine Plasma Proteins Compared with Other Protein Concentrates, Application in a Hamburger Formulation. Am. J. Food Technol. 2011, 6, 717–729. [Google Scholar] [CrossRef]
- Chang, C.; Li, X.; Li, J.; Niu, F.; Zhang, M.; Zhou, B.; Su, Y.; Yang, Y. Effect of enzymatic hydrolysis on characteristics and synergistic efficiency of pectin on emulsifying properties of egg white protein. Food Hydrocoll. 2017, 65, 87–95. [Google Scholar] [CrossRef]
- Amine, C.; Dreher, J.; Helgason, T.; Tadros, T. Investigation of emulsifying properties and emulsion stability of plant and milk proteins using interfacial tension and interfacial elasticity. Food Hydrocoll. 2014, 39, 180–186. [Google Scholar] [CrossRef]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Totosaus, A.; Montejano, J.G.; Salazar, J.A.; Guerrero, I. A review of physical and chemical protein-gel induction. Int. J. Food Sci. Tech. 2002, 37, 589–601. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. Factors Influencing Gel Formation by Myofibrillar Proteins in Muscle Foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Shan, L.; Li, Y.; Wang, Q.; Wang, B.; Guo, L.; Sun, J.; Xiao, J.; Zhu, Y.; Zhang, X.; Huang, M.; et al. Profiles of gelling characteristics of myofibrillar proteins extracted from chicken breast: Effects of temperatures and phosphates. LWT 2020, 129, 109525. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, W.; Huang, J.; Xiong, Y.L. Effects of sodium pyrophosphate coupled with catechin on the oxidative stability and gelling properties of myofibrillar protein. Food Hydrocoll. 2020, 104, 105722. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Y.L. Gelation of Chicken Muscle Myofibrillar Proteins Treated with Protease Inhibitors and Phosphates. J. Agric. Food Chem. 1997, 45, 3437–3442. [Google Scholar] [CrossRef]
- Çarkcioğlu, E.; Rosenthal, A.J.; Candoğan, K. Rheological and Textural Properties of Sodium Reduced Salt Soluble Myofibrillar Protein Gels Containing Sodium Tri-Polyphosphate. J. Texture Stud. 2016, 47, 181–187. [Google Scholar] [CrossRef]
- Pietrasik, Z. Binding and textural properties of beef gels processed with κ-carrageenan, egg albumin and microbial transglutaminase. Meat Sci. 2003, 63, 317–324. [Google Scholar] [CrossRef]
- Hunt, A.; Park, J.W.; Handa, A. Effect of Various Types of Egg White on Characteristics and Gelation of Fish Myofibrillar Proteins. J. Food Sci. 2009, 74, C683–C692. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, J.; Jiang, L.; Li, Y.; Wang, J.; Zhang, H.; Li, D.; Han, F.; Li, Q.; Wang, R.; et al. Effect of the interaction between myofibrillar protein and heat-induced soy protein isolates on gel properties. CYTA J. Food 2015, 13, 527–534. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Dong, X.; Li, K.; Wang, Y.; Wang, Y.; Du, M.; Zhang, J.; Bai, Y. Effect of chickpea (Cicer arietinumL.) protein isolate on the heat-induced gelation properties of pork myofibrillar protein. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- AUTIO, K.; MIETSCH, F. Heat-induced Gelation of Myofibrillar Proteins and Sausages: Effect of Blood Plasma and Globin. J. Food Sci. 1990, 55, 1494–1496. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt-extracted pea protein gels. Food Hydrocoll. 2012, 28, 325–332. [Google Scholar] [CrossRef]
- Gordon, A.; Barbut, S.; Schmidt, G. Mechanisms of meat batter stabilization: A review. Crit. Rev. Food Sci. Nutr. 1992, 32, 299–332. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, G.A. Utilizing functional meat-based proteins in processed meat applications. In Proceedings of the 55th Annual Reciprocal Meat Conference, American Meat Science Association, Savoy, IL, USA, 29 July 2002; pp. 29–34. [Google Scholar]
- Offer, G.; Trinick, J. On the mechanism of water holding in meat: The swelling and shrinking of myofibrils. Meat Sci. 1983, 8, 245–281. [Google Scholar] [CrossRef]
- Velemir, A.; Mandić, S.; Vučić, G.; Savanović, D. Effects of non-meat proteins on the quality of fermented sausages. Foods Raw Mater. 2020, 8, 259–267. [Google Scholar] [CrossRef]
- Fernandez, P.; Cofrades, S.; Solas, M.T.; Carballo, J.; Colmenero, F.J. High Pressure-Cooking of Chicken Meat Batters with Starch, Egg White, and Iota Carrageenan. J. Food Sci. 1998, 63, 267–271. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).